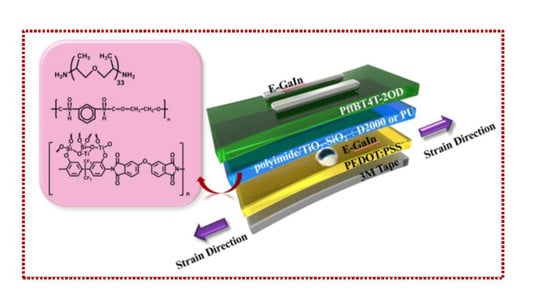
Preparation and Application of Organic-Inorganic Nanocomposite Materials in Stretched Organic Thin Film Transistors
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Dielectric Gate Dielectric
2.2. OTFT Device Preparation
2.3. Characterization
3. Results and Discussion
3.1. Analysis of Optical and Thermal Properties
3.2. Analysis of Stretching Properties
3.3. Analysis of Surface Morphology and Surface Energy
3.4. Analysis of Electrical Properties
4. Conclusion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Qian, Y.; Zhang, X.W.; Xie, L.H.; Qi, D.P.; Chandran, B.K.; Chen, X.D.; Huang, W. Stretchable Organic Semiconductor Devices. Adv. Mater. 2016, 28, 9243–9265. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.T.; Chen, J.Y.; Fukuta, S.; Lin, P.C.; Higashihara, T.; Chueh, C.C.; Chen, W.C. Realization of Intrinsically Stretchable Organic Solar Cells Enabled by Charge-Extraction Layer and Photoactive Material Engineering. ACS Appl. Mater. Interfaces 2018, 10, 21712–21720. [Google Scholar] [CrossRef]
- Shekar, B.C.; Lee, J.Y.; Rhee, S.W. Organic thin film transistors: Materials, process and devices. Korean J. Chem. Eng. 2004, 21, 267–285. [Google Scholar] [CrossRef]
- Kumar, B.; Kaushik, B.K.; Negi, Y.S. Organic Thin Film Transistors: Structures, Models, Materials, Fabrication, and Applications: A Review. Polym. Rev. 2014, 54, 33–111. [Google Scholar] [CrossRef]
- Golmar, F.; Gobbi, M.; Llopis, R.; Stoliar, P.; Casanova, F.; Hueso, L.E. Non-conventional metallic electrodes for organic field-effect transistors. Org. Electron. 2012, 13, 2301–2306. [Google Scholar] [CrossRef]
- Wen, H.F.; Wu, H.C.; Aimi, J.; Hung, C.C.; Chiang, Y.C.; Kuo, C.C.; Chen, W.C. Soft Poly(butyl acrylate) Side Chains toward Intrinsically Stretchable Polymeric Semiconductors for Field-Effect Transistor Applications. Macromolecules 2017, 50, 4982–4992. [Google Scholar] [CrossRef]
- Wang, J.T.; Takshima, S.; Wu, H.C.; Shih, C.C.; Isono, T.; Kakuchi, T.; Satoh, T.; Chen, W.C. Stretchable Conjugated Rod Coil Poly(3-hexylthiophene)-block-poly(butyl acrylate) Thin Films for Field Effect Transistor Applications. Macromolecules 2017, 50, 1442–1452. [Google Scholar] [CrossRef]
- Lee, W.Y.; Wu, H.C.; Lu, C.; Naab, B.D.; Chen, W.C.; Bao, Z.N. n-Type Doped Conjugated Polymer for Nonvolatile Memory. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Oh, J.Y.; Rondeau-Gagne, S.; Chiu, Y.C.; Chortos, A.; Lissel, F.; Wang, G.J.N.; Schroeder, B.C.; Kurosawa, T.; Lopez, J.; Katsumata, T.; et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature 2016, 539, 411–415. [Google Scholar] [CrossRef]
- Kumar, B.; Kaushik, B.K.; Negi, Y.S. Perspectives and challenges for organic thin film transistors: Materials, devices, processes and applications. J. Mater. Sci. Mater. Electron. 2014, 25, 1–30. [Google Scholar] [CrossRef]
- Wang, B.H.; Huang, W.; Chi, L.F.; Al-Hashimi, M.; Marks, T.J.; Facchetti, A. High-k Gate Dielectrics for Emerging Flexible and Stretchable Electronics. Chem. Rev. 2018, 118, 5690–5754. [Google Scholar] [CrossRef] [PubMed]
- Han, G.Q.; Wang, X.M.; Zhang, J.; Zhang, G.C.; Yang, H.H.; Hu, D.B.; Sun, D.W.; Wu, X.M.; Ye, Y.; Chen, H.P.; et al. Interface engineering with double-network dielectric structure for flexible organic thin film transistors. Org. Electron. 2018, 52, 213–221. [Google Scholar] [CrossRef]
- Hung, C.C.; Wu, H.C.; Chiu, Y.C.; Tung, S.H.; Chen, W.C. Crosslinkable high dielectric constant polymer dielectrics for low voltage organic field-effect transistor memory devices. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3224–3236. [Google Scholar] [CrossRef]
- McCoul, D.; Hu, W.L.; Gao, M.M.; Mehta, V.; Pei, Q.B. Recent Advances in Stretchable and Transparent Electronic Materials. Adv. Electron. Mater. 2016, 2. [Google Scholar] [CrossRef]
- Han, D.D.; Chen, Z.F.; Cong, Y.Y.; Yu, W.; Zhang, X.; Wang, Y. High-Performance Flexible Tin-Zinc-Oxide Thin-Film Transistors Fabricated on Plastic Substrates. IEEE Trans. Electron Devices 2016, 63, 3360–3363. [Google Scholar] [CrossRef]
- Sekine, T.; Fukuda, K.; Kumaki, D.; Tokito, S. Highly stable flexible printed organic thin-film transistor devices under high strain conditions using semiconducting polymers. Jpn. J. Appl. Phys. 2015, 54. [Google Scholar] [CrossRef]
- Lim, J.W.; Koo, J.B.; Yun, S.J.; Kim, H.T. Characteristics of pentacene thin film transistor with Al2O3 gate dielectrics on plastic substrate. Electrochem. Solid-State Lett. 2007, 10, J136–J138. [Google Scholar] [CrossRef]
- Shih, C.C.; Lee, W.Y.; Lu, C.; Wu, H.C.; Chen, W.C. Enhancing the Mechanical Durability of an Organic Field Effect Transistor through a Fluoroelastomer Substrate with a Crosslinking-Induced Self-Wrinkled Structure. Adv. Electron. Mater. 2017, 3. [Google Scholar] [CrossRef]
- Song, L.; Wang, Y.; Gao, Q.; Guo, Y.; Wang, Q.J.; Qian, J.; Jiang, S.; Wu, B.; Wang, X.R.; Shi, Y.; et al. Speed up Ferroelectric Organic Transistor Memories by Using Two-Dimensional Molecular Crystalline Semiconductors. ACS Appl. Mater. Interfaces 2017, 9, 18127–18133. [Google Scholar] [CrossRef]
- Hsu, H.H.; Chang, C.Y.; Cheng, C.H. Room-temperature flexible thin film transistor with high mobility. Curr. Appl. Phys. 2013, 13, 1459–1462. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Liu, C.L.; Chen, Y.C.; Chiu, Y.C.; Chen, W.C. Tunable dielectric constant of polyimide-barium titanate nanocomposite materials as the gate dielectrics for organic thin film transistor applications. RSC Adv. 2014, 4, 62132–62139. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Wang, H.; Shi, Z.S.; Cui, Z.C.; Yan, D.H. UV-directly patternable organic-inorganic hybrid composite dielectrics for organic thin-film transistors. Org. Electron. 2012, 13, 3302–3309. [Google Scholar] [CrossRef]
- Lee, W.H.; Wang, C.C.; Ho, J.C. Influence of nano-composite gate dielectrics on OTFT characteristics. Thin Solid Films 2009, 517, 5305–5310. [Google Scholar] [CrossRef]
- Jung, S.W.; Koo, J.B.; Park, C.W.; Na, B.S.; Park, N.M.; Oh, J.Y.; Moon, Y.G.; Lee, S.S.; Koo, K.W. Non-volatile organic ferroelectric memory transistors fabricated using rigid polyimide islands on an elastomer substrate. J. Mater. Chem. C 2016, 4, 4485–4490. [Google Scholar] [CrossRef]
- Damaceanu, M.D.; Constantin, C.P.; Bruma, M.; Belomoina, N.M. Highly fluorinated polyimide blends—Insights into physico-chemical characterization. Polymer 2014, 55, 4488–4497. [Google Scholar] [CrossRef]
- Ahn, T.; Kim, J.W.; Choi, Y.; Yi, M.H. Hybridization of a low-temperature processable polyimide gate insulator for high performance pentacene thin-film transistors. Org. Electron. 2008, 9, 711–720. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, J.; Kim, Y.S.; Lee, J.K. TiO2-poly(4-vinylphenol) nanocomposite dielectrics for organic thin film transistors. Org. Electron. 2013, 14, 3406–3414. [Google Scholar] [CrossRef]
- Chu, C.W.; Li, S.H.; Chen, C.W.; Shrotriya, V.; Yang, Y. High-performance organic thin-film transistors with metal oxide/metal bilayer electrode. Appl. Phys. Lett. 2005, 87. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.L.; Chen, C.J.; Wang, P.H.; Lin, J.J.; Liou, G.S. Novel solution-processable fluorene-based polyimide/TiO2 hybrids with tunable memory properties. Polym. Chem. 2013, 4, 4570–4573. [Google Scholar] [CrossRef]
- Zhao, J.B.; Li, Y.K.; Yang, G.F.; Jiang, K.; Lin, H.R.; Ade, H.; Ma, W.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1. [Google Scholar] [CrossRef]
- Scenev, V.; Cosseddu, P.; Bonfiglio, A.; Salzmann, I.; Severin, N.; Oehzelt, M.; Koch, N.; Rabe, J.P. Origin of mechanical strain sensitivity of pentacene thin-film transistors. Org. Electron. 2013, 14, 1323–1329. [Google Scholar] [CrossRef]
- Tsai, M.H.; Huang, Y.C.; Tseng, I.H.; Yu, H.P.; Lin, Y.K.; Huang, S.L. Thermal and mechanical properties of polyimide/nano-silica hybrid films. Thin Solid Films 2011, 519, 5238–5242. [Google Scholar] [CrossRef]
- Chou, W.Y.; Ho, T.Y.; Cheng, H.L.; Tang, F.C.; Chen, J.H.; Wang, Y.W. Gate field induced ordered electric dipoles in a polymer dielectric for low-voltage operating organic thin-film transistors. RSC Adv. 2013, 3, 20267–20272. [Google Scholar] [CrossRef]
- Huang, T.S.; Su, Y.K.; Wang, P.C. Study of organic thin film transistor with polymethylmethacrylate as a dielectric layer. Appl. Phys. Lett. 2007, 91. [Google Scholar] [CrossRef] [Green Version]
- Miskiewicz, P.; Kotarba, S.; Jung, J.; Marszalek, T.; Mas-Torrent, M.; Gomar-Nadal, E.; Amabilino, D.B.; Rovira, C.; Veciana, J.; Maniukiewicz, W.; et al. Influence of SiO2 surface energy on the performance of organic field effect transistors based on highly oriented, zone-cast layers of a tetrathiafulvalene derivative. J. Appl. Phys. 2008, 104. [Google Scholar] [CrossRef]
- Savagatrup, S.; Chan, E.; Renteria-Garcia, S.M.; Printz, A.D.; Zaretski, A.V.; O’Connor, T.F.; Rodriquez, D.; Valle, E.; Lipomi, D.J. Plasticization of PEDOT:PSS by Common Additives for Mechanically Robust Organic Solar Cells and Wearable Sensors. Adv. Funct. Mater. 2015, 25, 427–436. [Google Scholar] [CrossRef]
- Lu, C.; Lee, W.Y.; Shih, C.C.; Wen, M.Y.; Chen, W.C. Stretchable Polymer Dielectrics for Low-Voltage-Driven Field-Effect Transistors. ACS Appl. Mater. Interfaces 2017, 9, 25522–25532. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lee, W.Y.; Nakajima, R.; Mei, J.G.; Kim, D.H.; Bao, Z.A. Thiol-ene Cross-Linked Polymer Gate Dielectrics for Low-Voltage Organic Thin-Film Transistors. Chem. Mater. 2013, 25, 4806–4812. [Google Scholar] [CrossRef]
- Jiang, B.H.; Peng, Y.J.; Chen, C.P. Simple structured polyetheramines, Jeffamines, as efficient cathode interfacial layers for organic photovoltaics providing power conversion efficiencies up to 9.1%. J. Mater. Chem. A 2017, 5, 10424–10429. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Hu, X.T.; Liu, C.; Tan, L.C.; Chen, Y.W. Nucleation and Crystallization Control via Polyurethane to Enhance the Bendability of Perovskite Solar Cells with Excellent Device Performance. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Huang, T.J.; Lee, W.Y.; Chen, Y.C.; Kuo, C.C. Highly transparent polyimide/nanocrystalline-zirconium dioxide hybrid materials for organic thin film transistor applications. Org. Electron. 2017, 48, 19–28. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Chiu, C.T.; Chueh, C.C. Solution-Processable, Transparent Polyimide for High-Performance High-k Nanocomposite: Synthesis, Characterization, and Dielectric Applications in Transistors. Asian J. Org. Chem. 2018, 7, 2263–2270. [Google Scholar] [CrossRef]
- Yang, B.X.; Tseng, C.Y.; Chiang, A.S.T.; Liu, C.L. A sol-gel titanium-silicon oxide/organic hybrid dielectric for low-voltage organic thin film transistors. J. Mater. Chem. C 2015, 3, 968–972. [Google Scholar] [CrossRef]
- Baeg, K.J.; Noh, Y.Y.; Sirringhaus, H.; Kim, D.Y. Controllable Shifts in Threshold Voltage of Top-Gate Polymer Field-Effect Transistors for Applications in Organic Nano Floating Gate Memory. Adv. Funct. Mater. 2010, 20, 224–230. [Google Scholar] [CrossRef]
- Naber, R.C.G.; Tanase, C.; Blom, P.W.M.; Gelinck, G.H.; Marsman, A.W.; Touwslager, F.J.; Setayesh, S.; De Leeuw, D.M. High-performance solution-processed polymer ferroelectric field-effect transistors. Nat. Mater. 2005, 4, 243–248. [Google Scholar] [CrossRef] [Green Version]





| No. | H (nm) a | Ra (nm) b | Ra/h (%) | Td (°C) | Tg (°C) | T (%) |
|---|---|---|---|---|---|---|
| A0 | 215 | 0.44 | 0.20 | 418 | 266 | >90% |
| A10 | 200 | 0.56 | 0.28 | 427 | - | |
| A20 | 215 | 0.61 | 0.28 | 431 | - | |
| A30 | 220 | 0.65 | 0.29 | 450 | - | |
| A40 | 210 | 0.75 | 0.35 | 461 | - | |
| B0 | 200 | 0.31 | 0.15 | 426 | 286 | >90% |
| B10 | 210 | 0.35 | 0.17 | 431 | - | |
| B20 | 220 | 0.41 | 0.19 | 440 | - | |
| B30 | 215 | 0.57 | 0.27 | 461 | - | |
| B40 | 215 | 0.64 | 0.29 | 477 | - | |
| C0 | 220 | 0.43 | 0.19 | 405 | 270 | >90% |
| C10 | 210 | 0.49 | 0.23 | 419 | - | |
| C20 | 220 | 0.53 | 0.24 | 432 | - | |
| C30 | 215 | 0.60 | 0.28 | 443 | - | |
| C40 | 210 | 0.69 | 0.33 | 454 | - |
| No. | Dielectric Constant [-] | Water Contact Angle [o] | Diidomethane Contact Angle [o] | Surface Energy [mJ·m−2] | |||
|---|---|---|---|---|---|---|---|
| 1 kHz | 10 kHz | 100 kHz | 1 MHz | ||||
| A0 | 4.53 | 4.49 | 4.43 | 4.10 | 79.78 | 35.39 | 51.02 |
| A10 | 5.78 | 5.20 | 4.86 | 4.27 | 80.26 | 42.65 | 47.66 |
| A20 | 6.93 | 6.30 | 6.11 | 5.02 | 80.76 | 43.09 | 45.73 |
| A30 | 7.24 | 7.08 | 6.75 | 6.35 | 83.99 | 50.53 | 44.17 |
| A40 | 9.51 | 8.88 | 7.47 | 6.53 | 78.30 | 29.23 | 52.33 |
| B0 | 4.47 | 4.44 | 4.41 | 4.07 | 78.46 | 39.10 | 48.74 |
| B10 | 5.57 | 5.11 | 4.72 | 4.03 | 80.44 | 45.49 | 45.97 |
| B20 | 6.03 | 6.02 | 5.98 | 5.00 | 81.17 | 51.38 | 44.68 |
| B30 | 7.14 | 7.01 | 6.72 | 5.98 | 84.91 | 51.83 | 42.07 |
| B40 | 8.58 | 7.96 | 7.20 | 6.31 | 76.12 | 37.04 | 51.37 |
| C0 | 4.22 | 4.10 | 4.01 | 3.98 | 77.38 | 39.24 | 49.47 |
| C10 | 5.44 | 5.07 | 4.69 | 4.00 | 78.60 | 46.55 | 47.20 |
| C20 | 5.99 | 5.79 | 5.69 | 5.12 | 78.80 | 51.27 | 44.70 |
| C30 | 6.91 | 6.83 | 6.59 | 5.45 | 82.00 | 51.38 | 43.34 |
| C40 | 7.87 | 7.78 | 7.01 | 6.29 | 74.49 | 38.22 | 51.02 |
| No | LCD [A·cm−2] (at −2 MV·cm−1) | Vt [V] | μ [cm2·V−1·s−1] | ION/IOFF [-] |
|---|---|---|---|---|
| A0 | 7.5 × 10−10 | −2.1 | 1.81 × 10−2 | 1.13 × 103 |
| A10 | 1.5 × 10−9 | 4.1 | 7.01 × 10−2 | 3.24 × 103 |
| A20 | 2.7 × 10−9 | −2.3 | 1.21 × 10−1 | 5.57 × 103 |
| A30 | 4.8 × 10−9 | −7.3 | 2.42 × 10−1 | 9.04 × 103 |
| A40 | 7.7 × 10−9 | 3.2 | 1.07 × 10−2 | 1.16 × 103 |
| B0 | 6.3 × 10−10 | 3.3 | 5.04 × 10−2 | 1.51 × 104 |
| B10 | 8.6 × 10−10 | −1.5 | 2.09 × 10−1 | 2.24 × 104 |
| B20 | 1.7 × 10−9 | 3.9 | 4.23 × 10−1 | 3.01 × 104 |
| B30 | 2.5 × 10−9 | −8.1 | 8.17 × 10−1 | 4.27 × 105 |
| B40 | 5.8 × 10−9 | 2.2 | 5.51 × 10−1 | 8.50 × 104 |
| C0 | 5.9 × 10−10 | 4.6 | 4.81 × 10−2 | 8.13 × 103 |
| C10 | 7.7 × 10−10 | 2.4 | 1.89 × 10−1 | 1.24 × 104 |
| C20 | 1.6 × 10−9 | −2.6 | 3.21 × 10−1 | 2.57 × 104 |
| C30 | 3.4 × 10−9 | 3.8 | 5.62 × 10−1 | 2.04 × 105 |
| C40 | 8.5 × 10−9 | 2.2 | 2.07 × 10−1 | 2.16 × 104 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.-Y.; Yang, C.-H. Preparation and Application of Organic-Inorganic Nanocomposite Materials in Stretched Organic Thin Film Transistors. Polymers 2020, 12, 1058. https://doi.org/10.3390/polym12051058
Yu Y-Y, Yang C-H. Preparation and Application of Organic-Inorganic Nanocomposite Materials in Stretched Organic Thin Film Transistors. Polymers. 2020; 12(5):1058. https://doi.org/10.3390/polym12051058
Chicago/Turabian StyleYu, Yang-Yen, and Cheng-Huai Yang. 2020. "Preparation and Application of Organic-Inorganic Nanocomposite Materials in Stretched Organic Thin Film Transistors" Polymers 12, no. 5: 1058. https://doi.org/10.3390/polym12051058