Biofunctional Polymer Coated Au Nanoparticles Prepared via RAFT-Assisted Encapsulating Emulsion Polymerization and Click Chemistry
Abstract
:1. Introduction
2. Experimental
2.1. Reagents
2.2. Synthesis
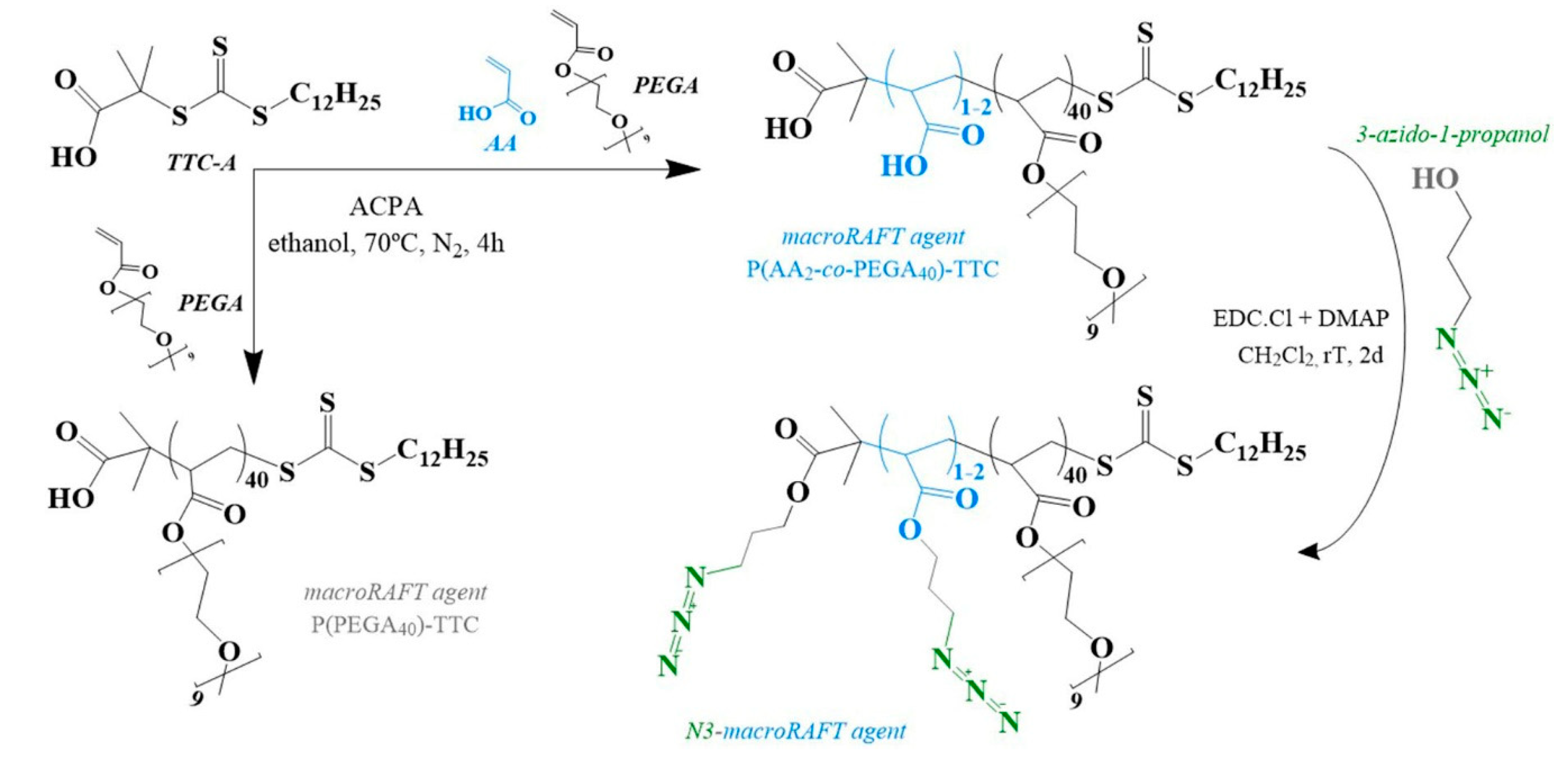
2.2.1. Synthesis of MacroRAFT Agents
2.2.2. Preparation of N3-MacroRAFT Agent
2.2.3. Preparation of Au NPs by the Citrate Method (Au-Cit NPs)
2.2.4. Copolymerization of MacroRAFT Agent via RAFT Emulsion Polymerization
2.2.5. Preparation of N3-Copolymer@Au NPs via REEP
2.2.6. Preparation of Copolymer@Au NPs via REEP
2.2.7. Synthesis of Alkynated Biotin
2.2.8. Preparation of Biotin-Copolymer@Au NPs via Click Reaction
2.2.9. Biosensing Tests
2.2.10. Preparation of Langmuir Monolayers to Study Copolymer–Biotin–Avidin Interactions
3. Characterization
4. Results and Discussion
4.1. Synthesis of MacroRAFT Agents
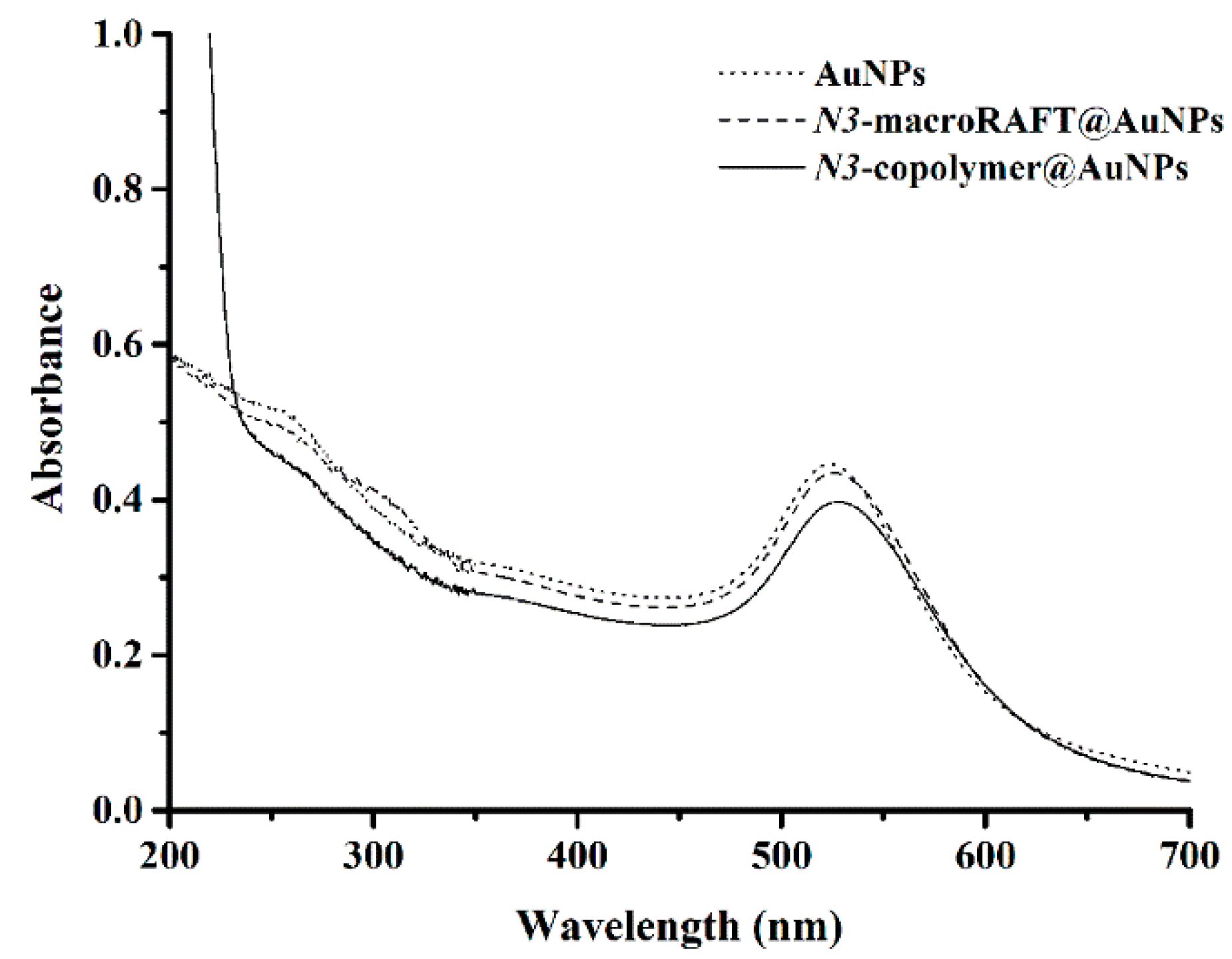
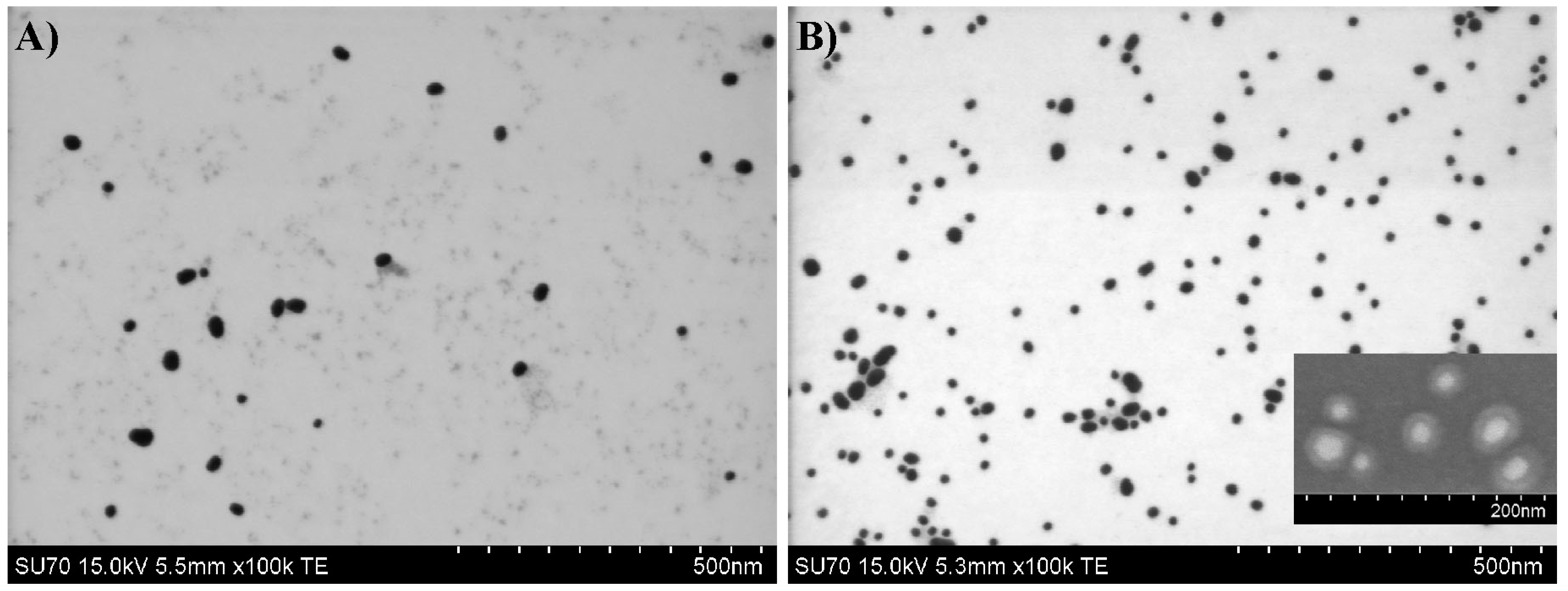
4.2. Preparation of Copolymer@Au NPs
4.3. Preparation of Copolymer@Au NPs at 44 °C
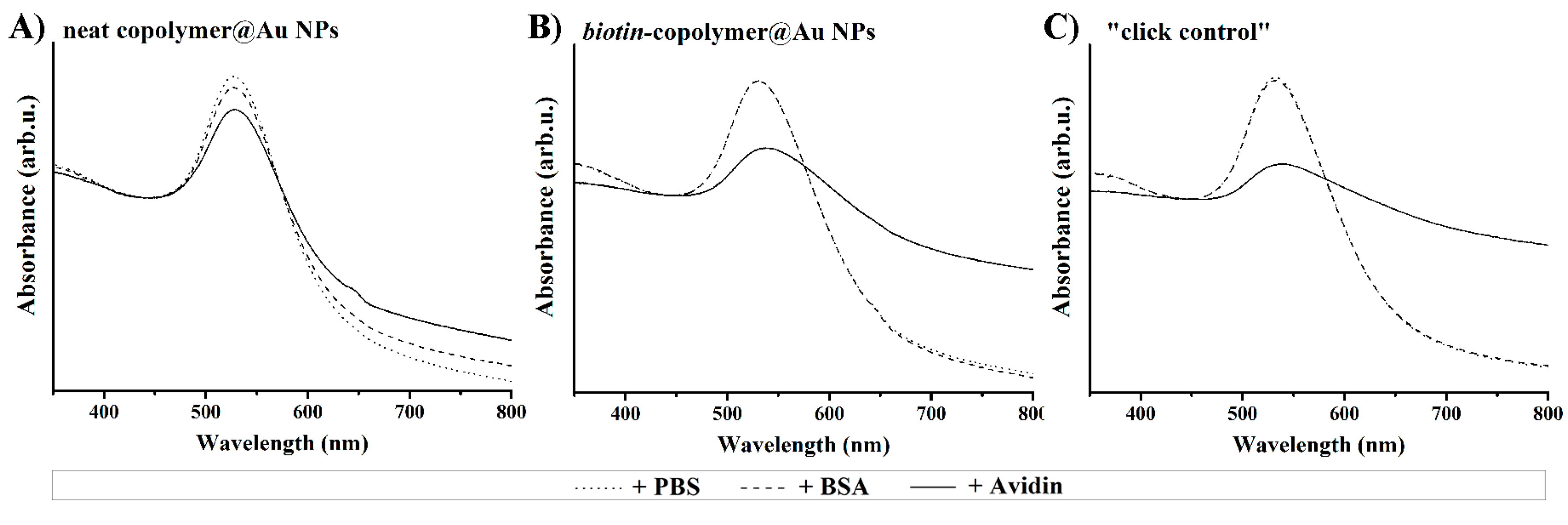
4.4. Biosensing Response
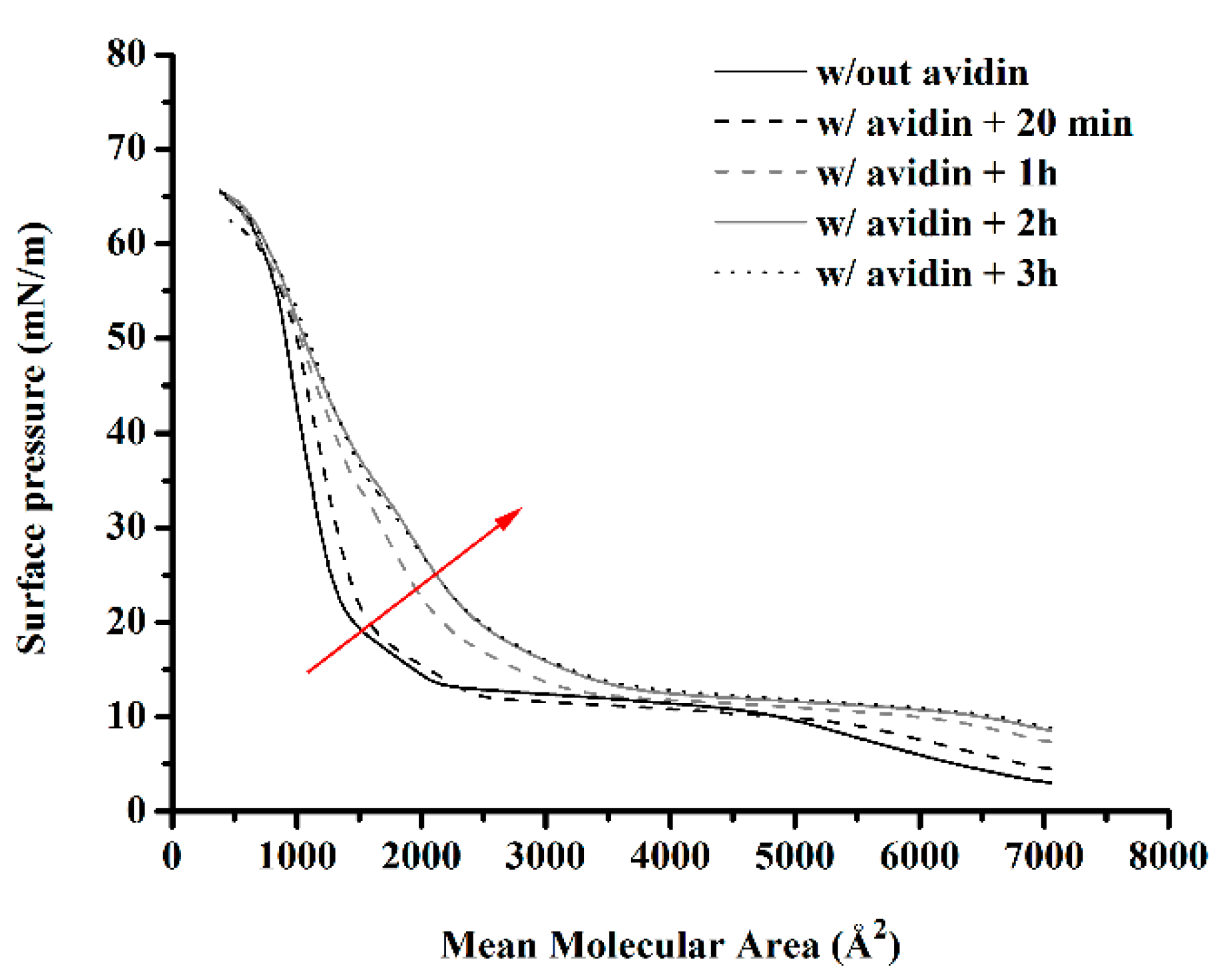
4.5. Langmuir–Blodgett Studies at the Air/Water Interface
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- York, A.; Kirkland, S.; McCormick, C. Advances in the synthesis of amphiphilic block copolymers via RAFT polymerization: Stimuli-responsive drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1018–1036. [Google Scholar] [CrossRef]
- Lowe, A.B.; McCormick, C.L. Reversible addition–fragmentation chain transfer (RAFT) radical polymerization and the synthesis of water-soluble (co)polymers under homogeneous conditions in organic and aqueous media. Prog. Polym. Sci. 2007, 32, 283–351. [Google Scholar] [CrossRef]
- Zetterlund, P.B.; Thickett, S.C.; Perrier, S.; Bourgeat-Lami, E.; Lansalot, M. Controlled/Living Radical Polymerization in Dispersed Systems: An Update. Chem. Rev. 2015, 115, 9745–9800. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-H.; Yao, H.; Ma, J. Recent advances in RAFT-mediated surfactant-free emulsion polymerization. Polym. Chem. 2018, 9, 2532–2561. [Google Scholar] [CrossRef]
- Nguyen, D.; Zondanos, H.S.; Farrugia, J.M.; Serelis, A.K.; Such, C.H.; Hawkett, B.S. Pigment Encapsulation by Emulsion Polymerization Using Macro-RAFT Copolymers. Langmuir 2008, 24, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Zeuna, J.N.; Claverie, J.P. A versatile encapsulation method of noncovalently modified carbon nanotubes by RAFT polymerization. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4403–4407. [Google Scholar] [CrossRef]
- Nguyen, D.; Such, C.H.; Hawkett, B.S. Polymer coating of carboxylic acid functionalized multiwalled carbon nanotubes via reversible addition-fragmentation chain transfer mediated emulsion polymerization. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 250–257. [Google Scholar] [CrossRef]
- Huynh, V.T.; Nguyen, D.; Such, C.H.; Hawkett, B.S. Polymer coating of graphene oxide via reversible addition-fragmentation chain transfer mediated emulsion polymerization. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1413–1421. [Google Scholar] [CrossRef]
- Das, P.; Claverie, J.P. Synthesis of single-core and multiple-core core-shell nanoparticles by RAFT emulsion polymerization: Lead sulfide-copolymer nanocomposites. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2802–2808. [Google Scholar] [CrossRef]
- Garnier, J.; Warnant, J.; Lacroix-Desmazes, P.; Dufils, P.-E.; Vinas, J.; van Herk, A.M. Sulfonated macro-RAFT agents for the surfactant-free synthesis of cerium oxide-based hybrid latexes. J. Colloid Interface Sci. 2013, 407, 273–281. [Google Scholar] [CrossRef]
- Zgheib, N.; Putaux, J.-L.; Thill, A.; Bourgeat-Lami, E.; D’Agosto, F.; Lansalot, M. Cerium oxide encapsulation by emulsion polymerization using hydrophilic macroRAFT agents. Polym. Chem. 2013, 4, 607–614. [Google Scholar] [CrossRef]
- Bourgeat-Lami, E.; França, A.J.P.G.; Chaparro, T.C.; Silva, R.D.; Dugas, P.-Y.; Alves, G.M.; Santos, A. Synthesis of Polymer/Silica Hybrid Latexes by Surfactant-Free RAFT-Mediated Emulsion Polymerization. Macromolecules 2016, 49, 4431–4440. [Google Scholar] [CrossRef]
- Zou, H.; Melro, L.; Chaparro, T.D.C.; Filho, I.R.D.S.; Ananias, D.; Bourgeat-Lami, E.; Santos, A.; Barros-Timmons, A. Adsorption study of a macro-RAFT agent onto SiO2-coated Gd2O3: Eu3+ nanorods: Requirements and limitations. Appl. Surf. Sci. 2017, 394, 519–527. [Google Scholar] [CrossRef]
- Silva, R.; Monteiro, I.S.; Chaparro, T.D.C.; Hardt, R.S.; Giudicci, R.; Barros-Timmons, A.; Bourgeat-Lami, E.; Santos, A. Investigation of the Adsorption of Amphipathic macroRAFT Agents onto Montmorillonite Clay. Langmuir 2017, 33, 9598–9608. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.O.; Barros-Timmons, A.; Trindade, T. A Comparative Study of Chemical Routes for Coating Gold Nanoparticles via Controlled RAFT Emulsion Polymerization. Part. Part. Syst. Charact. 2017, 34, 1600202. [Google Scholar] [CrossRef]
- Pereira, S.O.; Trindade, T.; Barros-Timmons, A. Impact of critical micelle concentration of macroRAFT agents on the encapsulation of colloidal Au nanoparticles. J. Colloid Interface Sci. 2019, 545, 251–258. [Google Scholar] [CrossRef]
- Pereira, S.O.; Barros-Timmons, A.; Trindade, T. Polymer@gold Nanoparticles Prepared via RAFT Polymerization for Opto-Biodetection. Polymers 2018, 10, 189. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Glebe, U.; Böker, A. Surface-initiated controlled radical polymerizations from silica nanoparticles, gold nanocrystals, and bionanoparticles. Polym. Chem. 2015, 6, 5143–5184. [Google Scholar] [CrossRef] [Green Version]
- Santos, A.C.; Alves, S.; Godinho, M.H.; Baleizão, C.; Farinha, J.P.S. Temperature-responsive fibres of cellulose-based copolymers. Polym. Chem. 2018, 9, 3615–3623. [Google Scholar] [CrossRef]
- Golas, P.L.; Matyjaszewski, K. Marrying click chemistry with polymerization: Expanding the scope of polymeric materials. Chem. Soc. Rev. 2010, 39, 1338–1354. [Google Scholar] [CrossRef]
- Campos, J.; Ferraria, A.M.; Rego, A.M.B.D.; Ribeiro, M.D.R.; Barros-Timmons, A. Studies on PLA grafting onto graphene oxide and its effect on the ensuing composite films. Mater. Chem. Phys. 2015, 166, 122–132. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G.; Hanson, J. Determination of Size and Concentration of Gold Nanoparticles from UV−Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Siegwart, D.J.; Oh, J.K.; Gao, H.; Bencherif, S.A.; Perineau, F.; Bohaty, A.K.; Hollinger, J.O.; Matyjaszewski, K. Biotin-, Pyrene-, and GRGDS-Functionalized Polymers and Nanogels via ATRP and End Group Modification. Macromol. Chem. Phys. 2008, 209, 2179–2193. [Google Scholar] [CrossRef]
- Su, J.; Qian, C.; Luo, N.; Xiang, X.; Xu, Y.; Chen, X. Experimental Measurement and Modeling of the Solubility of Biotin in Six Pure Solvents at Temperatures from 298.15 K to 333.85 K. J. Chem. Eng. Data 2014, 59, 3894–3899. [Google Scholar] [CrossRef]
- Feller, L.M.; Cerritelli, S.; Textor, M.; Hubbell, J.A.; Tosatti, S.G.P. Influence of Poly(propylene sulfide-block-ethylene glycol) Di- and Triblock Copolymer Architecture on the Formation of Molecular Adlayers on Gold Surfaces and Their Effect on Protein Resistance: A Candidate for Surface Modification in Biosensor Research. Macromolecules 2005, 38, 10503–10510. [Google Scholar] [CrossRef]
- Vaisocherová, H.; Brynda, E.; Homola, J. Functionalizable low-fouling coatings for label-free biosensing in complex biological media: Advances and applications. Anal. Bioanal. Chem. 2015, 407, 3927–3953. [Google Scholar] [CrossRef]
- Lowe, S.; Connal, L.A.; O’Brien-Simpson, N.M. Antibiofouling polymer interfaces: Poly(ethylene glycol) and other promising candidates. Polym. Chem. 2015, 6, 198–212. [Google Scholar] [CrossRef] [Green Version]
- Gondi, S.R.; Vogt, A.P.; Sumerlin, B.S. Versatile Pathway to Functional Telechelics via RAFT Polymerization and Click Chemistry. Macromolecules 2007, 40, 474–481. [Google Scholar] [CrossRef]
- Li, Y.; Benicewicz, B.C. Functionalization of Silica Nanoparticles via the Combination of Surface-Initiated RAFT Polymerization and Click Reactions. Macromolecules 2008, 41, 7986–7992. [Google Scholar] [CrossRef]
- Ladmiral, V.; Legge, T.M.; Zhao, Y.; Perrier, S. “Click” Chemistry and Radical Polymerization: Potential Loss of Orthogonality. Macromolecules 2008, 41, 6728–6732. [Google Scholar] [CrossRef]
- Gody, G.; Maschmeyer, T.; Zetterlund, P.B.; Perrier, S. Exploitation of the Degenerative Transfer Mechanism in RAFT Polymerization for Synthesis of Polymer of High Livingness at Full Monomer Conversion. Macromolecules 2014, 47, 639–649. [Google Scholar] [CrossRef]
- Gody, G.; Maschmeyer, T.; Zetterlund, P.B.; Perrier, S. Pushing the Limit of the RAFT Process: Multiblock Copolymers by One-Pot Rapid Multiple Chain Extensions at Full Monomer Conversion. Macromolecules 2014, 47, 3451–3460. [Google Scholar] [CrossRef]
- Rostovtsev, V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Patra, A.; Ding, T.; Engudar, G.; Wang, Y.; Dykas, M.M.; Liedberg, B.; Kah, J.C.Y.; Venkatesan, T.; Drum, C.L. Component-Specific Analysis of Plasma Protein Corona Formation on Gold Nanoparticles Using Multiplexed Surface Plasmon Resonance. Small 2016, 12, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Liu, R.; Deng, Z.; Ge, G.; Liu, Y.; Xie, L. Quantitative study of protein coronas on gold nanoparticles with different surface modifications. Nano Res. 2014, 7, 345–352. [Google Scholar] [CrossRef]
- Kato, N.; Caruso, F. Homogeneous, Competitive Fluorescence Quenching Immunoassay Based on Gold Nanoparticle/Polyelectrolyte Coated Latex Particles. J. Phys. Chem. B 2005, 109, 19604–19612. [Google Scholar] [CrossRef]
- McClellan, S.J.; Franses, E.I. Effect of concentration and denaturation on adsorption and surface tension of bovine serum albumin. Colloids Surfaces B Biointerfaces 2003, 28, 63–75. [Google Scholar] [CrossRef]
- Nobre, T.M.; Pavinatto, F.J.; Caseli, L.; Barros-Timmons, A.; Dynarowicz-Łątka, P.; Oliveira, O. Interactions of bioactive molecules & nanomaterials with Langmuir monolayers as cell membrane models. Thin Solid Films 2015, 593, 158–188. [Google Scholar] [CrossRef]
- Koda, Y.; Takahashi, D.; Sasaki, Y.; Akiyoshi, K. Amphiphilic Poly[poly(ethylene glycol) methacrylate]s with OH Groups in the PEG Side Chains for Controlling Solution/Rheological Properties and toward Bioapplication. ACS Appl. Bio Mater. 2019, 2, 1920–1930. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Xiang, X.; Heiden, P.A. Tuning thermoresponsive behavior of diblock copolymers and their gold core hybrids. Part 2. How properties change depending on block attachment to gold nanoparticles. J. Colloid Interface Sci. 2013, 396, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Trindade, T.; Barros-Timmons, A. Biotinylation of optically responsive gold/polyelectrolyte nanostructures. Gold Bull. 2015, 48, 3–11. [Google Scholar] [CrossRef]
- Stenzel, M.H. Hairy Core-Shell Nanoparticles via RAFT: Where are the Opportunities and Where are the Problems and Challenges? Macromol. Rapid Commun. 2009, 30, 1603–1624. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zheng, H.; Bai, R. A Facile Strategy for the Preparation of Azide Polymers via Room Temperature RAFT Polymerization by Redox Initiation. Macromol. Rapid Commun. 2009, 30, 442–447. [Google Scholar] [CrossRef]
- Gharatape, A.; Salehi, R. Recent progress in theranostic applications of hybrid gold nanoparticles. Eur. J. Med. Chem. 2017, 138, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent. Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef]




| NMR | GPC-SEC | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DP | Đ | DP | |||||||
| P(PEGA)40-TTC | 20,104 | 41 | 12,177 | 15,362 | 1.26 | 25 | |||
| Theoretical a | Gravimetry b | GPC-SEC | |||||||
| DPMMA:BA | % | DPMMA:BA | Đ | DPMMA:BA | |||||
| 70 °C controlled addition | 35,912 | 181 | 35 | 23,823 | 63 | 15,119 | 25,136 | 1.66 | 29 |
| 44 °C controlled addition | 35,079 | 173 | 24 | 21,577 | 41 | 12,246 | 15,532 | 1.27 | 1 |
| Au NPs | N3-MacroRAFT@Au NPs | N3-Copolymer@Au NPs | |
|---|---|---|---|
| λLSPR (nm) | 523 | 526 | 529 |
| z-average (nm) | 16.3 | 26.6 | 27.9 |
| PdI | 0.573 | 0.561 | 0.531 |
| ζ (mV) | −49.3 ± 1.8 | −25.1 ± 1.9 | −26.4 ± 1.2 |
| pH | 5.6 | 6.9 | 5.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, S.O.; Trindade, T.; Barros-Timmons, A. Biofunctional Polymer Coated Au Nanoparticles Prepared via RAFT-Assisted Encapsulating Emulsion Polymerization and Click Chemistry. Polymers 2020, 12, 1442. https://doi.org/10.3390/polym12071442
Pereira SO, Trindade T, Barros-Timmons A. Biofunctional Polymer Coated Au Nanoparticles Prepared via RAFT-Assisted Encapsulating Emulsion Polymerization and Click Chemistry. Polymers. 2020; 12(7):1442. https://doi.org/10.3390/polym12071442
Chicago/Turabian StylePereira, Sónia O., Tito Trindade, and Ana Barros-Timmons. 2020. "Biofunctional Polymer Coated Au Nanoparticles Prepared via RAFT-Assisted Encapsulating Emulsion Polymerization and Click Chemistry" Polymers 12, no. 7: 1442. https://doi.org/10.3390/polym12071442
APA StylePereira, S. O., Trindade, T., & Barros-Timmons, A. (2020). Biofunctional Polymer Coated Au Nanoparticles Prepared via RAFT-Assisted Encapsulating Emulsion Polymerization and Click Chemistry. Polymers, 12(7), 1442. https://doi.org/10.3390/polym12071442








