Study on Adsorption and Aggregation in the Mixed System of Polyacrylamide, Cu(II) Ions and Innovative Carbon–Silica Composite
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Surface Charge Density, Zeta Potential and Functional Groups of the Carbon–Silica Composite
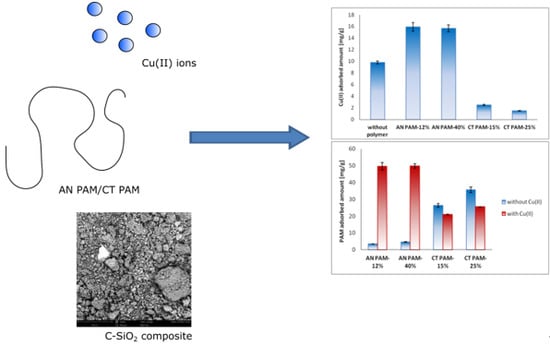
3.2. Adsorbed AMount of Ionic Polyacrylamide and Cu(II) Ions on the C-SiO2 Surface in the Single and Mixed Systems
3.3. C-SiO2 Aggregation without and with Ionic Polyacrylamide/Cu(II) Ions in the Simple and Mixed Systems
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ościk, J. Adsorption; UMCS: Lublin, Poland, 1969. (In Polish) [Google Scholar]
- Silva-Bermudez, P.; Rodil, S.E. An overview of protein adsorption on metal oxide coatings for biomedical implants. Surf. Coat. Technol. 2013, 233, 147–158. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Szewczuk-Karpisz, K. Removal possibilities of colloidal chromium(III) oxide from water using polyacrylic acid. Env. Sci. Pollut. Res. 2013, 20, 3657–3669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schramm, P.T.; Johnson, C.J.; McKenzie, D.; Aiken, J.M.; Pedersen, J.A. Potential role of soil in the transmission of prion disease. Geochemistry 2006, 64, 135–152. [Google Scholar]
- Kojima, C.; Watanabe, K. Adsorption and desorption of bioactive proteins on hydroxyapatite for protein delivery systems. J. Drug Deliv. 2012, 932461. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Jeurnink, T.J. Fouling of heat exchangers in the dairy industry. Exp. Therm. Fluid Sci. 1997, 14, 407–424. [Google Scholar] [CrossRef]
- Králik, M. Adsorption, chemisorptions, and catalysis. Chem. Pap. 2014, 68, 1625–1638. [Google Scholar] [CrossRef]
- Pham, T.D.; Tran, T.T.; Le, V.A.; Pham, T.T.; Dao, T.H.; Le, T.S. Adsorption characteristics of molecular oxytetracycline onto alumina particles: The role of surface modification with an anionic surfactant. J. Mol. Liq. 2019, 287, 110900. [Google Scholar] [CrossRef]
- Nguyen, T.M.T.; Do, T.P.T.; Hoang, T.S.; Nguyen, N.V.; Pham, H.D.; Nguyen, T.D.; Pham, T.N.M.; Le, T.S.; Pham, T.D. Adsorption of anionic surfactants onto alumina: Characteristics, mechanisms, and application for heavy metal removal. Int. J. Polymer Sci. 2018, 2830286, 1–11. [Google Scholar] [CrossRef]
- Pham, T.D.; Do, T.U.; Pham, T.T.; Nguyen, T.A.H.; Nguyen, T.K.T.; Vu, N.D.; Vu, C.M.; Kobayashi, M. Adsorption of poly(styrenesulfonate) onto different-sized alumina particles: Characteristics and mechanisms. Colloid Polymer Sci. 2019, 297, 13–22. [Google Scholar] [CrossRef]
- Pham, T.D.; Bui, T.T.; Nguyen, V.T.; Bui, T.K.V.; Tran, T.T.; Phan, Q.C.; Pham, T.D.; Hoang, T.H. Adsorption of polyelectrolyte onto nanosilica synthesized from rice husk: Characteristics, mechanisms, and applications for antibiotic removal. Polymers 2018, 10, 220. [Google Scholar] [CrossRef] [Green Version]
- Pham, T.D.; Bui, T.T.; Truong, T.T.T.; Hoang, T.H.; Le, T.S.; Duong, V.D.; Yamaguchi, A.; Kobayashi, M.; Adachi, Y. Adsorption characteristics of beta-lactam cefixime onto nanosilica fabricated from rice HUSK with surface modification by polyelectrolyte. J. Mol. Liq. 2020, 298, 111981. [Google Scholar] [CrossRef]
- Pham, T.D.; Vu, T.N.; Nguyen, H.L.; Le, P.H.P.; Hoang, T.S. Adsorptive removal of antibiotic ciprofloxacin from aqueous solution using protein-modified nanosilica. Polymers 2020, 12, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.D.; Pham, T.T.; Phan, M.N.; Ngo, T.M.V.; Dang, V.D.; Vu, C.M. Adsorption characteristics of anionic surfactant onto laterite soil with differently charged surfaces and application for cationic dye removal. J. Mol. Liq. 2020, 301, 112456. [Google Scholar] [CrossRef]
- Tomczyk, A.; Boguta, P.; Sokołowska, Z. Biochar efficiency in copper removal from Haplic soils. Int. J. Env. Sci. Technol. 2019, 16, 4899–4912. [Google Scholar] [CrossRef] [Green Version]
- Adamczuk, A.; Kołodyńska, D. Utilization of fly ashes from the coal burning processes to produce effective low-cost sorbents. Energ. Fuel. 2017, 31, 2095–2105. [Google Scholar] [CrossRef]
- Adamczuk, A.; Kołodyńska, D. Equilibrium, thermodynamic and kinetic studies on removal of chromium, copper, zinc and arsenic from aqueous solutions onto fly ash coated by chitosan. Chem. Eng. J. 2015, 274, 200–212. [Google Scholar] [CrossRef]
- Nowicki, P.; Skibiszewska, P.; Pietrzak, R. Hydrogen sulphide removal on carbonaceous adsorbents prepared from coffee waste materials. Chem. Eng. J. 2014, 248, 208–215. [Google Scholar] [CrossRef]
- Nowicki, P.; Kazmierczak-Razna, J.; Pietrzak, R. Physicochemical and adsorption properties of carbonaceous sorbents prepared by activation of tropi cal fruit skins with potassium carbonate. Mater. Design 2016, 90, 578–585. [Google Scholar]
- Orooji, Y.; Ghanbari, M.; Amiri, O.; Salavati-Niasari, M. Facile fabrication of silver iodide/graphitic carbon nitride nanocomposites by notable photo-catalytic performance through sunlight and antimicrobial activity. J. Hazard. Mater. 2020, 389, 122079. [Google Scholar] [CrossRef]
- Orooji, Y.; Ghasali, E.; Emami, N.; Noorisafa, F.; Razmjou, A. ANOVA design for the optimalization of TiO2 coating on polyether sulfone membranes. Molecules 2019, 24, 2924. [Google Scholar] [CrossRef] [Green Version]
- Irani-nezhad, M.H.; Khataee, A.; Hassanzadeh, J.; Orooji, Y. A chemiluminescent method for the detection of H2O2 on intrinsic peroxidase-like activity of WS2 quantum dots. Molecules 2019, 24, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassandoost, R.; Pouran, S.R.; Khataee, A.; Orooji, Y.; Joo, S.W. Hirarchically structured ternary heterojunctions based on Ce3+/Cr4+ modified Fe3O4 nanoparticles anchored onto graphene oxide sheets as magnetic visible-light-active photocatalysts for decontamination of oxytetracycline. J. Hazard. Mater. 2019, 376, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Shafieizadeh, M.; Taher, M.A.; Opoku, F.; Kiarii, E.M.; Govender, P.P.; Ranjbari, S.; Rezapour, M.; Orooji, Y. The role of magnetite/grapheme oxide nanocomposite as a high-efficiency adsorbent for removal of phenazopyridine residues from water samples, an experimental/theoretical investigation. J. Mol. Liq. 2020, 298, 112040. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, M.; Li, J.; Li, J.; Xu, J.; Wang, X. Porous magnetic carbon sheets from biomass as an adsorbent for the fast removal of organic pollutants from aqueous solution. J. Mater. Chem. A 2014, 2, 4391–4397. [Google Scholar] [CrossRef]
- Fijałkowska, G.; Szewczuk-Karpisz, K.; Wiśniewska, M. Anionic polyacrylamide as a substance strengthening the Pb(II) immobilization on the kaolinite surface. Int. J. Env. Technol. 2020, 17, 1101–1112. [Google Scholar] [CrossRef] [Green Version]
- Fijałkowska, G.; Szewczuk-Karpisz, K.; Wiśniewska, M. Chromium(VI) and lead(II) accumulation at the montmorillonite/aqueous solution interface in the presence of polyacrylamide containing quaternary amine groups. J. Mol. Liq. 2019, 293, 111514. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Nowicki, P. Peat-based activated carbons as adsorbents for simultaneous separation of organic molecules from mixed solution of poly(acrylic acid) polymer and sodium dodecyl sulfate surfactant. Colloid. Surf. A. 2019, 585, 124179. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Nowicki, P. Simultaneous removal of lead(II) ions and poly(acrylic acid) macromolecules from liquid phase using biocarbons obtained from corncob and peanut shell precursors. J. Mol. Liq. 2019, 296, 111806. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Nowicki, P.; Sokołowska, Z.; Pietrzak, R. Hay-based activated biochars obtained using two different heating methods as effective low-cost sorbents: Solid surface charactristics, adsorptive properties and aggregation in the mixed Cu(II)/PAM system. Chemosphere 2020, 250, 126312. [Google Scholar] [CrossRef]
- Janusz, W. Electrical double layer at metal oxide-electrolyte interface. In Interfacial Forces and Fields Theory and Applications; M. Dekker: New York, NY, USA, 1999. [Google Scholar]
- Oshima, H. A simple expansion for Henry’s function for the retardation effect in electrophoresis of spherical colloidal particles. J. Colloid Interface Sci. 1994, 168, 269–271. [Google Scholar] [CrossRef]
- Minczewski, J.; Marczenko, Z. Analytical Chemistry; PWN: Warsaw, Poland, 1965. [Google Scholar]
- Kang, J.; Sowers, T.D.; Duckworth, O.W.; Amoozegar, A.; Heitman, J.L.; McLaughlin, R.A. Turbidimetric determination of anionic polyacrylamide in low biochar soil extracts. J. Environ. Qual. 2013, 42, 1902–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehlig, J. Colorimetric determination of copper with ammonia. Ind. Eng. Chem. Anal. Ed. 1941, 13, 533–535. [Google Scholar] [CrossRef]
- Janusz, W.; Skwarek, E. Effect of Co(II) ions adsorption in the hydroxyapatite/aqueous NaClO4 solution system in particles electrokinetics. Physicochem. Probl. Mi. 2018, 54, 31–39. [Google Scholar]
- Skwarek, E.; Janusz, W.; Sternik, D. The influence of the hydroxyapatite synthesis method on the electrochemical, surface and adsorption properties of hydroxyapatite. Adsorption Sci. Technol. 2017, 35, 507–518. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.; You, H.; Li, Z.; Zhou, R.; Zhang, M.; Yu, H. Infrared spectroscopy study of silicone oil C-Si stretching vibration. Mater. Rev. 2016, 30, 81–84. [Google Scholar] [CrossRef]
- Gild, M.; Sobrados, I.; Rhaiem, H.B.; Sanz, J.; Amara, A.B.H. Alkaline activation of metakaolinite-silica mixtures: Role of dissolved silica concentration on the formation of geopolymers. Ceramics Int. 2017, 43, 12641–12650. [Google Scholar] [CrossRef]
- Orooji, Y.; Irani-nezhad, M.H.; Hassandoost, R.; Khataee, A.; Pouran, S.R.; Joo, S.W. Cerium doped magnetite nanoparticles for highly sensitive detection of metronidazole via chemiluminescence assay. Spectrochim. Acta A 2020, 234, 118272. [Google Scholar] [CrossRef]
- Pefferkorn, E. Polyacrylamide at soli/liquid interfaces. J. Colloid Interface Sci. 1999, 216, 197–220. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Fijałkowska, G.; Szewczuk-Karpisz, K. The mechanism of anionic polyacrylamide adsorption on montmorillonite surface in the presence of Cr(VI) ions. Chemosphere 2018, 211, 524–534. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Chibowski, S.; Urban, T.; Sternik, D.; Terpiłowski, K. Impact of anionic polyacrylamide on stability and surface properties of the Al2O3-polymer solution system at different temperatures. Colloid. Polym. Sci. 2016, 294, 1511–1517. [Google Scholar] [CrossRef] [Green Version]
- Wiśniewska, M.; Chibowski, S.; Urban, T. Impact of polyacrylamide with different contents of carboxyl groups on the chromium(III) oxide adsorption properties in aqueous solution. J. Hazard. Mater. 2015, 283, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ye, L.; Li, Y.; Wu, T. Investigation into the adsorption of partially hydrolyzed polyacrylamide onto in situ formed magnesium hydroxide particles. RSC Adv. 2016, 6, 31092–31100. [Google Scholar] [CrossRef]
- Chiem, L.T.; Huynh, L.; Ralston, J.; Beattie, D.A. An in situ ATR-FTIR study of polyacrylamide adsorption at the talc surface. J. Colloid Interface Sci. 2006, 297, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Hanif, M.U.; Capareda, S.; Chang, Z.; Huang, H.; Ai, Y. Copper(II) removal potential from aqueous solution by pyrolysis biochar derived from anaerobically digested algae-dairy-manure and effect of KOH activation. J. Environ. Chem. Eng. 2016, 4, 365–372. [Google Scholar] [CrossRef]
- Shim, T.; Yoo, J.; Ryu, C.; Park, Y.-K.; Jung, J. Effect of steamactivation of biochar produced from a giant Miscanthus on copper sorption and toxicity. Bioresour. Technol. 2015, 197, 85–90. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazal, H.; Ahmad, D.; Jouhara, H. Removal of copper ions from aqueous solution using low temperature biochar derived from the pyrolysis of municipal solid waste. Sci. Total Environ. 2019, 673, 777–789. [Google Scholar] [CrossRef]
- Chhabra, R.; Basavaraj, M.G. Coulson and Richardson’s Chemical Engineering; Volume 2a: Particulate Systems and Particle Technology, Chapter 13: Colloidal Dispersions; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Wiśniewska, M. Polyacrylamide (Chapter 4) In High Performance Polymers and Their Nanocomposites; Wiley Online Library: New York, NY, USA, 2018. [Google Scholar]
- Birdi, K.S. Handbook of Surface and Colloid Chemistry, 2nd ed.; CRS Press: Boca Raton, FL, USA, 2003. [Google Scholar]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczuk-Karpisz, K.; Bogatyrov, V.M.; Galaburda, M.; Sokołowska, Z. Study on Adsorption and Aggregation in the Mixed System of Polyacrylamide, Cu(II) Ions and Innovative Carbon–Silica Composite. Polymers 2020, 12, 961. https://doi.org/10.3390/polym12040961
Szewczuk-Karpisz K, Bogatyrov VM, Galaburda M, Sokołowska Z. Study on Adsorption and Aggregation in the Mixed System of Polyacrylamide, Cu(II) Ions and Innovative Carbon–Silica Composite. Polymers. 2020; 12(4):961. https://doi.org/10.3390/polym12040961
Chicago/Turabian StyleSzewczuk-Karpisz, Katarzyna, Viktor M. Bogatyrov, Mariia Galaburda, and Zofia Sokołowska. 2020. "Study on Adsorption and Aggregation in the Mixed System of Polyacrylamide, Cu(II) Ions and Innovative Carbon–Silica Composite" Polymers 12, no. 4: 961. https://doi.org/10.3390/polym12040961
APA StyleSzewczuk-Karpisz, K., Bogatyrov, V. M., Galaburda, M., & Sokołowska, Z. (2020). Study on Adsorption and Aggregation in the Mixed System of Polyacrylamide, Cu(II) Ions and Innovative Carbon–Silica Composite. Polymers, 12(4), 961. https://doi.org/10.3390/polym12040961