Recent Applications of Advanced Atomic Force Microscopy in Polymer Science: A Review
Abstract
:1. Introduction
2. The Principle of AFM and AFM-IR
2.1. AFM Scanning/Operation Modes
2.1.1. Contact Mode
2.1.2. Non-Contact Mode
2.1.3. Tapping Mode
2.2. Phase Imaging
2.3. Atomic Force Microscopy
2.4. Cantilever-Tip Systems
2.5. Principle of AFM-IR Technique
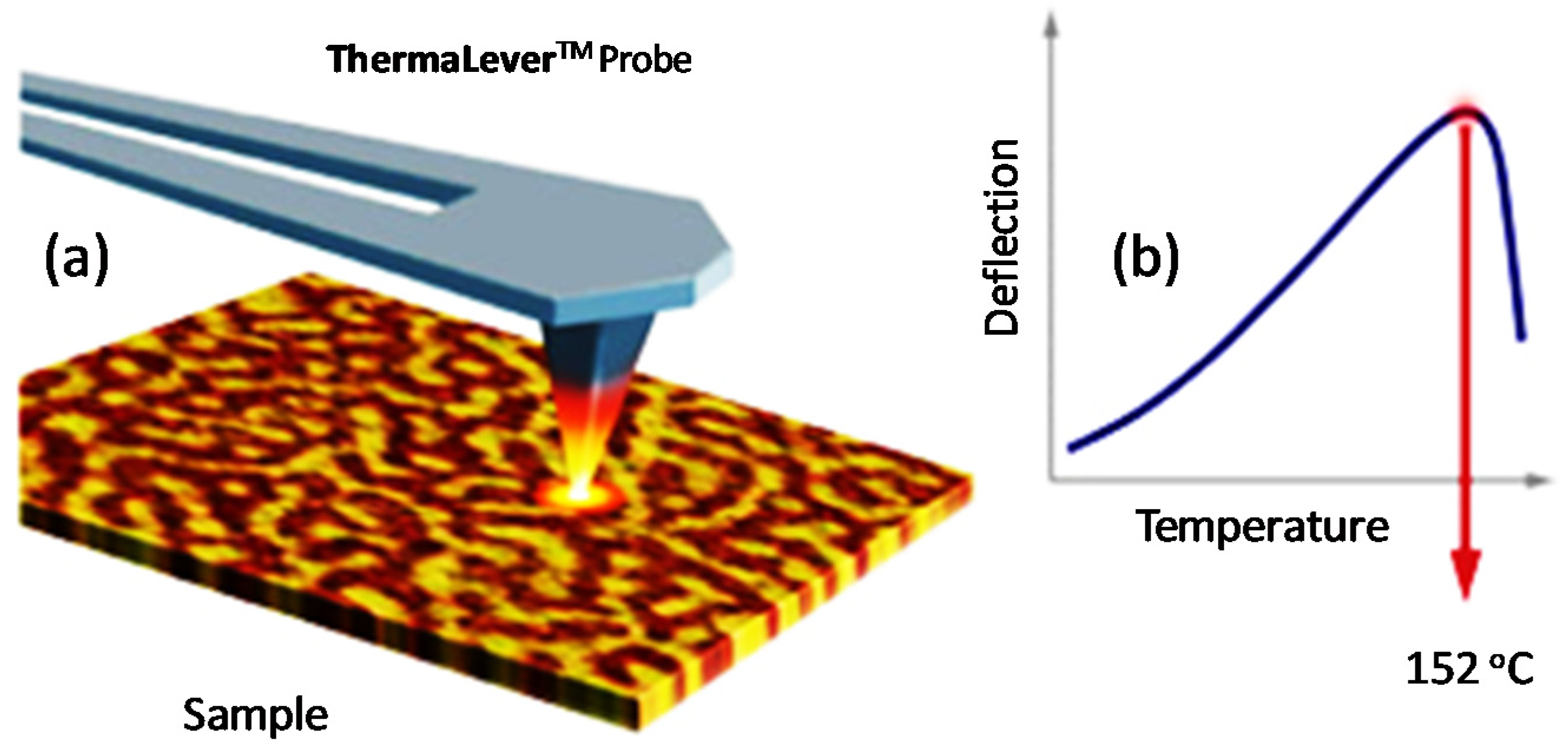
2.6. Nano-Thermal Analysis (Nano-TA)
3. Application of Advanced AFM in the Crystallization of Polymers
3.1. AFM in Polymer Crystallization
3.1.1. Primary Stages of Crystallization
3.1.2. Effects of Film Thickness
3.2. AFM-IR in Polymers
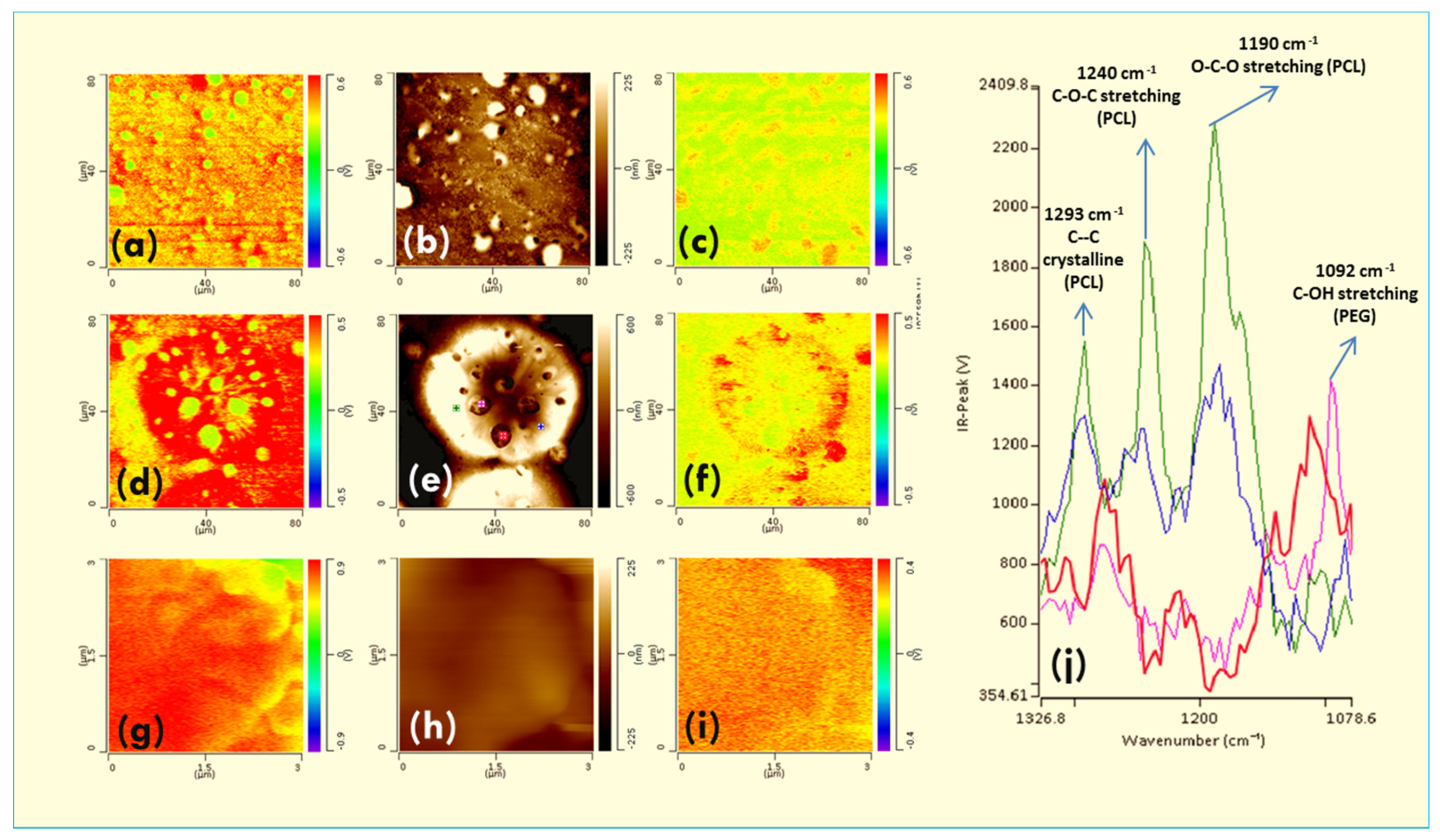
3.2.1. Polymers Blends
3.2.2. Crystallization of Miscible Blends
3.2.3. AFM-IR in the Crystallization of Immiscible Blend
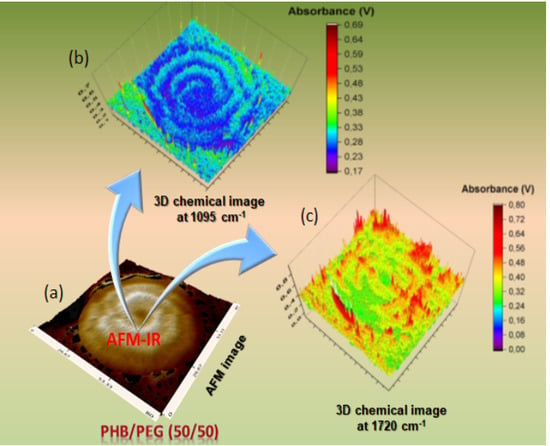
3.3. Other Applications of AFM-IR in Polymer Science
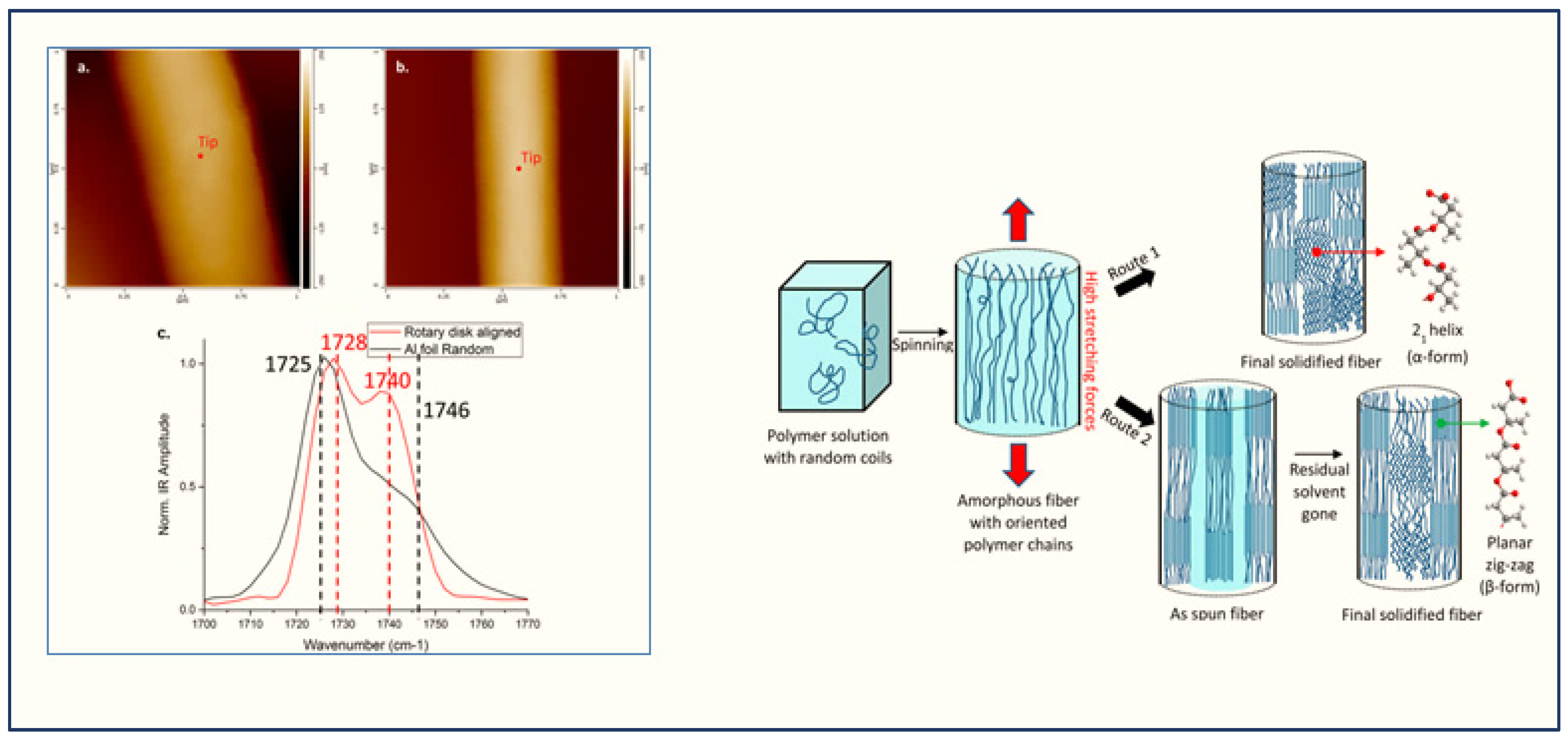
3.3.1. AFM-IR in Studying Polymer Aging
3.3.2. AFM-IR in Biopolymers and Multilayers
4. Summary and Outlooks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hobbs, J.K.; Farrance, O.E.; Kailas, L. How atomic force microscopy has contributed to our understanding of polymer crystallization. Polymer 2009, 50, 4281–4292. [Google Scholar] [CrossRef] [Green Version]
- Koenig, J.L.; Wang, S.-Q.; Bhargava, R. Peer Reviewed: FTIR Images. Anal. Chem. 2001, 73, 360A–369A. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popescu, M.-C.; Filip, D.; Vasile, C.; Cruz, C.; Rueff, J.; Marcos, M.; Serrano, J.; Singurel, G. Characterization by Fourier transform infrared spectroscopy (FT-IR) and 2D IR correlation spectroscopy of PAMAM dendrimer. J. Phys. Chem. B 2006, 110, 14198–14211. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A.M. Infrared microspectroscopy mapping studies of packaging materials: Experiment design and data profiling considerations. Vib. Spectrosc. 2000, 24, 47–62. [Google Scholar] [CrossRef]
- Tannenbaum, R.; Zubris, M.; David, K.; Ciprari, D.; Jacob, K.; Jasiuk, I.; Dan, N. FTIR characterization of the reactive interface of cobalt oxide nanoparticles embedded in polymeric matrices. J. Phys. Chem. B 2006, 110, 2227–2232. [Google Scholar] [CrossRef]
- Stelzer, E.H. Contrast, resolution, pixelation, dynamic range and signal-to-noise ratio: Fundamental limits to resolution in fluorescence light microscopy. J. Microsc. 1998, 189, 15–24. [Google Scholar] [CrossRef]
- Lehmann, T.; Oberschelp, W.; Pelikan, E.; Repges, R. Bildverarbeitung für die Medizin: Grundlagen, Modelle, Methoden, Anwendungen; Springer: Berling, Germany, 2013. [Google Scholar]
- Nishikida, K. Spatial Resolution in Infrared Microscopy and Imaging; Application Note; Thermo Electron Corporation: Waltham, MA, USA, 2004; p. 50717. [Google Scholar]
- Lasch, P.; Naumann, D. Spatial resolution in infrared microspectroscopic imaging of tissues. Biochim. Biophys. Acta Bba Biomembr. 2006, 1758, 814–829. [Google Scholar] [CrossRef] [Green Version]
- Kumar, T.S. Physical and Chemical Characterization of Biomaterials, in Characterization of Biomaterials; Elsevier: Amsterdam, The Netherlands, 2013; pp. 11–47. [Google Scholar]
- Ho, T.T.; Bremmell, K.E.; Krasowska, M.; MacWilliams, S.V.; Richard, C.l.J.; Stringer, D.N.; Beattie, D.A. In situ ATR FTIR spectroscopic study of the formation and hydration of a fucoidan/chitosan polyelectrolyte multilayer. Langmuir 2015, 31, 11249–11259. [Google Scholar] [CrossRef]
- Tejedor-Tejedor, M.I.; Paredes, L.; Anderson, M.A. Evaluation of ATR—FTIR spectroscopy as an “in situ” tool for following the hydrolysis and condensation of alkoxysilanes under rich H2O conditions. Chem. Mater. 1998, 10, 3410–3421. [Google Scholar] [CrossRef]
- Roy, S.; Perez-Guaita, D.; Andrew, D.W.; Richards, J.S.; McNaughton, D.; Heraud, P.; Wood, B.R. Simultaneous ATR-FTIR based determination of malaria parasitemia, glucose and urea in whole blood dried onto a glass slide. Anal. Chem. 2017, 89, 5238–5245. [Google Scholar] [CrossRef]
- Schuttlefield, J.D.; Grassian, V.H. ATR–FTIR spectroscopy in the undergraduate chemistry laboratory. Part I: Fundamentals and examples. J. Chem. Educ. 2008, 85, 279. [Google Scholar] [CrossRef]
- Ami, D.; Mereghetti, P.; Foli, A.; Tasaki, M.; Milani, P.; Nuvolone, M.; Palladini, G.; Merlini, G.; Lavatelli, F.; Natalello, A. ATR-FTIR spectroscopy supported by multivariate analysis for the characterization of adipose tissue aspirates from patients affected by systemic amyloidosis. Anal. Chem. 2019, 91, 2894–2900. [Google Scholar] [CrossRef] [PubMed]
- Maver, U.; Maver, T.; Peršin, Z.; Mozetič, M.; Vesel, A.; Gaberšček, M.; Stana-Kleinschek, K. Polymer characterization with the atomic force microscope. Polym. Sci. 2013, 4. [Google Scholar] [CrossRef]
- Magonov, S. Atomic force microscopy in analysis of polymers. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Vahabi, S.; Salman, B.N.; Javanmard, A. Atomic force microscopy application in biological research: A review study. Iran. J. Med Sci. 2013, 38, 76. [Google Scholar]
- Last, J.A.; Russell, P.; Nealey, P.F.; Murphy, C.J. The applications of atomic force microscopy to vision science. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6083–6094. [Google Scholar] [CrossRef]
- Stocker, W.; Schumacher, M.; Graff, S.; Thierry, A.; Wittmann, J.-C.; Lotz, B. Epitaxial crystallization and AFM investigation of a frustrated polymer structure: Isotactic poly (propylene), β phase. Macromolecules 1998, 31, 807–814. [Google Scholar] [CrossRef]
- Hobbs, J.; Miles, M. Direct observation of polyethylene shish-kebab crystallization using in-situ atomic force microscopy. Macromolecules 2001, 34, 353–355. [Google Scholar] [CrossRef]
- Hobbs, J.; Humphris, A.; Miles, M. In-situ atomic force microscopy of polyethylene crystallization. 1. crystallization from an oriented backbone. Macromolecules 2001, 34, 5508–5519. [Google Scholar] [CrossRef]
- Ono, Y.; Kumaki, J. In situ real-time observation of polymer folded-chain crystallization by atomic force microscopy at the molecular level. Macromolecules 2018, 51, 7629–7636. [Google Scholar] [CrossRef]
- Schulz, M.; Seidlitz, A.; Kurz, R.; Baärenwald, R.; Petzold, A.; Saalwachter, K.; Thurn-Albrecht, T. The Underestimated Effect of Intracrystalline Chain Dynamics on the Morphology and Stability of Semicrystalline Polymers. Macromolecules 2018, 51, 8377–8385. [Google Scholar] [CrossRef]
- Sun, H.; Yu, D.M.; Shi, S.; Yuan, Q.; Fujinami, S.; Sun, X.; Wang, D.; Russell, T.P. Configurationally Constrained Crystallization of Brush Polymers with Poly (ethylene oxide) Side Chains. Macromolecules 2019, 52, 592–600. [Google Scholar] [CrossRef]
- Boisseé, S.p.; Kryuchkov, M.A.; Tien, N.-D.; Bazuin, C.G.r.; Prud’homme, R.E. PLLA Crystallization in Linear AB and BAB Copolymers of l-Lactide and 2-Dimethylaminoethyl Methacrylate. Macromolecules 2016, 49, 6973–6986. [Google Scholar] [CrossRef]
- Roland, S.b.; Gamys, C.G.; Grosrenaud, J.; Boisseé, S.p.; Pellerin, C.; Prud’homme, R.E.; Bazuin, C.G. Solvent Influence on thickness, composition, and morphology variation with dip-coating rate in supramolecular PS-b-P4VP thin films. Macromolecules 2015, 48, 4823–4834. [Google Scholar] [CrossRef]
- Mareau, V.H.; Prud’Homme, R.E. In-situ hot stage atomic force microscopy study of poly (ε-caprolactone) crystal growth in ultrathin films. Macromolecules 2005, 38, 398–408. [Google Scholar] [CrossRef]
- Maillard, D.; Prud’Homme, R.E. Crystallization of ultrathin films of polylactides: From chain chirality to lamella curvature and twisting. Macromolecules 2008, 41, 1705–1712. [Google Scholar] [CrossRef]
- Vielmuth, F.; Spindler, V.; Waschke, J. Atomic force microscopy provides new mechanistic insights into the pathogenesis of pemphigus. Front. Immunol. 2018, 9, 485. [Google Scholar] [CrossRef] [Green Version]
- Baker, A.; Helbert, W.; Sugiyama, J.; Miles, M. New insight into cellulose structure by atomic force microscopy shows the Iα crystal phase at near-atomic resolution. Biophys. J. 2000, 79, 1139–1145. [Google Scholar] [CrossRef] [Green Version]
- Dufrêne, Y.F. Atomic force microscopy in microbiology: New structural and functional insights into the microbial cell surface. MBio 2014, 5, e01363-14. [Google Scholar] [CrossRef] [Green Version]
- Maver, U.; Velnar, T.; Gaberšček, M.; Planinšek, O.; Finšgar, M. Recent progressive use of atomic force microscopy in biomedical applications. Trac Trends Anal. Chem. 2016, 80, 96–111. [Google Scholar] [CrossRef] [Green Version]
- Edwardson, J.M.; Henderson, R.M. Atomic force microscopy and drug discovery. Drug Discov. Today 2004, 9, 64–71. [Google Scholar] [CrossRef]
- Senapati, S.; Lindsay, S. Recent progress in molecular recognition imaging using atomic force microscopy. Acc. Chem. Res. 2016, 49, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Soon, R.L.; Nation, R.L.; Hartley, P.G.; Larson, I.; Li, J. Atomic force microscopy investigation of the morphology and topography of colistin-heteroresistant Acinetobacter baumannii strains as a function of growth phase and in response to colistin treatment. Antimicrob. Agents Chemother. 2009, 53, 4979–4986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, Y.; Wang, H. The structure and function of cell membranes examined by atomic force microscopy and single-molecule force spectroscopy. Chem. Soc. Rev. 2015, 44, 3617–3638. [Google Scholar] [CrossRef] [PubMed]
- Marrese, M.; Guarino, V.; Ambrosio, L. Atomic force microscopy: A powerful tool to address scaffold design in tissue engineering. J. Funct. Biomater. 2017, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Frisbie, C.D.; Rozsnyai, L.F.; Noy, A.; Wrighton, M.S.; Lieber, C.M. Functional group imaging by chemical force microscopy. Science 1994, 265, 2071–2074. [Google Scholar] [CrossRef]
- Piner, R.D.; Mirkin, C.A. Effect of water on lateral force microscopy in air. Langmuir 1997, 13, 6864–6868. [Google Scholar] [CrossRef]
- Davis, J.J. Engineering the Bioelectronic Interface: Applications to Analyte Biosensing and Protein Detection; Royal Society of Chemistry: London, UK, 2009. [Google Scholar]
- Smith, D.; Connell, S.; Robinson, C.; Kirkham, J. Chemical force microscopy: Applications in surface characterisation of natural hydroxyapatite. Anal. Chim. Acta 2003, 479, 39–57. [Google Scholar] [CrossRef]
- Steffens, C.; Leite, F.L.; Bueno, C.C.; Manzoli, A.; Herrmann, P.S.D.P. Atomic force microscopy as a tool applied to nano/biosensors. Sensors 2012, 12, 8278–8300. [Google Scholar] [CrossRef]
- Jarzembski, A.; Shaskey, C.; Park, K. Tip-based vibrational spectroscopy for nanoscale analysis of emerging energy materials. Front. Energy 2018, 12, 43–71. [Google Scholar] [CrossRef]
- Liu, Y.; Collins, L.; Proksch, R.; Kim, S.; Watson, B.R.; Doughty, B.; Calhoun, T.R.; Ahmadi, M.; Ievlev, A.V.; Jesse, S. Chemical nature of ferroelastic twin domains in CH3 NH3 PbI3 perovskite. Nat. Mater. 2018, 17, 1013–1019. [Google Scholar] [CrossRef]
- Yang, J.; Hatcherian, J.; Hackley, P.C.; Pomerantz, A.E. Nanoscale geochemical and geomechanical characterization of organic matter in shale. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, M.; Lu, F.; Belkin, M.A. High-sensitivity infrared vibrational nanospectroscopy in water. Light: Sci. Appl. 2017, 6, e17096. [Google Scholar] [CrossRef]
- Hassenkam, T.; Andersson, M.; Dalby, K.; Mackenzie, D.; Rosing, M. Elements of Eoarchean life trapped in mineral inclusions. Nature 2017, 548, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Zhang, S.; Jiang, J.; Corder, S.G.; Qian, Z.; Zhou, Z.; Lee, W.; Liu, K.; Wang, X.; Li, X.; et al. Nanoscale probing of electron-regulated structural transitions in silk proteins by near-field IR imaging and nano-spectroscopy. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Khanikaev, A.B.; Arju, N.; Fan, Z.; Purtseladze, D.; Lu, F.; Lee, J.; Sarriugarte, P.; Schnell, M.; Hillenbrand, R.; Belkin, M. Experimental demonstration of the microscopic origin of circular dichroism in two-dimensional metamaterials. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- O’callahan, B.T.; Jones, A.C.; Park, J.H.; Cobden, D.H.; Atkin, J.M.; Raschke, M.B. Inhomogeneity of the ultrafast insulator-to-metal transition dynamics of VO 2. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Ruggeri, F.; Longo, G.; Faggiano, S.; Lipiec, E.; Pastore, A.; Dietler, G. Infrared nanospectroscopy characterization of oligomeric and fibrillar aggregates during amyloid formation. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Kouamé, N.A.; Ramos, L.; Remita, S.; Dazzi, A.; Deniset-Besseau, A.; Beaunier, P.; Goubard, F.; Aubert, P.-H.; Remita, H. Conducting polymer nanostructures for photocatalysis under visible light. Nat. Mater. 2015, 14, 505–511. [Google Scholar] [CrossRef]
- Lu, F.; Jin, M.; Belkin, M.A. Tip-enhanced infrared nanospectroscopy via molecular expansion force detection. Nat. Photonics 2014, 8, 307. [Google Scholar] [CrossRef]
- Dazzi, A.; Prater, C.B. AFM-IR: Technology and applications in nanoscale infrared spectroscopy and chemical imaging. Chem. Rev. 2017, 117, 5146–5173. [Google Scholar] [CrossRef]
- Gong, L.; Chase, D.B.; Noda, I.; Liu, J.; Martin, D.C.; Ni, C.; Rabolt, J.F. Discovery of β-form crystal structure in electrospun poly [(R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate](PHBHx) nanofibers: From fiber mats to single fibers. Macromolecules 2015, 48, 6197–6205. [Google Scholar] [CrossRef]
- Tang, F.; Bao, P.; Su, Z. Analysis of nanodomain composition in high-impact polypropylene by atomic force microscopy-infrared. Anal. Chem. 2016, 88, 4926–4930. [Google Scholar] [CrossRef] [PubMed]
- Purohit, H.S.; Taylor, L.S. Miscibility of itraconazole–hydroxypropyl methylcellulose blends: Insights with high resolution analytical methodologies. Mol. Pharm. 2015, 12, 4542–4553. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdenbrugh, B.; Lo, M.; Kjoller, K.; Marcott, C.; Taylor, L.S. Nanoscale mid-infrared evaluation of the miscibility behavior of blends of dextran or maltodextrin with poly (vinylpyrrolidone). Mol. Pharm. 2012, 9, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, X.; Yu, A.; Zhao, W.; Zhang, W.; Zhang, S.; Wei, L.; Cook, D.J.; Roy, A. Functionalized metal-organic framework nanocomposites for dispersive solid phase extraction and enantioselective capture of chiral drug intermediates. J. Chromatoraph. A 2018, 1537, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mikhalchan, A.; Banas, A.M.; Banas, K.; Borkowska, A.M.; Nowakowski, M.; Breese, M.B.; Kwiatek, W.M.; Paluszkiewicz, C.; Tay, T.E. Revealing chemical heterogeneity of CNT fiber nanocomposites via nanoscale chemical imaging. Chem. Mater. 2018, 30, 1856–1864. [Google Scholar] [CrossRef]
- Ali, A.; Morrow, P.; Henda, R.; Fagerberg, R. Deposition of cobalt doped zinc oxide thin film nano-composites via pulsed electron beam ablation. Mrs Adv. 2016, 1, 433–439. [Google Scholar] [CrossRef]
- Van Assche, G.; Van Mele, B. Interphase formation in model composites studied by micro-thermal analysis. Polymer 2002, 43, 4605–4610. [Google Scholar] [CrossRef]
- Kelchtermans, M.; Lo, M.; Dillon, E.; Kjoller, K.; Marcott, C. Characterization of a polyethylene–polyamide multilayer film using nanoscale infrared spectroscopy and imaging. Vib. Spectrosc. 2016, 82, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Hinrichs, K.; Shaykhutdinov, T. Polarization-Dependent Atomic Force Microscopy–Infrared Spectroscopy (AFM-IR): Infrared Nanopolarimetric Analysis of Structure and Anisotropy of Thin Films and Surfaces. Appl. Spectrosc. 2018, 72, 817–832. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, C.-T.; Xu, F.; Yang, J.; Liu, J.; Cai, W.; Zhu, G. Resistive switching and nanoscale chemical mapping of phase separation in PVDF/PMMA/F8T2 ternary thin films. Polymer 2018, 153, 498–506. [Google Scholar] [CrossRef]
- Pancani, E.; Mathurin, J.; Bilent, S.; Bernet-Camard, M.F.; Dazzi, A.; Deniset-Besseau, A.; Gref, R. High-Resolution Label-Free Detection of Biocompatible Polymeric Nanoparticles in Cells. Part. Part. Syst. Charact. 2018, 35, 1700457. [Google Scholar] [CrossRef]
- Perez-Guaita, D.; Kochan, K.; Batty, M.; Doerig, C.; Garcia-Bustos, J.; Espinoza, S.; McNaughton, D.; Heraud, P.; Wood, B.R. Multispectral atomic force microscopy-infrared nano-imaging of malaria infected red blood cells. Anal. Chem. 2018, 90, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Shaykhutdinov, T.; Pop, S.D.; Furchner, A.; Hinrichs, K. Supramolecular orientation in anisotropic assemblies by infrared nanopolarimetry. ACS Macro Lett. 2017, 6, 598–602. [Google Scholar] [CrossRef]
- Centrone, A. Infrared imaging and spectroscopy beyond the diffraction limit. Annu. Rev. Anal. Chem. 2015, 8, 101–126. [Google Scholar] [CrossRef] [Green Version]
- Fu, W.; Zhang, W. Hybrid AFM for nanoscale physicochemical characterization: Recent development and emerging applications. Small 2017, 13, 1603525. [Google Scholar] [CrossRef]
- Xiao, L.; Schultz, Z.D. Spectroscopic imaging at the nanoscale: Technologies and recent applications. Anal. Chem. 2018, 90, 440–458. [Google Scholar] [CrossRef]
- Crist, B.; Schultz, J.M. Atomic Force Microscopy Studies of Polymer Crystals: Nucleation, Growth, Annealing, and Melting. In Encyclopedia of Polymers and Composites; Palsule, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–25. [Google Scholar]
- Zhu, D.; Shou, X.; Liu, Y.; Chen, E.; Cheng, S.Z. AFM-tip-induced crystallization of poly (ethylene oxide) melt droplets. Front. Chem. China 2007, 2, 174–177. [Google Scholar] [CrossRef]
- Magonov, S.N.; Reneker, D.H. Characterization of polymer surfaces with atomic force microscopy. Annu. Rev. Mater. Sci. 1997, 27, 175–222. [Google Scholar] [CrossRef] [Green Version]
- Klinov, D.; Magonov, S. True molecular resolution in tapping-mode atomic force microscopy with high-resolution probes. Appl. Phys. Lett. 2004, 84, 2697–2699. [Google Scholar] [CrossRef]
- Pearce, R.; Vancso, G.J. Real-time imaging of melting and crystallization in poly (ethylene oxide) by atomic force microscopy. Polymer 1998, 39, 1237–1242. [Google Scholar] [CrossRef] [Green Version]
- Pearce, R.; Vancso, G.J. Imaging of melting and crystallization of poly (ethylene oxide) in real-time by hot-stage atomic force microscopy. Macromolecules 1997, 30, 5843–5848. [Google Scholar] [CrossRef] [Green Version]
- McMaster, T.; Hobbs, J.; Barham, P.; Miles, M. AFM study of in situ real time polymer crystallization and spherulite structure. Probe Microsc. 1997, 1, 43–56. [Google Scholar]
- Hobbs, J.K.; McMaster, T.J.; Miles, M.J.; Barham, P.J. Direct observations of the growth of spherulites of poly (hydroxybutyrate-co-valerate) using atomic force microscopy. Polymer 1998, 39, 2437–2446. [Google Scholar] [CrossRef]
- Braga, P.C.; Ricci, D. Atomic Force Microscopy: Biomedical Methods and Applications; Springer Science & Business Media: Berlin, Germany, 2004; Volume 242. [Google Scholar]
- Eaton, P.; West, P. Atomic Force Miscroscopy; Oxford University Press: Oxford, UK, 2009; ISBN-13: 978-0199570454. [Google Scholar]
- Jagtap, R.; Ambre, A. Overview literature on atomic force microscopy (AFM): Basics and its important applications for polymer characterization. Indian J. Eng. Mater. Sci. 2006, 13, 368–384. [Google Scholar]
- MikroMasch, N.G. Contact Mode, Ambient Conditions. Available online: https://www.spmtips.com/how-to-choose-by-AFM-technique-contact-mode.html (accessed on 10 April 2020).
- Park Systems. Phase Imaging/Phase Detection Microscopy. 2020. Available online: https://parksystems.com/park-spm-modes/91-standard-imaging-mode/221-phase-imaging-phase-detection-microscopy-pdm (accessed on 10 April 2020).
- Schmitz, I.; Schreiner, M.; Friedbacher, G.; Grasserbauer, M. Phase imaging as an extension to tapping mode AFM for the identification of material properties on humidity-sensitive surfaces. Appl. Surf. Sci. 1997, 115, 190–198. [Google Scholar] [CrossRef]
- Lin, W.-C.; Blanchette, C.D.; Ratto, T.V.; Longo, M.L. Lipid asymmetry in DLPC/DSPC-supported lipid bilayers: A combined AFM and fluorescence microscopy study. Biophys. J. 2006, 90, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Simon, M.; Wittmar, M.; Bakowsky, U.; Kissel, T. Self-assembling nanocomplexes from insulin and water-soluble branched polyesters, poly [(vinyl-3-(diethylamino)-propylcarbamate-co-(vinyl acetate)-co-(vinyl alcohol)]-graft-poly (l-lactic acid): A novel carrier for transmucosal delivery of peptides. Bioconjugate Chem. 2004, 15, 841–849. [Google Scholar] [CrossRef]
- Butt, H.-J.; Cappella, B.; Kappl, M. Force measurements with the atomic force microscope: Technique, interpretation and applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.; Chilkoti, A.; Moy, V.T. Direct force measurements of the streptavidin–biotin interaction. Biomol. Eng. 1999, 16, 45–55. [Google Scholar] [CrossRef]
- Radmacher, M.; Cleveland, J.P.; Fritz, M.; Hansma, H.G.; Hansma, P.K. Mapping interaction forces with the atomic force microscope. Biophys. J. 1994, 66, 2159–2165. [Google Scholar] [CrossRef] [Green Version]
- Quist, A.P.; Bergman, A.A.; Reimann, C.T.; Oscarsson, S.O.; Sundqvist, B. Imaging of single antigens, antibodies, and specific immunocomplex formation by scanning force microscopy. Scanning Microsc. 1995, 9, 395–400. [Google Scholar] [PubMed]
- Florin, E.-L.; Moy, V.T.; Gaub, H.E. Adhesion forces between individual ligand-receptor pairs. Science 1994, 264, 415–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.; Moy, V.T. Cross-linking of cell surface receptors enhances cooperativity of molecular adhesion. Biophys. J. 2000, 78, 2814–2820. [Google Scholar] [CrossRef] [Green Version]
- Rimondini, L.; Bianchi, C.L.; Vernè, E. Surface Tailoring of Inorganic Materials for Biomedical Applications; Bentham Science Publishers: Sharjah, UAE, 2012. [Google Scholar]
- MicroscopeMaster. The Atomic Force Microscope (AFM): What Are Its Uses in Microscopy Today? Advantages and Disadvantages. 2020. Available online: https://www.microscopemaster.com/atomic-force-microscope.html (accessed on 10 April 2020).
- Villarrubia, J.S. Morphological estimation of tip geometry for scanned probe microscopy. Surf. Sci. 1994, 321, 287–300. [Google Scholar] [CrossRef]
- Wilson, R.A.; Bullen, H.A. Basic Theory Atomic Force Microscopy (AFM), Department of Chemistry, Northern Kentucky University. Available online: Users.metu.edu.tr (accessed on 10 April 2020).
- Dai, H.; Hafner, J.H.; Rinzler, A.G.; Colbert, D.T.; Smalley, R.E. Nanotubes as nanoprobes in scanning probe microscopy. Nature 1996, 384, 147–150. [Google Scholar] [CrossRef]
- Hui, F.; Chen, S.; Liang, X.; Yuan, B.; Jing, X.; Shi, Y.; Lanza, M. Graphene Coated Nanoprobes: A Review. Crystals 2017, 7, 269. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Chen, J.; Guo, Y.; Wu, B.; Yu, G.; Liu, Y. Multilayer graphene-coated atomic force microscopy tips for molecular junctions. Adv. Mater. 2012, 24, 3482–3485. [Google Scholar] [CrossRef]
- Lanza, M.; Bayerl, A.; Gao, T.; Porti, M.; Nafria, M.; Jing, G.; Zhang, Y.; Liu, Z.; Duan, H. Graphene-Coated Atomic Force Microscope Tips for Reliable Nanoscale Electrical Characterization. Adv. Mater. 2013, 25, 1440–1444. [Google Scholar] [CrossRef]
- Shim, W.; Brown, K.A.; Zhou, X.; Rasin, B.; Liao, X.; Mirkin, C.A. Multifunctional cantilever-free scanning probe arrays coated with multilayer graphene. Proc. Natl. Acad. Sci. USA 2012, 109, 18312–18317. [Google Scholar] [CrossRef] [Green Version]
- Martin-Olmos, C.; Rasool, H.I.; Weiller, B.H.; Gimzewski, J.K. Graphene MEMS: AFM probe performance improvement. ACS Nano 2013, 7, 4164–4170. [Google Scholar] [CrossRef] [PubMed]
- Pacios, M.; Hosseini, P.; Fan, Y.; He, Z.; Krause, O.; Hutchison, J.; Warner, J.H.; Bhaskaran, H. Direct manufacturing of ultrathin graphite on three-dimensional nanoscale features. Sci. Rep. 2016, 6, 22700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, F.; Vajha, P.; Shi, Y.; Ji, Y.; Duan, H.; Padovani, A.; Larcher, L.; Li, X.R.; Xu, J.J.; Lanza, M. Moving graphene devices from lab to market: Advanced graphene-coated nanoprobes. Nanoscale 2016, 8, 8466–8473. [Google Scholar] [CrossRef]
- Keller, D.J.; Chih-Chung, C. Imaging steep, high structures by scanning force microscopy with electron beam deposited tips. Surf. Sci. 1992, 268, 333–339. [Google Scholar] [CrossRef]
- Micic, M.; Chen, A.; Leblanc, R.M.; Moy, V.T. Scanning electron microscopy studies of protein-functionalized atomic force microscopy cantilever tips. Scanning 1999, 21, 394–397. [Google Scholar] [CrossRef]
- NanoWorld AG. a) AFM Si3N4 Tip with Cantilever and d) Diamond Coated AFM Tip. Available online: https://www.nanoandmore.com/AFM-Probe-ARROW-CONTR (accessed on 10 April 2020).
- Mathurin, J.; Dartois, E.; Engrand, C.; Duprat, J.; Deniset Besseau, A.; Dazzi, A.; Kebukawa, Y.; Noguchi, T.; Troadec, D. Nanometre-scale infrared chemical imaging (AFM-IR) of organic matter in ultra-carbonaceous antarctic micrometeorites (UCAMMS) and future analyses of hayabusa 2 samples. In Proceedings of the 51st Lunar and Planetary Science Conference, The Woodlands, TX, USA, 16–21 March 2020. [Google Scholar]
- Lahiri, B.; Holland, G.; Centrone, A. Chemical imaging beyond the diffraction limit: Experimental validation of the PTIR technique. Small 2013, 9, 439–445. [Google Scholar] [CrossRef]
- Dazzi, A.; Prazeres, R.; Glotin, F.; Ortega, J. Local infrared microspectroscopy with subwavelength spatial resolution with an atomic force microscope tip used as a photothermal sensor. Opt. Lett. 2005, 30, 2388–2390. [Google Scholar] [CrossRef]
- Baden, N.; Yasuda, M.; Yoshida, A.; Muraki, N. New method for chemical characterization of polymer materials in industrial devices: AFM-IR with FIB sample preparation. In Proceedings of the IEEE 22nd International Symposium on the Physical and Failure Analysis of Integrated Circuits, Hsinchu, Taiwan, 29 June–2 July 2015; IEEE: Picataway, NJ, USA, 2015; pp. 496–499. [Google Scholar]
- Lu, F.; Belkin, M.A. Infrared absorption nano-spectroscopy using sample photoexpansion induced by tunable quantum cascade lasers. Opt. Express 2011, 19, 19942–19947. [Google Scholar] [CrossRef]
- Frogley, M.D.; Lekkas, I.; Kelley, C.S.; Cinque, G. Performances for broadband synchrotron photothermal infrared nano-spectroscopy at Diamond Light Source. Infrared Phys. Technol. 2020, 105, 103238. [Google Scholar] [CrossRef]
- Fukuoka, N.; Tanabe, K. Lightning-Rod Effect of Plasmonic Field Enhancement on Hydrogen-Absorbing Transition Metals. Nanomaterials 2019, 9, 1235. [Google Scholar] [CrossRef] [Green Version]
- Rahaman, M.; Milekhin, A.G.; Mukherjee, A.; Rodyakina, E.E.; Latyshev, A.V.; Dzhagan, V.M.; Zahn, D.R. The role of a plasmonic substrate on the enhancement and spatial resolution of tip-enhanced Raman scattering. Faraday Discuss. 2019, 214, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Mathurin, J.; Pancani, E.; Deniset-Besseau, A.; Kjoller, K.; Prater, C.B.; Gref, R.; Dazzi, A. How to unravel the chemical structure and component localization of individual drug-loaded polymeric nanoparticles by using tapping AFM-IR. Analyst 2018, 143, 5940–5949. [Google Scholar] [CrossRef] [PubMed]
- Wieland, K.; Ramer, G.; Weiss, V.U.; Allmaier, G.; Lendl, B.; Centrone, A. Nanoscale chemical imaging of individual chemotherapeutic cytarabine-loaded liposomal nanocarriers. Nano Res. 2019, 12, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Tuteja, M.; Kang, M.; Leal, C.; Centrone, A. Nanoscale partitioning of paclitaxel in hybrid lipid–polymer membranes. Analyst 2018, 143, 3808–3813. [Google Scholar] [CrossRef]
- Dontsova, K.; Balogh-Brunstad, Z.; Roux, G.L. Biogeochemical Cycles: Ecological Drivers and Environmental Impact; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Harding, L.; King, W.P.; Dai, X.; Craig, D.Q.; Reading, M. Nanoscale characterisation and imaging of partially amorphous materials using local thermomechanical analysis and heated tip AFM. Pharm. Res. 2007, 24, 2048–2054. [Google Scholar] [CrossRef]
- Bruker, A.I.C. nanoIR Nanoscale Thermal Analysis (nanoTA), nano-TA Uses a Heated AFM Tip to Measure Glass Transition and Melt Temperatures. Available online: https://www.anasysinstruments.com/products/nanoir2/ (accessed on 14 April 2020).
- Mamun, A.; Bazuin, C.G.; Prud’homme, R.E. Morphologies of Various Polycaprolactone/Polymer Blends in Ultrathin Films. Macromolecules 2015, 48, 1412–1417. [Google Scholar] [CrossRef]
- Roland, S.; Gaspard, D.; Prud’homme, R.E.; Bazuin, C.G. Morphology Evolution in Slowly Dip-Coated Supramolecular PS-b-P4VP Thin Films. Macromolecules 2012, 45, 5463–5476. [Google Scholar] [CrossRef]
- Chan, C.-M.; Li, L. Direct Observation of the Growth of Lamellae and Spherulites by AFM. In Intrinsic Molecular Mobility and Toughness of Polymers II; Springer: Berlin, Germany, 2005; pp. 1–41. [Google Scholar]
- Schönherr, H.; Waymouth, R.M.; Frank, C.W. Nucleation and Crystallization of Low-Crystallinity Polypropylene Followed in Situ by Hot Stage Atomic Force Microscopy. Macromolecules 2003, 36, 2412–2418. [Google Scholar] [CrossRef]
- Lei, Y.-G.; Chan, C.-M.; Li, J.-X.; Ng, K.-M.; Wang, Y.; Jiang, Y.; Li, L. The Birth of an Embryo and Development of the Founding Lamella of Spherulites As Observed by Atomic Force Microscopy. Macromolecules 2002, 35, 6751–6753. [Google Scholar] [CrossRef]
- Schönherr, H.; Frank, C.W. Ultrathin Films of Poly(ethylene oxides) on Oxidized Silicon. 2. In Situ Study of Crystallization and Melting by Hot Stage AFM. Macromolecules 2003, 36, 1199–1208. [Google Scholar] [CrossRef]
- Lei, Y.-G.; Chan, C.-M.; Wang, Y.; Ng, K.-M.; Jiang, Y.; Lin, L. Growth process of homogeneously and heterogeneously nucleated spherulites as observed by atomic force microscopy. Polymer 2003, 44, 4673–4679. [Google Scholar] [CrossRef]
- Shtukenberg, A.G.; Punin, Y.O.; Gunn, E.; Kahr, B. Spherulites. Chem. Rev. 2012, 112, 1805–1838. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-X.; Chen, E.-Q. Polymer crystallization of ultrathin films on solid substrates. Coord. Chem. Rev. 2010, 254, 1011–1037. [Google Scholar] [CrossRef]
- Taguchi, K.; Miyaji, H.; Izumi, K.; Hoshino, A.; Miyamoto, Y.; Kokawa, R. Crystal growth of isotactic polystyrene in ultrathin films: Film thickness dependence. J. Macromol. Sci. Part B 2002, 41, 1033–1042. [Google Scholar] [CrossRef]
- Mullins, W.W.; Sekerka, R.F. Morphological Stability of a Particle Growing by Diffusion or Heat Flow. J. Appl. Phys. 1963, 34, 323–329. [Google Scholar] [CrossRef]
- Prud’homme, R.E. Crystallization and morphology of ultrathin films of homopolymers and polymer blends. Prog. Polym. Sci. 2016, 54–55, 214–231. [Google Scholar]
- Dazzi, A.; Prater, C.B.; Hu, Q.; Chase, D.B.; Rabolt, J.F.; Marcott, C. AFM–IR: Combining atomic force microscopy and infrared spectroscopy for nanoscale chemical characterization. Appl. Spectrosc. 2012, 66, 1365–1384. [Google Scholar] [CrossRef] [Green Version]
- Prater, C.; Kjoller, K.; Cook, D.; Shetty, R.; Meyers, G.; Reinhardt, C.; Felts, J.; King, W.; Vodopyanov, K.; Dazzi, A. Nanoscale infrared spectroscopy of materials by atomic force microscopy. Microsc. Anal. -UK 2010, 24, 5–8. [Google Scholar]
- Dazzi, A.; Glotin, F.; Carminati, R. Theory of infrared nanospectroscopy by photothermal induced resonance. J. Appl. Phys. 2010, 107, 124519. [Google Scholar] [CrossRef]
- Katzenmeyer, A.M.; Aksyuk, V.; Centrone, A. Nanoscale infrared spectroscopy: Improving the spectral range of the photothermal induced resonance technique. Anal. Chem. 2013, 85, 1972–1979. [Google Scholar] [CrossRef]
- Felts, J.R.; Kjoller, K.; Lo, M.; Prater, C.B.; King, W.P. Nanometer-scale infrared spectroscopy of heterogeneous polymer nanostructures fabricated by tip-based nanofabrication. ACS Nano 2012, 6, 8015–8021. [Google Scholar] [CrossRef] [PubMed]
- Mayet, C.; Deniset-Besseau, A.; Prazeres, R.; Ortega, J.-M.; Dazzi, A. Analysis of bacterial polyhydroxybutyrate production by multimodal nanoimaging. Biotechnol. Adv. 2013, 31, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Kjoller, K.; Prater, C.; Shetty, R. Polymer Characterization: Using Nanoscale Infrared Spectroscopy. Am. Lab. 2010, 42, 20–23. [Google Scholar]
- Ye, J.; Midorikawa, H.; Awatani, T.; Marcott, C.; Lo, M.; Kjoller, K.; Shetty, R. Nanoscale infrared spectroscopy and AFM imaging of a polycarbonate/acrylonitrile-styrene/butadiene blend. Microsc. Anal 2012, 26, 24–27. [Google Scholar]
- Felts, J.R.; Somnath, S.; Ewoldt, R.H.; King, W.P. Nanometer-scale flow of molten polyethylene from a heated atomic force microscope tip. Nanotechnology 2012, 23, 215301. [Google Scholar] [CrossRef] [PubMed]
- Kenkel, S.; Mittal, A.; Mittal, S.; Bhargava, R. Probe–Sample Interaction-Independent Atomic Force Microscopy–Infrared Spectroscopy: Toward Robust Nanoscale Compositional Mapping. Anal. Chem. 2018, 90, 8845–8855. [Google Scholar] [CrossRef] [PubMed]
- Van Eerdenbrugh, B.; Lo, M.; Kjoller, K.; Marcott, C.; Taylor, L.S. Nanoscale mid-infrared imaging of phase separation in a drug–polymer blend. J. Pharm. Sci. 2012, 101, 2066–2073. [Google Scholar] [CrossRef]
- Barsbay, M.; Güner, A. Miscibility of dextran and poly (ethylene glycol) in solid state: Effect of the solvent choice. Carbohydr. Polym. 2007, 69, 214–223. [Google Scholar] [CrossRef]
- Borodko, Y.; Habas, S.E.; Koebel, M.; Yang, P.; Frei, H.; Somorjai, G.A. Probing the Interaction of Poly (vinylpyrrolidone) with Platinum Nanocrystals by UV−Raman and FTIR. J. Phys. Chem. B 2006, 110, 23052–23059. [Google Scholar] [CrossRef]
- Marcott, C.; Awatani, T.; Ye, J.; Gerrard, D.; Lod, M.; Kjollerd, K. Review of nanoscale infrared spectroscopy applications to energy related materials. Spectrosc. Eur. 2014, 19–23. [Google Scholar]
- Tipduangta, P.; Belton, P.; Fabian, L.; Wang, L.Y.; Tang, H.; Eddleston, M.; Qi, S. Electrospun polymer blend nanofibers for tunable drug delivery: The role of transformative phase separation on controlling the release rate. Mol. Pharm. 2016, 13, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Taylor, L.S. Nanoscale infrared, thermal, and mechanical characterization of telaprevir–polymer miscibility in amorphous solid dispersions prepared by solvent evaporation. Mol. Pharm. 2016, 13, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Bao, P.; Roy, A.; Wang, Y.; Su, Z. In-situ spectroscopic and thermal analyses of phase domains in high-impact polypropylene. Polymer 2018, 142, 155–163. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef] [Green Version]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Tognana, S.A.; Salgueiro, W.A.; Silva, L. A SAXS study of PHB/DGEBA blends crystallized at different temperatures. Procedia Mater. Sci. 2015, 8, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.R.; Ogawa, S.; Sato, H.; Takahashi, I.; Ozaki, Y. Evolution of intermediate and highly ordered crystalline states under spatial confinement in poly (3-hydroxybutyrate) ultrathin films. Macromolecules 2016, 49, 4202–4210. [Google Scholar]
- Rosenthal, M.; Burghammer, M.; Bar, G.; Samulski, E.T.; Ivanov, D.A. Switching chirality of hybrid left–right crystalline helicoids built of achiral polymer chains: When right to left becomes left to right. Macromolecules 2014, 47, 8295–8304. [Google Scholar] [CrossRef]
- Ivanov, D.A.; Rosenthal, M. Microstructure of banded polymer spherulites: New insights from synchrotron nanofocus X-ray scattering. In Polymer Crystallization II; Springer: Berlin, Germany, 2016; pp. 95–126. [Google Scholar]
- Nguyen-Tri, P.; Prud’homme, R.E. Nanoscale lamellar assembly and segregation mechanism of poly (3-hydroxybutyrate)/poly (ethylene glycol) blends. Macromolecules 2018, 51, 181–188. [Google Scholar]
- Nguyen Tri, P.; Prud’homme, R.E. Crystallization and Segregation Behavior at the Submicrometer Scale of PCL/PEG Blends. Macromolecules 2018, 51, 7266–7273. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; Prud’homme, R.E. Nanoscale analysis of the photodegradation of Polyester fibers by AFM-IR. J. Photochem. Photobiol. A Chem. 2019, 371, 196–204. [Google Scholar] [CrossRef]
- Zeb, G.; Nguyen-Tri., P.; Palacin, S.; Le, X.T. Pulse potential deposition of thick polyvinylpyridine-like film on the surface of titanium nitride. RSC Adv. 2016, 6, 80825–80829. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Le, X.H.; Dao, P.H.; Decker, C.; Nguyen-Tri, P. Stability of acrylic polyurethane coatings under accelerated aging tests and natural outdoor exposure: The critical role of the used photo-stabilizers. Prog. Org. Coat. 2018, 124, 137–146. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Tri, P.N.; Nguyen, T.D.; El Aidani, R.; Decker, C. Accelerated degradation of water borne acrylic nanocomposites used in outdoor protective coatings. Polym. Degrad. Stab. 2016, 128, 65–76. [Google Scholar] [CrossRef]
- Nguyen-Tri, P.; El Aidani, R.; Leborgne, É.; Pham, T.; Vu-Khanh, T. Chemical ageingaging of a polyester nonwoven membrane used in aerosol and drainage filter. Polym. Degrad. Stab. 2014, 101, 71–80. [Google Scholar] [CrossRef]
- El Aidani, R.; Nguyen-Tri, P.; Malajati, Y.; Lara, J.; Vu-Khanh, T. Photochemical aging of an e-PTFE/NOMEX® membrane used in firefighter protective clothing. Polym. Degrad. Stab. 2013, 98, 1300–1310. [Google Scholar] [CrossRef]
- Yan, C.; Huang, W.; Ma, J.; Xu, J.; Lv, Q.; Lin, P. Characterizing the SBS polymer degradation within high content polymer modified asphalt using ATR-FTIR. Constr. Build. Mater. 2020, 233, 117708. [Google Scholar] [CrossRef]
- Tsuji, H.; Sato, S.; Masaki, N.; Arakawa, Y.; Yoshizaki, Y.; Kuzuya, A.; Ohya, Y. Thermal properties and degradation of enantiomeric copolyesteramides poly(lactic acid-co-alanine)s. Polym. Degrad. Stab. 2020, 171, 109047. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Tailoring the toughness of sustainable polymer blends from biodegradable plastics via morphology transition observed by atomic force microscopy. Polym. Degrad. Stab. 2020, 173, 109066. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Li, L.; Dai, J.; Ran, Q.; Gu, Y. Thermal degradation mechanism of a cured acetylene/aldehyde functional benzoxazine with high thermal stability. Polym. Degrad. Stab. 2020, 171, 109041. [Google Scholar] [CrossRef]
- Numata, K.; Asano, A.; Nakazawa, Y. Solid-state and time domain NMR to elucidate degradation behavior of thermally aged poly (urea-urethane). Polym. Degrad. Stab. 2020, 172, 109052. [Google Scholar] [CrossRef]
- Wei, T.; Tang, Z.; Yu, Q.; Chen, H. Smart antibacterial surfaces with switchable bacteria-killing and bacteria-releasing capabilities. ACS Appl. Mater. Interfaces 2017, 9, 37511–37523. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.V.; Davanco, M.; Sun, Z.; Kretinin, A.; Chen, Y.; Matson, J.R.; Vurgaftman, I.; Sharac, N.; Giles, A.J.; Fogler, M.M. Nanoscale mapping and spectroscopy of nonradiative hyperbolic modes in hexagonal boron nitride nanostructures. Nano Lett. 2018, 18, 1628–1636. [Google Scholar] [CrossRef] [Green Version]
- Morsch, S.; Lyon, S.; Greensmith, P.; Smith, S.; Gibbon, S. Mapping water uptake in organic coatings using AFM-IR. Faraday Discuss. 2015, 180, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Morsch, S.; Lyon, S.; Gibbon, S. The degradation mechanism of an epoxy-phenolic can coating. Prog. Org. Coat. 2017, 102, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Morsch, S.; Liu, Y.; Greensmith, P.; Lyon, S.B.; Gibbon, S.R. Molecularly controlled epoxy network nanostructures. Polymer 2017, 108, 146–153. [Google Scholar] [CrossRef]
- Morsch, S.; Van Driel, B.A.; van den Berg, K.J.; Dik, J. Investigating the photocatalytic degradation of oil paint using ATR-IR and AFM-IR. ACS Appl. Mater. Interfaces 2017, 9, 10169–10179. [Google Scholar] [CrossRef] [Green Version]
- Morsch, S.; Bastidas, P.D.; Rowland, S.M. AFM-IR insights into the chemistry of interfacial tracking. J. Mater. Chem. A 2017, 5, 24508–24517. [Google Scholar] [CrossRef] [Green Version]
- Shiraishi, F.; Maruoka, D.; Tanoue, Y. A better UV light and TiO2-PET sheet arrangement for enhancing photocatalytic decomposition of volatile organic compounds. Separat. Purif. Technol. 2017, 175, 185–193. [Google Scholar] [CrossRef]
- Hurley, C.R.; Leggett, G.J. Quantitative investigation of the photodegradation of polyethylene terephthalate film by friction force microscopy, contact-angle goniometry, and X-ray photoelectron spectroscopy. ACS Appl. Mater. Interfaces 2009, 1, 1688–1697. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen-Tri, P.; Ghassemi, P.; Carriere, P.; Nanda, S.; Assadi, A.A.; Nguyen, D.D. Recent Applications of Advanced Atomic Force Microscopy in Polymer Science: A Review. Polymers 2020, 12, 1142. https://doi.org/10.3390/polym12051142
Nguyen-Tri P, Ghassemi P, Carriere P, Nanda S, Assadi AA, Nguyen DD. Recent Applications of Advanced Atomic Force Microscopy in Polymer Science: A Review. Polymers. 2020; 12(5):1142. https://doi.org/10.3390/polym12051142
Chicago/Turabian StyleNguyen-Tri, Phuong, Payman Ghassemi, Pascal Carriere, Sonil Nanda, Aymen Amine Assadi, and Dinh Duc Nguyen. 2020. "Recent Applications of Advanced Atomic Force Microscopy in Polymer Science: A Review" Polymers 12, no. 5: 1142. https://doi.org/10.3390/polym12051142
APA StyleNguyen-Tri, P., Ghassemi, P., Carriere, P., Nanda, S., Assadi, A. A., & Nguyen, D. D. (2020). Recent Applications of Advanced Atomic Force Microscopy in Polymer Science: A Review. Polymers, 12(5), 1142. https://doi.org/10.3390/polym12051142