Glycopolymer Brushes by Reversible Deactivation Radical Polymerization: Preparation, Applications, and Future Challenges
Abstract
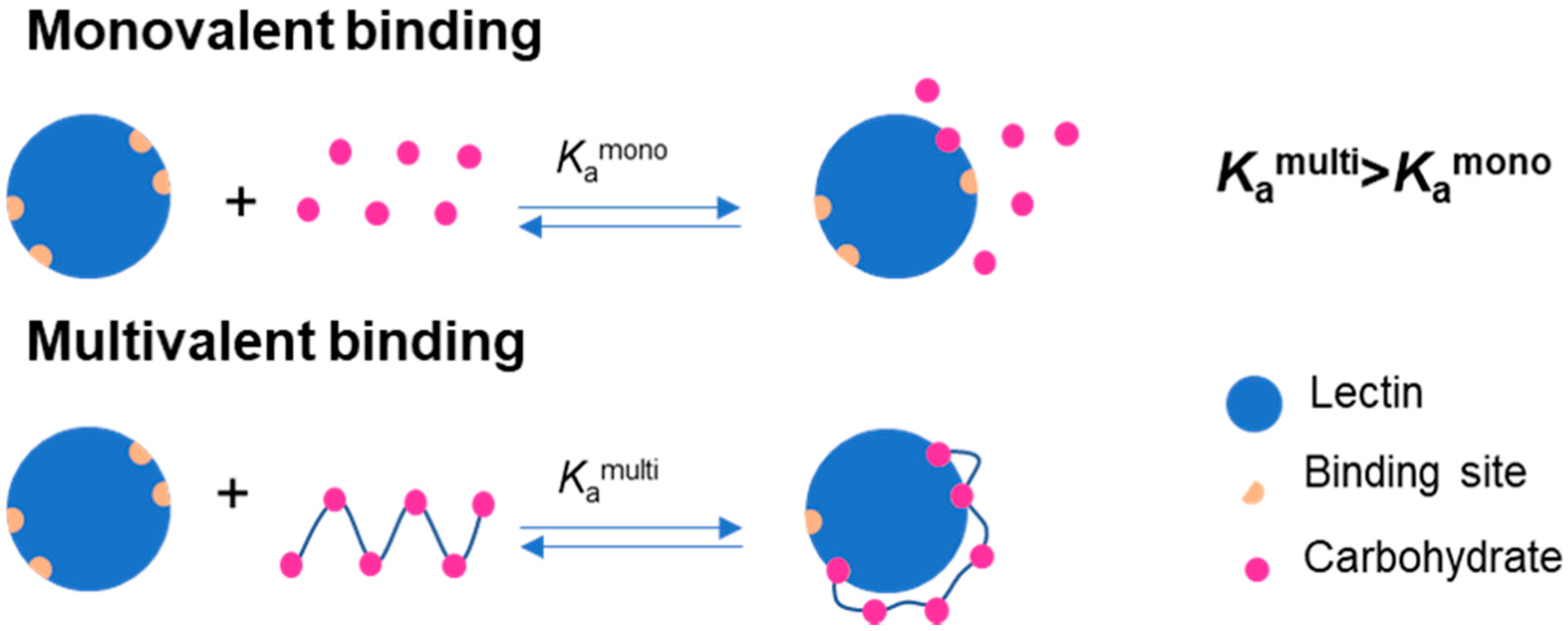
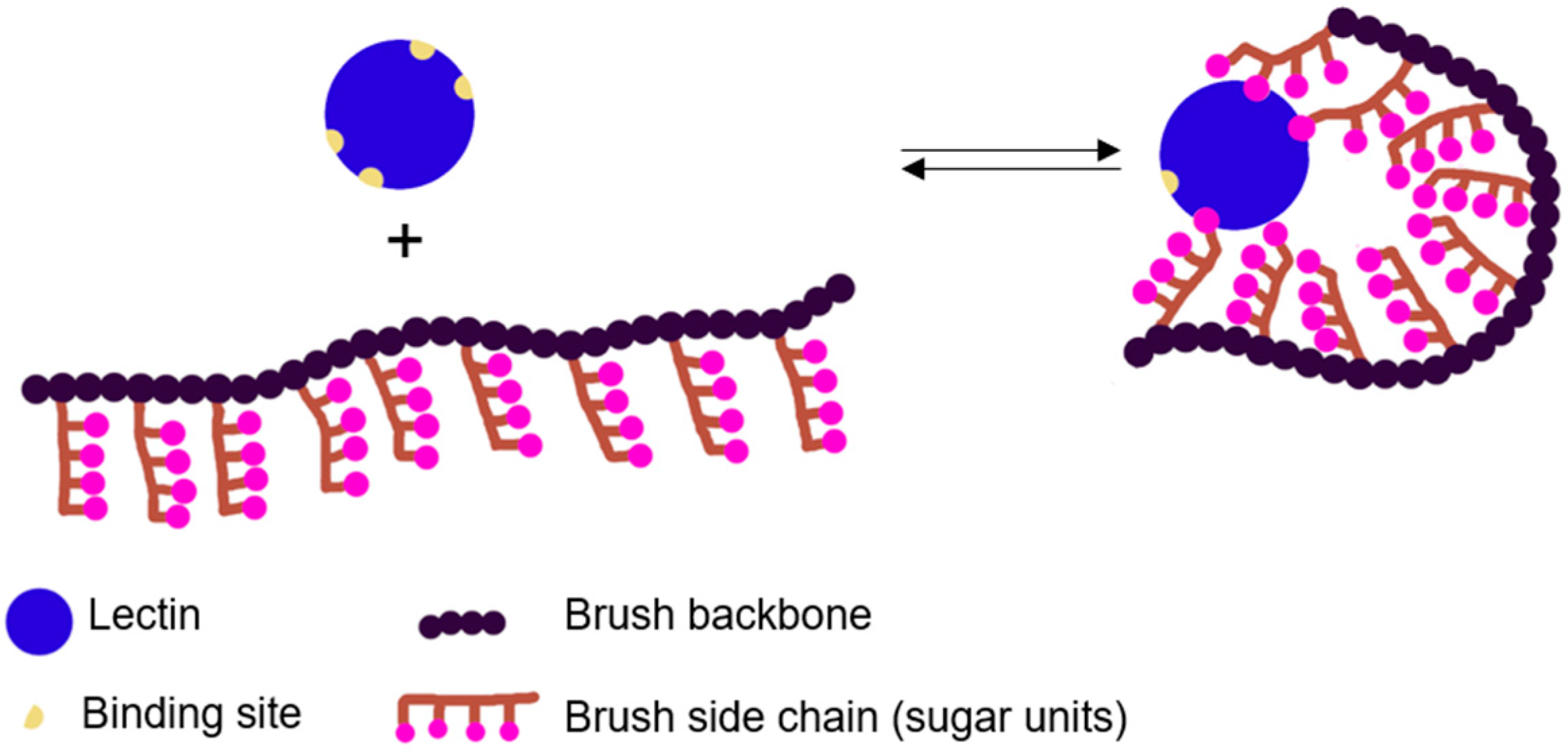
:1. Introduction
2. Preparation of Polymer Brushes by RDRP
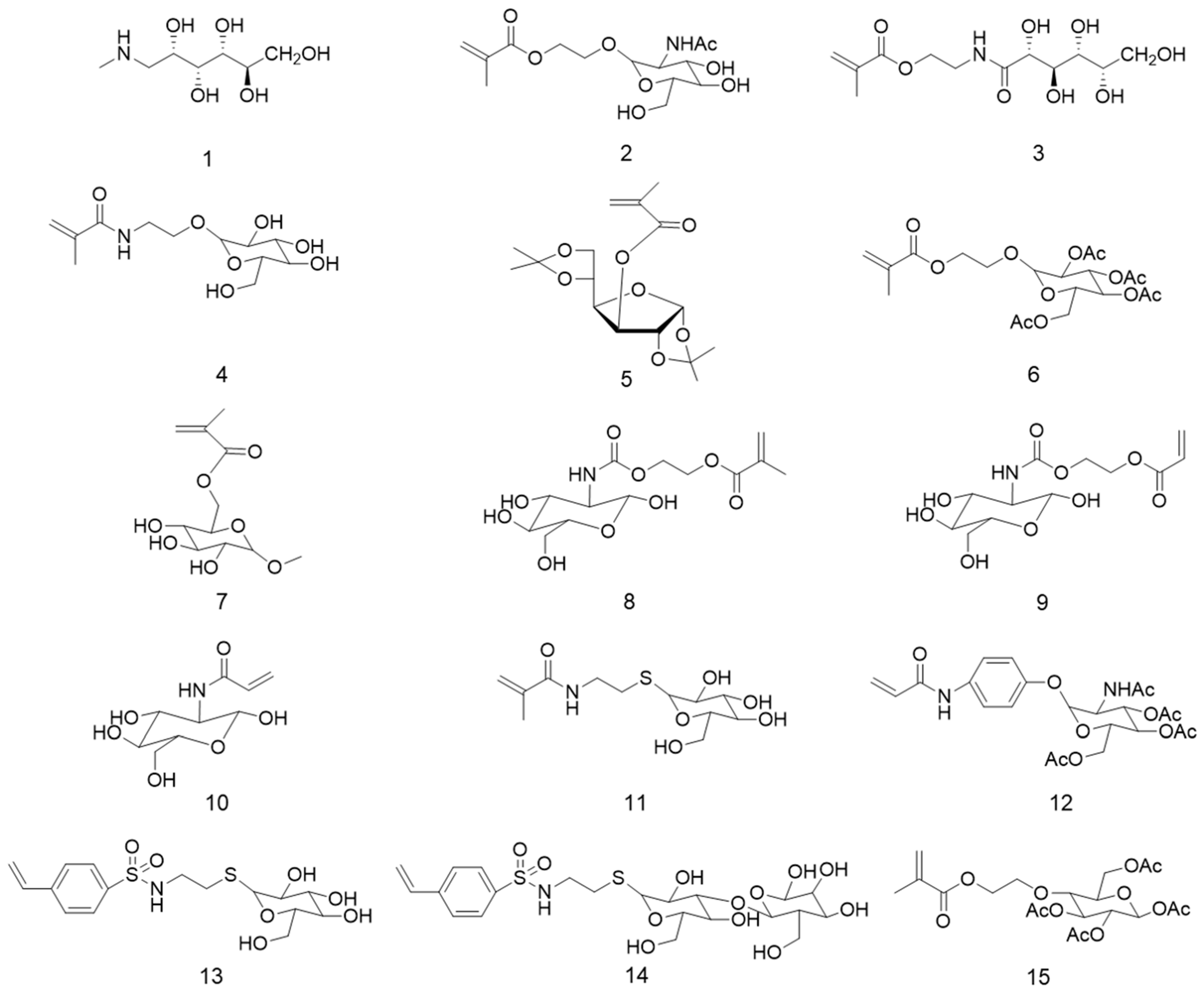
3. Glucose-Containing Polymer Brushes
4. Mannose-Containing Polymer Brushes
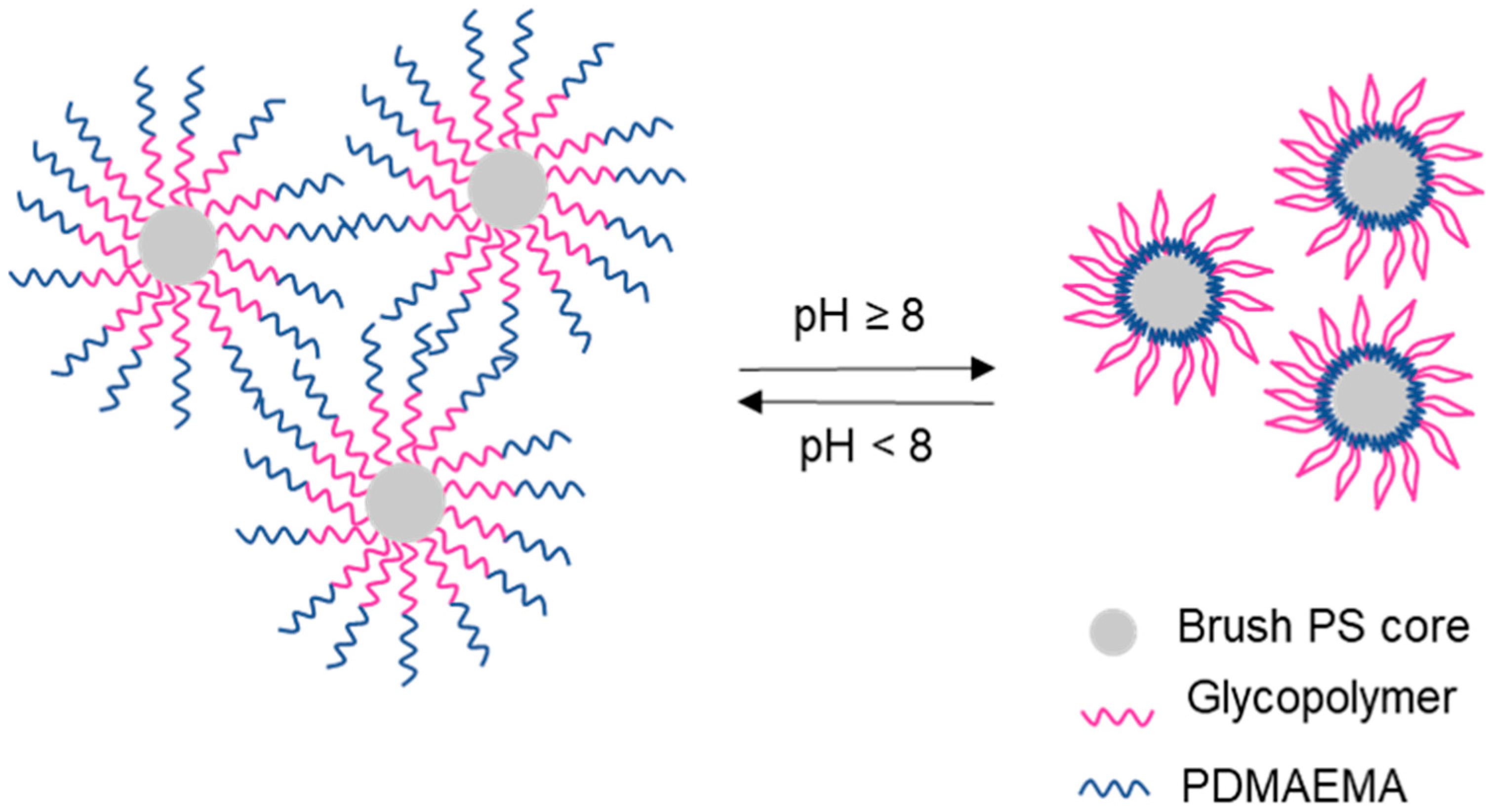
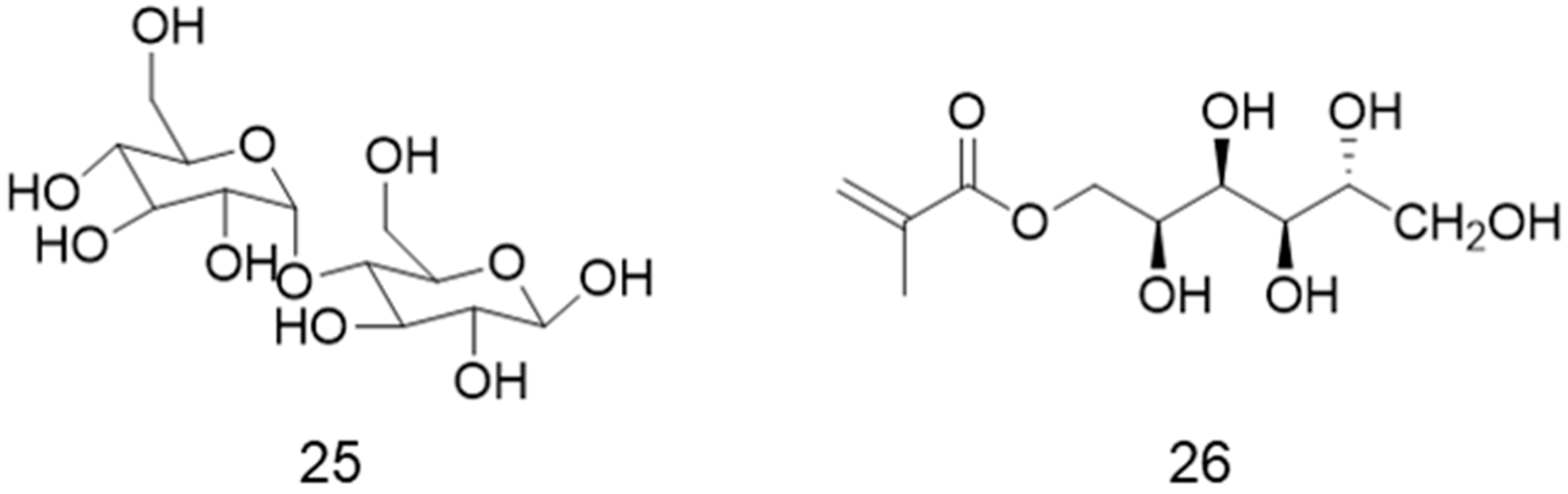
5. Galactose-Containing Polymer Brushes
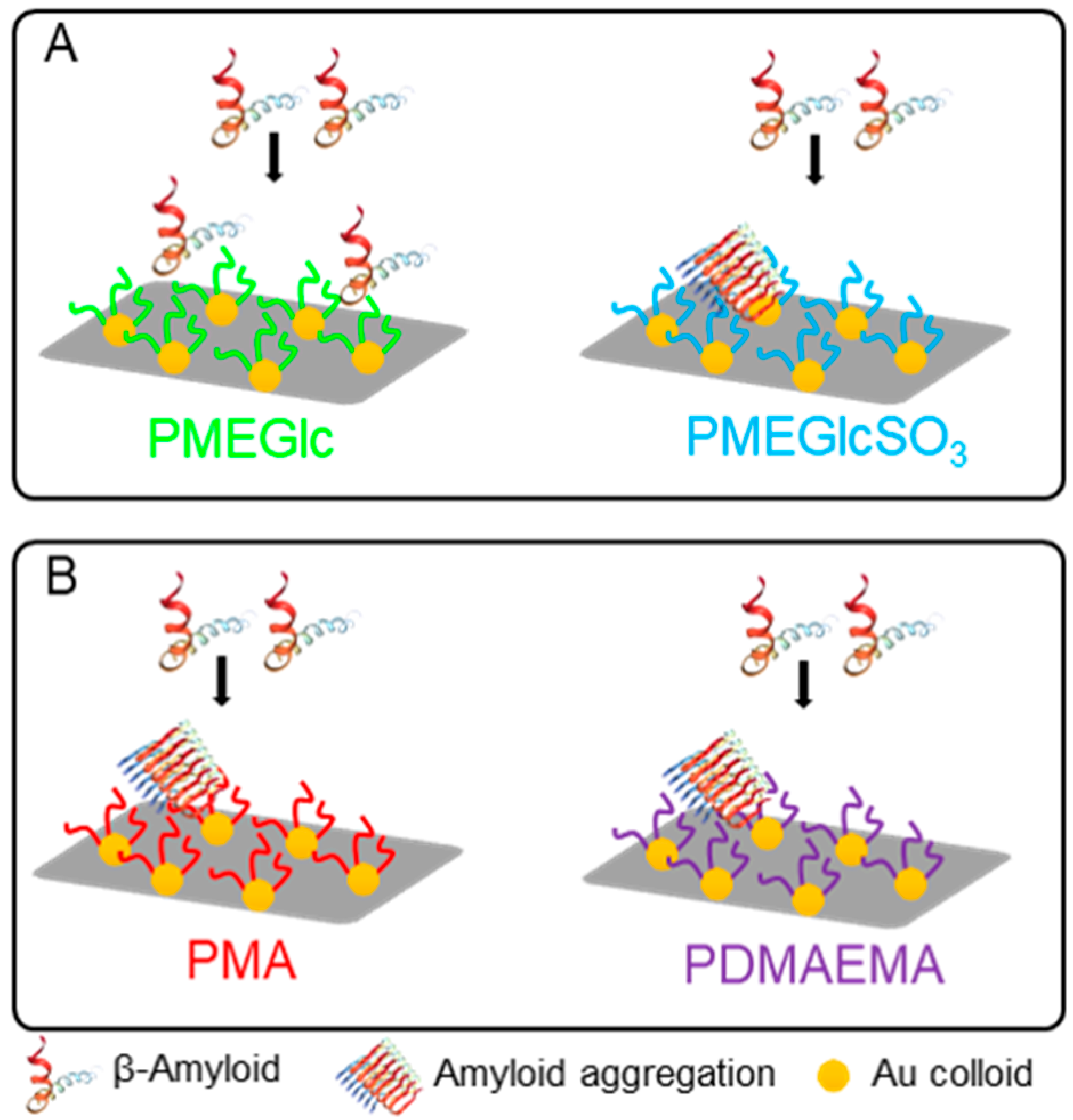
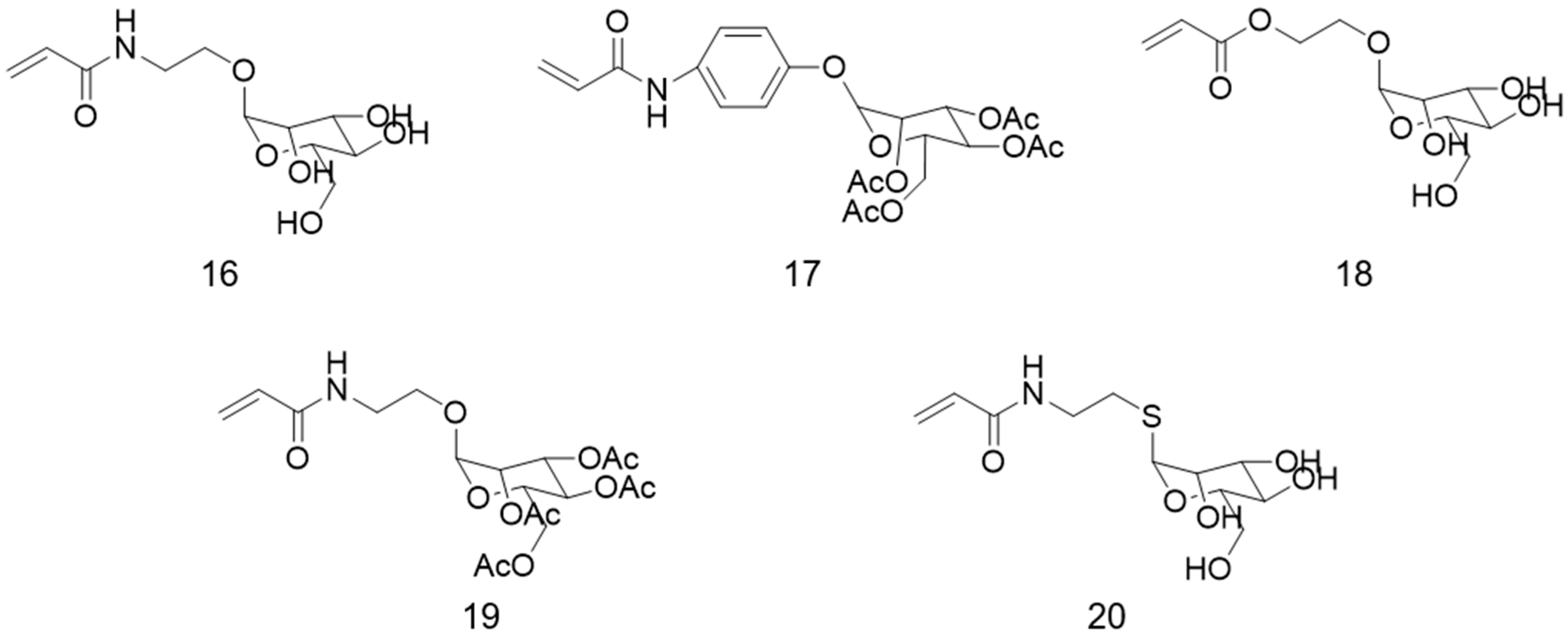
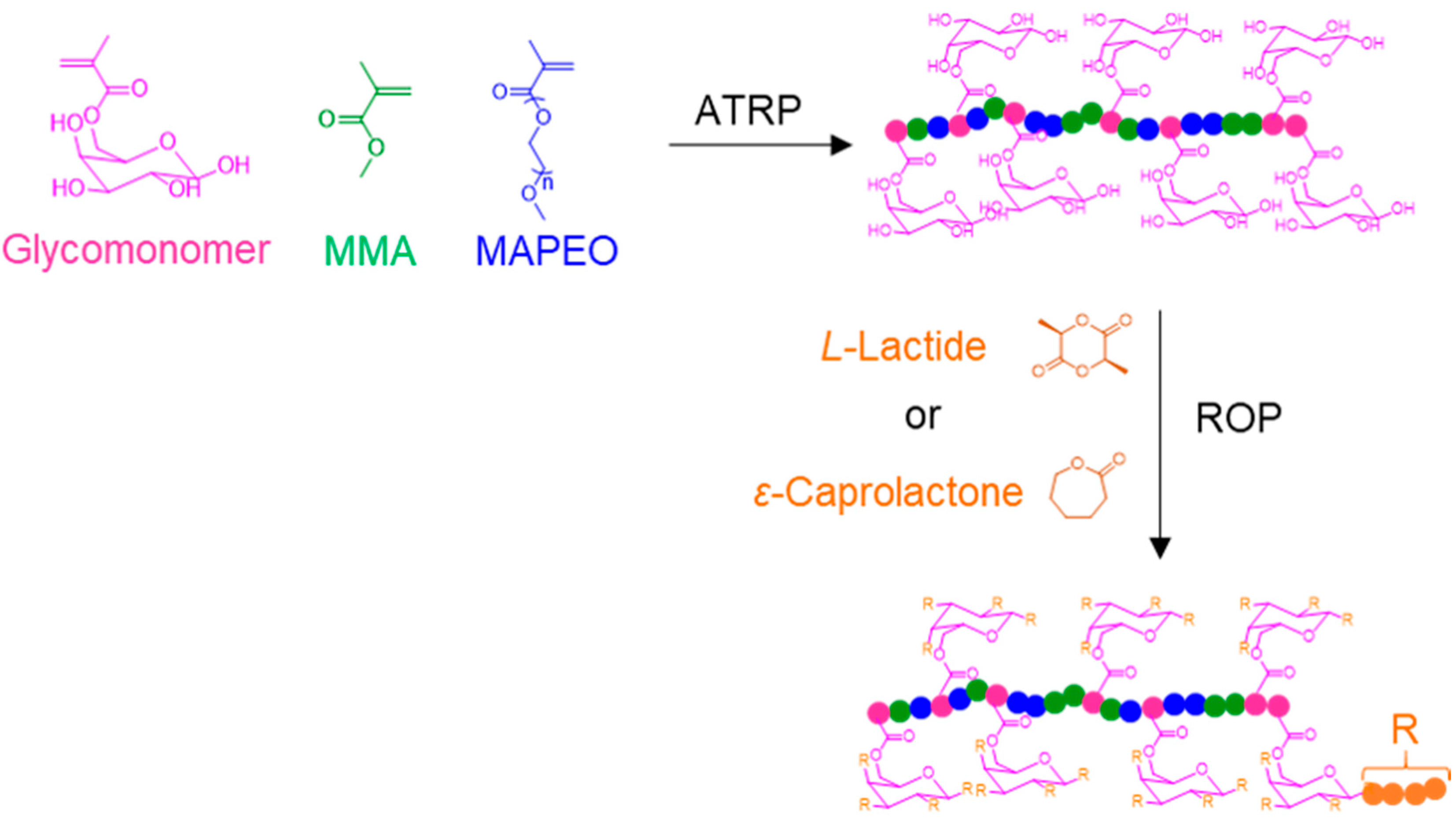
6. Other Carbohydrate-Based Polymer Brushes
7. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miura, Y.; Hoshino, Y.; Seto, H. Glycopolymer Nanobiotechnology. Chem. Rev. 2016, 116, 1673–1692. [Google Scholar] [CrossRef] [PubMed]
- Ernst, B.; Magnani, J.L. From Carbohydrate Leads to Glycomimetic Drugs. Nat. Rev. Drug Discov. 2009, 8, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Murphy, P.V.; Gabius, H.J. Multivalent Carbohydrate-Lectin Interactions: How Synthetic Chemistry Enables Insights into Nanometric Recognition. Molecules 2016, 21, 629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, H.; Van Damme, E.J.M.; Peumans, W.J.; Goldstein, I.J. Purification and Characterization of a Mannose-Specific Lectin from Shallot (Allium Ascalonicum) Bulbs. Arch. Biochem. Biophys. 1993, 306, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, I.J.; Winter, H.C.; Poretz, R.D. Plant Lectins: Tools for the Study of Complex Carbohydrates. In New Comprehensive Biochemistry; Montreuil, J., Vliegenthart, J.F.G., Schachter, H., Eds.; Elsevier Masson SAS: Amesterdam, The Netherlands, 1997; Chapter 12; Volume 29, pp. 1–652. [Google Scholar] [CrossRef]
- Yilmaz, G.; Becer, C.R. Glyconanoparticles and Their Interactions with Lectins. Polym. Chem. 2015, 6, 5503–5514. [Google Scholar] [CrossRef] [Green Version]
- Lis, H.; Sharon, N. Lectins: Carbohydrate-Specific Proteins That Mediate Cellular Recognition. Chem. Rev. 1998, 98, 637–674. [Google Scholar] [CrossRef]
- Goldstein, I.J.; Hollerman, C.E.; Smith, E.E. Protein-Carbohydrate Interaction. II. Inhibition Studies on the Interaction of Concanavalin A with Polysaccharides. Biochemistry 1965, 4, 876–883. [Google Scholar] [CrossRef]
- Ma, Z.; Zhu, X.X. Copolymers Containing Carbohydrates and Other Biomolecules: Design, Synthesis and Applications. J. Mater. Chem. B 2019, 7, 1361–1378. [Google Scholar] [CrossRef]
- Pramudya, I.; Chung, H. Recent Progress of Glycopolymer Synthesis for Biomedical Applications. Biomater. Sci. 2019, 7, 4848–4872. [Google Scholar] [CrossRef]
- Zhang, Q.; Haddleton, D.M. Synthetic Glycopolymers: Some Recent Developments. In Hierarchical Macromolecular Structures: 60 Years after the Staudinger Nobel Prize II; Percec, V., Ed.; Springer International Publishing: Cham, Switzerland, 2013; Volume 626, pp. 39–59. [Google Scholar] [CrossRef] [Green Version]
- Ghadban, A.; Albertin, L. Synthesis of Glycopolymer Architectures by Reversible-Deactivation Radical Polymerization. Polymers 2013, 5, 431–526. [Google Scholar] [CrossRef] [Green Version]
- Pfaff, A.; Shinde, V.S.; Lu, Y.; Wittemann, A.; Ballauff, M.; Müller, A.H.E. Glycopolymer-Grafted Polystyrene Nanospheres. Macromol. Biosci. 2011, 11, 199–210. [Google Scholar] [CrossRef]
- Narumi, A.; Kakuchi, T. Synthesis of Glycoconjugated Branched Macromolecular Architectures. Polym. J. 2008, 40, 383–397. [Google Scholar] [CrossRef] [Green Version]
- Miura, Y. Design and Synthesis of Well-Defined Glycopolymers for the Control of Biological Functionalities. Polym. J. 2012, 44, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Martinez, M.R.; Olszewski, M.; Sheiko, S.S.; Matyjaszewski, K. Molecular Bottlebrushes as Novel Materials. Biomacromolecules 2019, 20, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Hadjicharalambous, C.; Flouraki, C.; Narain, R.; Chatzinikolaidou, M.; Vamvakaki, M. Controlling Pre-Osteoblastic Cell Adhesion and Spreading on Glycopolymer Brushes of Variable Film Thickness. J. Mater. Sci. Mater. Med. 2018, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kratzer, D.; Cheng, K.; Prisby, J.; Sugai, J.; Giannobile, W.V.; Lahann, J. Carbohydrate-Based Polymer Brushes Prevent Viral Adsorption on Electrostatically Heterogeneous Interfaces. Macromol. Rapid Commun. 2019, 40, 1800530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Brittain, W.J. Polymer Brushes: Surface-Immobilized Macromolecules. Prog. Polym. Sci. 2000, 25, 677–710. [Google Scholar] [CrossRef]
- Verduzco, R.; Li, X.; Pesek, S.L.; Stein, G.E. Structure, Function, Self-Assembly, and Applications of Bottlebrush Copolymers. Chem. Soc. Rev. 2015, 44, 2405–2420. [Google Scholar] [CrossRef] [Green Version]
- Minko, S. Grafting on Solid Surfaces: “Grafting to” and “Grafting from” Methods. In Polymer Surfaces and Interfaces: Characterization, Modification and Applications; Stamm, M., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2008; pp. 215–234. [Google Scholar] [CrossRef]
- Von Der Ehe, C.; Weber, C.; Gottschaldt, M.; Schubert, U.S. Immobilized Glycopolymers: Synthesis, Methods and Applications. Prog. Polym. Sci. 2016, 57, 64–102. [Google Scholar] [CrossRef]
- Mohammadi Sejoubsari, R.; Martinez, A.P.; Kutes, Y.; Wang, Z.; Dobrynin, A.V.; Adamson, D.H. “Grafting-Through”: Growing Polymer Brushes by Supplying Monomers through the Surface. Macromolecules 2016, 49, 2477–2483. [Google Scholar] [CrossRef]
- Pei, D.; Li, Y.; Huang, Q.; Ren, Q.; Li, F.; Shi, T. Biomimetic Glycopolymers Tethered Gold Nanoparticles: Preparation, Self-Assembly and Lectin Recognition Properties. Colloids Surf. B Biointerfaces 2015, 126, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Rosencrantz, R.R.; Nguyen, V.H.; Park, H.; Schulte, C.; Böker, A.; Schnakenberg, U.; Elling, L. Lectin Binding Studies on a Glycopolymer Brush Flow-through Biosensor by Localized Surface Plasmon Resonance. Anal. Bioanal. Chem. 2016, 408, 5633–5640. [Google Scholar] [CrossRef] [PubMed]
- Chernyy, S.; Jensen, B.E.B.; Shimizu, K.; Ceccato, M.; Pedersen, S.U.; Zelikin, A.N.; Daasbjerg, K.; Iruthayaraj, J. Surface Grafted Glycopolymer Brushes to Enhance Selective Adhesion of HepG2 Cells. J. Colloid Interface Sci. 2013, 404, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Idota, N.; Ebara, M.; Kotsuchibashi, Y.; Narain, R.; Aoyagi, T. Novel Temperature-Responsive Polymer Brushes with Carbohydrate Residues Facilitate Selective Adhesion and Collection of Hepatocytes. Sci. Technol. Adv. Mater. 2012, 13, 064206. [Google Scholar] [CrossRef]
- Meng, X.L.; Fang, Y.; Wan, L.S.; Huang, X.J.; Xu, Z.K. Glycopolymer Brushes for the Affinity Adsorption of RCA120: Effects of Thickness, Grafting Density, and Epitope Density. Langmuir 2012, 28, 13616–13623. [Google Scholar] [CrossRef]
- Lepoittevin, B.; Costa, L.; Pardoue, S.; Dragoé, D.; Mazerat, S.; Roger, P. Hydrophilic PET Surfaces by Aminolysis and Glycopolymer Brushes Chemistry. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2689–2697. [Google Scholar] [CrossRef]
- Stenzel, M.H.; Zhang, L.; Huck, W.T.S. Temperature-Responsive Glycopolymer Brushes Synthesized via RAFT Polymerization Using the Z-Group Approach. Macromol. Rapid Commun. 2006, 27, 1121–1126. [Google Scholar] [CrossRef]
- Sun, P.; Wang, G.; Hou, H.; Yuan, P.; Deng, W.; Wang, C.; Lu, X.; Fan, Q.; Huang, W. A Water-Soluble Phosphorescent Conjugated Polymer Brush for Tumor-Targeted Photodynamic Therapy. Polym. Chem. 2017, 8, 5836–5844. [Google Scholar] [CrossRef]
- Ordanini, S.; Celentano, W.; Bernardi, A.; Cellesi, F. Mannosylated Brush Copolymers Based on Poly (Ethylene Glycol) and Poly (ε-Caprolactone) as Multivalent Lectin-Binding Nanomaterials. Beilstein J. Nanotechnol. 2019, 10, 2192–2206. [Google Scholar] [CrossRef]
- Fleet, R.; Van den Dungen, E.; Klumperman, B. Synthesis of Novel Glycopolymer Brushes via a Combination of RAFT-Mediated Polymerisation and ATRP. S. Afr. J. Sci. 2011, 107, 1–11. [Google Scholar] [CrossRef]
- Yebra-Biurrun, M.C. Food and Nutritional Analysis | Sweeteners. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Townshend, A., Poole, C., Miró, M., Eds.; Elsevier: Oxford, UK, 2013; Volume 3, pp. 471–481. [Google Scholar] [CrossRef]
- La Fleur, S.E.; Fliers, E.; Kalsbeek, A. Neuroscience of Glucose Homeostasis. In Handbook of Clinical Neurology; Zochodne, D.W., Malik, R.A.B.T., Eds.; Elsevier: London, UK, 2014; Chapter 24; Volume 126, pp. 341–351. [Google Scholar] [CrossRef]
- Nelson, D.L.; Lehninger, A.L.; Cox, M.M. Lehninger Principles of Biochemistry, 4th ed.; W. H. Freeman: New York, NY, USA, 2005; pp. 1–1119. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 6th ed.; W. H. Freeman: New York, NY, USA, 2007; pp. 1–1120. [Google Scholar]
- Yu, K.; Kizhakkedathu, J.N. Synthesis of Functional Polymer Brushes Containing Carbohydrate Residues in the Pyranose Form and Their Specific and Nonspecific Interactions with Proteins. Biomacromolecules 2010, 11, 3073–3085. [Google Scholar] [CrossRef] [PubMed]
- Schulte-Osseili, C.; Kleinert, M.; Keil, N.; Rosencrantz, R.R. Rapid Drop-Test for Lectin Binding with Glycopolymer-Coated Optical Ring Resonators. Biosensors 2019, 9, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaff, A.; Müller, A.H.E. Hyperbranched Glycopolymer Grafted Microspheres. Macromolecules 2011, 44, 1266–1272. [Google Scholar] [CrossRef]
- Ogata, Y.; Seto, H.; Murakami, T.; Hoshino, Y.; Miura, Y. Affinity Separation of Lectins Using Porous Membranes Immobilized with Glycopolymer Brushes Containing Mannose or N-Acetyl-D-Glucosamine. Membranes (Basel) 2013, 3, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Kitano, H.; Saito, D.; Kamada, T.; Gemmei-Ide, M. Binding of β-Amyloid to Sulfated Sugar Residues in a Polymer Brush. Colloids Surf. B Biointerfaces 2012, 93, 219–225. [Google Scholar] [CrossRef]
- Yang, Q.; Tian, J.; Hu, M.X.; Xu, Z.K. Construction of a Comb-like Glycosylated Membrane Surface by a Combination of UV-Induced Graft Polymerization and Surface-Initiated ATRP. Langmuir 2007, 23, 6684–6690. [Google Scholar] [CrossRef]
- Yuan, J.; Meng, J.Q.; Kang, Y.L.; Du, Q.Y.; Zhang, Y.F. Facile Surface Glycosylation of PVDF Microporous Membrane via Direct Surface-Initiated AGET ATRP and Improvement of Antifouling Property and Biocompatibility. Appl. Surf. Sci. 2012, 258, 2856–2863. [Google Scholar] [CrossRef]
- Muthukrishnan, S.; Zhang, M.; Burkhardt, M.; Drechsler, M.; Mori, H.; Müller, A.H.E. Molecular Sugar Sticks: Cylindrical Glycopolymer Brushes. Macromolecules 2005, 38, 7926–7934. [Google Scholar] [CrossRef]
- Fleet, R.; Van Den Dungen, E.T.A.; Klumperman, B. Novel Glycopolymer Brushes via ATRP: 1. Synthesis and Characterization. Macromol. Chem. Phys. 2011, 212, 2191–2208. [Google Scholar] [CrossRef]
- Fleet, R.; Van Den Dungen, E.T.A.; Klumperman, B. Novel Glycopolymer Brushes via ATRP: 2. Thermal an Mechanical Properties. Macromol. Chem. Phys. 2011, 212, 2209–2216. [Google Scholar] [CrossRef]
- Álvarez-Paino, M.; Bordegé, V.; Cuervo-Rodríguez, R.; Muñoz-Bonilla, A.; Fernández-García, M. Well-Defined Glycopolymers via Raft Polymerization: Stabilization of Gold Nanoparticles. Macromol. Chem. Phys. 2014, 215, 1915–1924. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Muthukrishnan, S.; Li, W.; Yuan, J.; Xu, Y.; Müller, A.H.E. Linear and Hyperbranched Glycopolymer-Functionalized Carbon Nanotubes: Synthesis, Kinetics, and Characterization. Macromolecules 2007, 40, 1803–1815. [Google Scholar] [CrossRef]
- Ma, G.; Luo, X.; Sun, X.; Wang, W.; Shou, Q.; Liang, X.; Liu, H. Glycopolymer Grafted Silica Gel as Chromatographic Packing Materials. Int. J. Mol. Sci. 2019, 20, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaff, A.; Müller, A.H.E. Surface Modification of Spherical Particles with Bioactive Glycopolymers. In Progress in Controlled Radical Polymerization: Materials and Applications; Matyjaszewski, K., Sumerlin, B.S., Tsarevsky, N.V., Eds.; American Chemical Society: Washington, DC, USA, 2012; Volume 1101, pp. 257–270. [Google Scholar] [CrossRef] [Green Version]
- Kohri, M.; Sato, M.; Abo, F.; Inada, T.; Kasuya, M.; Taniguchi, T.; Nakahira, T. Preparation and Lectin Binding Specificity of Polystyrene Particles Grafted with Glycopolymers Bearing S-Linked Carbohydrates. Eur. Polym. J. 2011, 47, 2351–2360. [Google Scholar] [CrossRef]
- Mateescu, A.; Ye, J.; Narain, R.; Vamvakaki, M. Synthesis and Characterization of Novel Glycosurfaces by ATRP. Soft Matter 2009, 5, 1621–1629. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Y.; Zhang, S.; Liu, W.; Wu, Z.; Chen, H. Protein-Resistant Properties of Poly (N-Vinylpyrrolidone)-Modified Gold Surfaces: The Advantage of Bottle-Brushes over Linear Brushes. Colloids Surf. B Biointerfaces 2019, 177, 448–453. [Google Scholar] [CrossRef]
- Le-Masurier, S.P.; Duong, H.T.T.; Boyer, C.; Granville, A.M. Surface Modification of Polydopamine Coated Particles via Glycopolymer Brush Synthesis for Protein Binding and FLIM Testing. Polym. Chem. 2015, 6, 2504–2511. [Google Scholar] [CrossRef]
- Kohri, M.; Taniguchi, T.; Kishikawa, K. Glycopolymer-Grafted Polymer Particles for Lectin Recognition. In Macro-Glycoligands. Methods and Protocols; Sun, X.-L., Ed.; Humana Press: New York, NY, USA, 2016; Volume 1367, pp. 183–193. [Google Scholar] [CrossRef]
- Ke, B.B.; Wan, L.S.; Zhang, W.X.; Xu, Z.K. Controlled Synthesis of Linear and Comb-like Glycopolymers for Preparation of Honeycomb-Patterned Films. Polymer (Guildf) 2010, 51, 2168–2176. [Google Scholar] [CrossRef]
- Pan, Y.; Ma, C.; Tong, W.; Fan, C.; Zhang, Q.; Zhang, W.; Tian, F.; Peng, B.; Qin, W.; Qian, X. Preparation of Sequence-Controlled Triblock Copolymer-Grafted Silica Microparticles by Sequential-ATRP for Highly Efficient Glycopeptides Enrichment. Anal. Chem. 2015, 87, 656–662. [Google Scholar] [CrossRef]
- Yang, Q.; Kaul, C.; Ulbricht, M. Anti-Nonspecific Protein Adsorption Properties of Biomimetic Glycocalyx-like Glycopolymer Layers: Effects of Glycopolymer Chain Density and Protein Size. Langmuir 2010, 26, 5746–5752. [Google Scholar] [CrossRef]
- Ejaz, M.; Ohno, K.; Tsujii, Y.; Fukuda, T. Controlled Grafting of a Well-Defined Glycopolymer on a Solid Surface by Surface-Initiated Atom Transfer Radical Polymerization. Macromolecules 2000, 33, 2870–2874. [Google Scholar] [CrossRef]
- Yu, K.; Lai, B.F.L.; Kizhakkedathu Prof., J.N. Carbohydrate Structure Dependent Hemocompatibility of Biomimetic Functional Polymer Brushes on Surfaces. Adv. Healthc. Mater. 2012, 1, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Lai, B.F.L.; Foley, J.H.; Krisinger, M.J.; Conway, E.M.; Kizhakkedathu, J.N. Modulation of Complement Activation and Amplification on Nanoparticle Surfaces by Glycopolymer Conformation and Chemistry. ACS Nano 2014, 8, 7687–7703. [Google Scholar] [CrossRef] [PubMed]
- Raynor, J.E.; Petrie, T.A.; Fears, K.P.; Latour, R.A.; García, A.J.; Collard, D.M. Saccharide Polymer Brushes to Control Protein and Cell Adhesion to Titanium. Biomacromolecules 2009, 10, 748–755. [Google Scholar] [CrossRef]
- Lazar, J.; Rosencrantz, R.R.; Elling, L.; Schnakenberg, U. Simultaneous Electrochemical Impedance Spectroscopy and Localized Surface Plasmon Resonance in a Microfluidic Chip: New Insights into the Spatial Origin of the Signal. Anal. Chem. 2016, 88, 9590–9596. [Google Scholar] [CrossRef] [PubMed]
- Lazar, J.; Park, H.; Rosencrantz, R.R.; Böker, A.; Elling, L.; Schnakenberg, U. Evaluating the Thickness of Multivalent Glycopolymer Brushes for Lectin Binding. Macromol. Rapid Commun. 2015, 36, 1472–1478. [Google Scholar] [CrossRef]
- Park, H.; Rosencrantz, R.R.; Elling, L.; Böker, A. Glycopolymer Brushes for Specific Lectin Binding by Controlled Multivalent Presentation of N-Acetyllactosamine Glycan Oligomers. Macromol. Rapid Commun. 2015, 36, 45–54. [Google Scholar] [CrossRef]
- Zhao, C.; Shi, Q.; Hou, J.; Xin, Z.; Jin, J.; Li, C.; Wong, S.C.; Yin, J. Capturing Red Blood Cells from the Blood by Lectin Recognition on a Glycopolymer-Patterned Surface. J. Mater. Chem. B 2016, 4, 4130–4137. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Synthesis of an Amphiphilic Glucose-Carrying Graft Copolymer and Its Use for Membrane Surface Modification. J. Appl. Polym. Sci. 2008, 109, 2914–2923. [Google Scholar] [CrossRef]
- Von Der Ehe, C.; Buś, T.; Weber, C.; Stumpf, S.; Bellstedt, P.; Hartlieb, M.; Schubert, U.S.; Gottschaldt, M. Glycopolymer-Functionalized Cryogels as Catch and Release Devices for the Pre-Enrichment of Pathogens. ACS Macro Lett. 2016, 5, 326–331. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface Hydration: Principles and Applications toward Low-Fouling/Nonfouling Biomaterials. Polymer (Guildf) 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.C.; Rho, Y.; Kim, G.; Kim, M.; Kim, H.; Kim, I.J.; Kim, J.R.; Ree, M. New Self-Assembled Brush Glycopolymers: Synthesis, Structure and Properties. Polym. Chem. 2013, 4, 2260–2271. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Liu, F.; Xue, L.; Wang, H.; Pan, J.; Cui, Y.; Chen, H.; Yuan, L. Recyclable Escherichia Coli-Specific-Killing AuNP-Polymer (ESKAP) Nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 11309–11317. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Matsumoto, E.; Onogi, S.; Miura, Y. Aggregation of Alzheimer Amyloid β Peptide (1-42) on the Multivalent Sulfonated Sugar Interface. Bioconjug. Chem. 2010, 21, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Yasuda, K.; Yamamoto, K.; Koike, M.; Nishida, Y.; Kobayashi, K. Inhibition of Alzheimer Amyloid Aggregation with Sulfated Gycopolymers. Biomacromolecules 2007, 8, 2129–2134. [Google Scholar] [CrossRef]
- Meng, J.; Yuan, J.; Kang, Y.; Zhang, Y.; Du, Q. Surface Glycosylation of Polysulfone Membrane towards a Novel Complexing Membrane for Boron Removal. J. Colloid Interface Sci. 2012, 368, 197–207. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, W.; Jiang, M.; Xie, X. Surface Glycopolymer-Modified Functional Macroporous PolyHIPE Obtained by ATRP for the Removal of Boron in Water. New J. Chem. 2018, 42, 2104–2112. [Google Scholar] [CrossRef]
- Shi, Q.; Meng, J.Q.; Xu, R.S.; Du, X.L.; Zhang, Y.F. Synthesis of Hydrophilic Polysulfone Membranes Having Antifouling and Boron Adsorption Properties via Blending with an Amphiphilic Graft Glycopolymer. J. Memb. Sci. 2013, 444, 50–59. [Google Scholar] [CrossRef]
- Hestrin, S.; Mager, J. Differentiation between Glucose, Galactose and Mannose by a Colour Reaction. Nature 1946, 158, 95. [Google Scholar] [CrossRef]
- Sharma, V.; Smolin, J.; Nayak, J.; Ayala, J.E.; Scott, D.A.; Peterson, S.N.; Freeze, H.H. Mannose Alters Gut Microbiome, Prevents Diet-Induced Obesity, and Improves Host Metabolism. Cell Rep. 2018, 24, 3087–3098. [Google Scholar] [CrossRef] [Green Version]
- Kranjčec, B.; Papeš, D.; Altarac, S. D-Mannose Powder for Prophylaxis of Recurrent Urinary Tract Infections in Women: A Randomized Clinical Trial. World J. Urol. 2014, 32, 79–84. [Google Scholar] [CrossRef]
- Patterson, M.C. Congenital Disorders of N-Linked Glycosylation. In Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease, 5th ed.; Rosenberg, R.N., Pascual, J.M.T., Eds.; Academic Press: Boston, MA, USA, 2015; Chapter 60; pp. 673–686. [Google Scholar] [CrossRef]
- Stayton, P.S.; Ghosn, B.; Wilson, J.T. B.3—Targeting. In Biomaterials Science, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 1028–1036. [Google Scholar] [CrossRef]
- Yu, K.; Creagh, A.L.; Haynes, C.A.; Kizhakkedathu, J.N. Lectin Interactions on Surface-Grafted Glycostructures: Influence of the Spatial Distribution of Carbohydrates on the Binding Kinetics and Rupture Forces. Anal. Chem. 2013, 85, 7786–7793. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Tsuji, S.; Miura, Y. Glycopolymer Preparation via Post-Polymerization Modification Using N-Succinimidyl Monomers. Polym. J. 2019, 51, 617–625. [Google Scholar] [CrossRef]
- Kitano, H.; Takahashi, Y.; Mizukami, K.; Matsuura, K. Kinetic Study on the Binding of Lectin to Mannose Residues in a Polymer Brush. Colloids Surf. B Biointerfaces 2009, 70, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Igde, S.; Röblitz, S.; Müller, A.; Kolbe, K.; Boden, S.; Fessele, C.; Lindhorst, T.K.; Weber, M.; Hartmann, L. Linear Precision Glycomacromolecules with Varying Interligand Spacing and Linker Functionalities Binding to Concanavalin A and the Bacterial Lectin FimH. Macromol. Biosci. 2017, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.A.; Thomas, W.D.; Kiessling, L.L.; Corn, R.M. Surface Plasmon Resonance Imaging Studies of Protein-Carbohydrate Interactions. J. Am. Chem. Soc. 2003, 125, 6140–6148. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, A.; Barner, L.; Müller, A.H.E.; Granville, A.M. Surface Modification of Polymeric Microspheres Using Glycopolymers for Biorecognition. Eur. Polym. J. 2011, 47, 805–815. [Google Scholar] [CrossRef]
- Beyer, V.P.; Monaco, A.; Napier, R.; Yilmaz, G.; Becer, C.R. Bottlebrush Glycopolymers from 2-Oxazolines and Acrylamides for Targeting DC-SIGN and MBL. Biomacromolecules 2020. [Google Scholar] [CrossRef]
- Pelley, J.W. Minor Carbohydrate Pathways. In Elsevier’s Integrated Review Biochemistry, 2nd ed.; Pelley, J.W., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2012; pp. 75–79. [Google Scholar] [CrossRef]
- Petry, K.G.; Reichardt, J.K.V. The Fundamental Importance of Human Galactose Metabolism: Lessons from Genetics and Biochemistry. Trends Genet. 1998, 14, 98–102. [Google Scholar] [CrossRef]
- Itakura, Y.; Nakamura-Tsuruta, S.; Kominami, J.; Sharon, N.; Kasai, K.I.; Hirabayashi, J. Systematic Comparison of Oligosaccharide Specificity of Ricinus Communis Agglutinin I and Erythrina Lectins: A Search by Frontal Affinity Chromatography. J. Biochem. 2007, 142, 459–469. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, G.; Han, Z.; Yang, B.; Hu, Y.; Zhao, X.; Wu, J.; Lv, Y.; Chai, W. Specificities of Ricinus Communis Agglutinin 120 Interaction with Sulfated Galactose. FEBS Lett. 2011, 585, 3927–3934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Tian, X.Y.; Zong, L.P.; Zhang, Q.; Zhang, X.J.; Marks, R.; Cosnier, S.; Shan, D. Uniform and Easy-To-Prepare Glycopolymer-Brush Interface for Rapid Protein (Anti-)Adhesion Sensing. ACS Appl. Mater. Interfaces 2019, 11, 32366–32372. [Google Scholar] [CrossRef] [PubMed]
- Tsao, D.; Kim, Y.S. Separation of Cell Surface Glycoproteins from Glycolipids by Ricinus Communis Agglutinin-Sepharose. J. Biol. Chem. 1981, 256, 4947–4950. [Google Scholar] [PubMed]
- Hageman, G.S.; Johnson, L.V. Biochemical Characterization of the Major Peanut-agglutinin-binding Glycoproteins in Vertebrate Retinae. J. Comp. Neurol. 1986, 249, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Reisner, Y.; Biniaminov, M.; Rosenthal, E.; Sharon, N.; Ramot, B. Interaction of Peanut Agglutinin with Normal Human Lymphocytes and with Leukemic Cells. Proc. Natl. Acad. Sci. USA 1979, 76, 447–451. [Google Scholar] [CrossRef] [Green Version]
- Mizukami, K.; Takakura, H.; Matsunaga, T.; Kitano, H. Binding of Ricinus Communis Agglutinin to a Galactose-Carrying Polymer Brush on a Colloidal Gold Monolayer. Colloids Surf. B Biointerfaces 2008, 66, 110–118. [Google Scholar] [CrossRef]
- Arslan, H.; Pfaff, A.; Lu, Y.; Stepanek, P.; Müller, A.H.E. Stimuli-Responsive Spherical Brushes Based on D-Galactopyranose and 2-(Dimethylamino)Ethyl Methacrylate. Macromol. Biosci. 2014, 14, 81–91. [Google Scholar] [CrossRef]
- Han, L.; Tang, C.; Yin, C. Enhanced Antitumor Efficacies of Multifunctional Nanocomplexes through Knocking down the Barriers for SiRNA Delivery. Biomaterials 2015, 44, 111–121. [Google Scholar] [CrossRef]
- Zhang, C.; Qu, X.; Li, J.; Hong, H.; Li, J.; Ren, J.; Payne, G.F.; Liu, C. Biofabricated Nanoparticle Coating for Liver-Cell Targeting. Adv. Healthc. Mater. 2015, 4, 1972–1981. [Google Scholar] [CrossRef]
- Plamper, F.A.; Ruppel, M.; Schmalz, A.; Borisov, O.; Ballauff, M.; Müller, A.H.E. Tuning the Thermoresponsive Properties of Weak Poly Electrolytes: Aqueous Solutions of Star-Shaped and Linear Poly(N,N-Dimethylaminoethyl Methacrylate). Macromolecules 2007, 40, 8361–8366. [Google Scholar] [CrossRef]
- Alonso, S. Exploiting the Bioengineering Versatility of Lactobionic Acid in Targeted Nanosystems and Biomaterials. J. Control. Release 2018, 287, 216–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ulbricht, M. Cylindrical Membrane Pores with Well-Defined Grafted Linear and Comblike Glycopolymer Layers for Lectin Binding. Macromolecules 2011, 44, 1303–1310. [Google Scholar] [CrossRef]
- Li, F.; Pei, D.; Huang, Q.; Shi, T.; Zhang, G. Synthesis and Properties of Novel Biomimetic and Thermo-Responsive Dextran-Based Biohybrids. Carbohydr. Polym. 2014, 99, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, Y.; Pei, D.; Huang, Q.; Zhang, H.; Yang, Z.; Li, F.; Shi, T. Glycopolymers/PEI Complexes as Serum-Tolerant Vectors for Enhanced Gene Delivery to Hepatocytes. Carbohydr. Polym. 2019, 205, 167–175. [Google Scholar] [CrossRef]
- Taniguchi, T.; Kunisada, Y.; Shinohara, M.; Kasuya, M.; Ogawa, T.; Kohri, M.; Nakahira, T. Preparation of Glycopolymer Hollow Particles by Sacrificial Dissolution of Colloidal Templates. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 240–245. [Google Scholar] [CrossRef]
- Cairo, C.W.; Gestwicki, J.E.; Kanai, M.; Kiessling, L.L. Control of Multivalent Interactions by Binding Epitope Density. J. Am. Chem. Soc. 2002, 124, 1615–1619. [Google Scholar] [CrossRef]
- Suriano, F.; Coulembier, O.; Dubois, P. Synthesis of Brush-like Copolymers Using Carbohydrates as Initiators: Benefits of Organic Catalysts for the ROP of Lactones. React. Funct. Polym. 2010, 70, 747–754. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, L.; Xu, F.; Xin, F.; Ma, J.; Jiang, M.; Fang, Y. Chemoenzymatic Synthesis of Branched Glycopolymer Brushes as the Artificial Glycocalyx for Lectin Specific Binding. Langmuir 2019, 35, 4445–4452. [Google Scholar] [CrossRef]
- Kumar, R.; Kopyeva, I.; Cheng, K.; Liu, K.; Lahann, J. Examining Nanoparticle Adsorption on Electrostatically “Patchy” Glycopolymer Brushes Using Real-Time ζ-Potential Measurements. Langmuir 2017, 33, 6322–6332. [Google Scholar] [CrossRef]
- Engelking, L.R. Carbohydrate Structure. In Textbook of Veterinary Physiological Chemistry, 3rd ed.; Engelking, L., Ed.; Academic Press: Boston, MA, USA, 2015; Chapter 18; pp. 118–123. [Google Scholar] [CrossRef]
- Bhagavan, N.V. Simple Carbohydrates. In Medical Biochemistry, 4th ed.; Bhagavan, N., Ed.; Academic Press: San Diego, CA, USA, 2002; Chapter 9; pp. 133–151. [Google Scholar] [CrossRef]
- McRorie, J.W. The Physics of Fiber in the Gastrointestinal Tract. In Dietary Interventions in Gastrointestinal Diseases; Watson, R.R., Preedy, V.R., Eds.; Elsevier Inc.: London, UK, 2019; pp. 19–32. [Google Scholar] [CrossRef]





| Architecture | Polymer | Ka (×10−3 Ms−1) | |
|---|---|---|---|
| MBL | DC-SIGN | ||
| Linear | PNIPAAm10-b-PManAA10 | 0.575 | 1.53 |
| PNIPAAm50-b-PManAA10 | 0.478 | 1.61 | |
| PNIPAAm10-r-PManAA10 | 0.364 | 1.31 | |
| PNIPAAm50-b-PManAA10 | 0.468 | 1.58 | |
| Homopolymer brush | PNIPAAm10 | - | - |
| PNIPAAm50 | - | - | |
| PManAA10 | 1.72 | 4.66 | |
| PManAA50 | 5.20 | 27.3 | |
| Random copolymer brush | PNIPAAm10-r-PManAA10 | 1.30 | 5.52 |
| PNIPAAm50-r-PManAA10 | 1.21 | 3.38 | |
| Block copolymer brush | PNIPAAm10-b-PManAA1 | 0.48 | 1.73 |
| PNIPAAm10-b-PManAA10 | 1.66 | 4.75 | |
| PNIPAAm50-b-PManAA1 | 0.43 | 1.62 | |
| PNIPAAm50-b-PManAA10 | 2.12 | 3.69 | |
| PManAA10-b- PNIPAAm10 | 1.61 | 4.16 | |
| PManAA10-b- PNIPAAm50 | 1.19 | 3.59 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, J.P.M.; Mendonça, P.V.; Coelho, J.F.J.; Matyjaszewski, K.; Serra, A.C. Glycopolymer Brushes by Reversible Deactivation Radical Polymerization: Preparation, Applications, and Future Challenges. Polymers 2020, 12, 1268. https://doi.org/10.3390/polym12061268
Ribeiro JPM, Mendonça PV, Coelho JFJ, Matyjaszewski K, Serra AC. Glycopolymer Brushes by Reversible Deactivation Radical Polymerization: Preparation, Applications, and Future Challenges. Polymers. 2020; 12(6):1268. https://doi.org/10.3390/polym12061268
Chicago/Turabian StyleRibeiro, Jessica P. M., Patrícia V. Mendonça, Jorge F. J. Coelho, Krzysztof Matyjaszewski, and Arménio C. Serra. 2020. "Glycopolymer Brushes by Reversible Deactivation Radical Polymerization: Preparation, Applications, and Future Challenges" Polymers 12, no. 6: 1268. https://doi.org/10.3390/polym12061268
APA StyleRibeiro, J. P. M., Mendonça, P. V., Coelho, J. F. J., Matyjaszewski, K., & Serra, A. C. (2020). Glycopolymer Brushes by Reversible Deactivation Radical Polymerization: Preparation, Applications, and Future Challenges. Polymers, 12(6), 1268. https://doi.org/10.3390/polym12061268







