Probing Interactions in Combined Hydroxide Base Solvents for Improving Dissolution of Cellulose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Dissolution of Cellulose
2.3. Maximum Dissolution Limit of Cellulose
2.4. Rheology
2.5. Solvatochromic Solvent Parameters
2.6. Differential Scanning Calorimetry (DSC)
2.7. ATR FTIR
3. Results and Discussion
3.1. Dissolution Capacity
3.2. Flow Behaviour of the Solutions
3.3. Solvatochromic Parameters
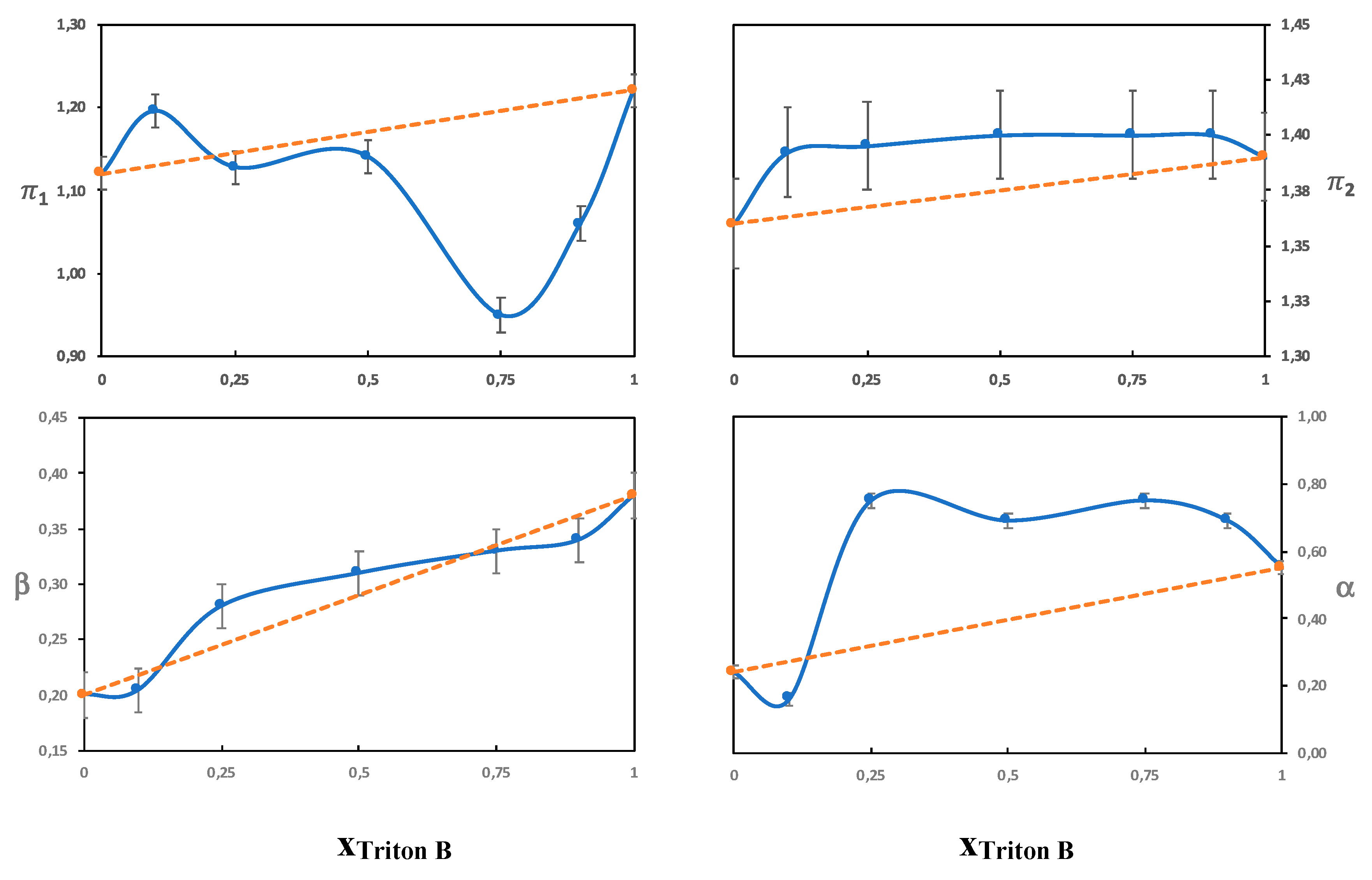
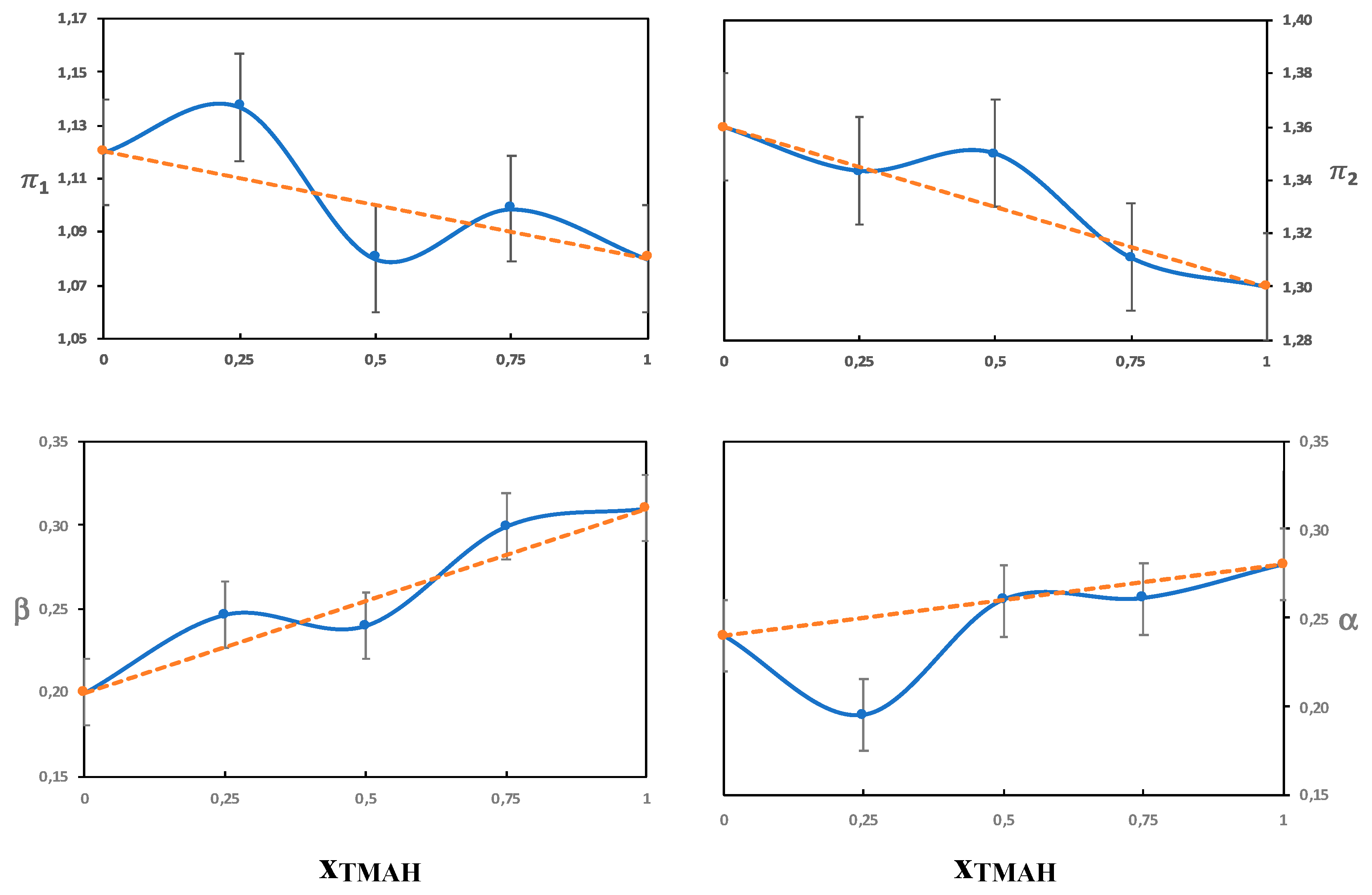
3.3.1. The π Parameter
Solutions without Urea
Solutions with Urea
3.3.2. The Beta Parameter
3.3.3. The α Parameter
Urea
On the Combinations
3.4. DSC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Budtova, T.; Navard, P. Cellulose in NaOH–water based solvents: A review. Cellulose 2016, 23, 5–55. [Google Scholar]
- Roy, C.; Budtova, T.; Navard, P. Rheological Properties and Gelation of Aqueous Cellulose-NaOH Solutions. Biomacromolecules 2003, 4, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Hagman, J.; Gentile, L.; Jessen, C.M.; Behrens, M.; Bergqvist, K.-E.; Olsson, U. On the dissolution state of cellulose in cold alkali solutions. Cellulose 2017, 24, 2003–2015. [Google Scholar] [CrossRef] [Green Version]
- Martin-Bertelsen, B.; Andersson, E.; Köhnke, T.; Hedlund, A.; Stigsson, L.; Olsson, U. Revisiting the Dissolution of Cellulose in NaOH as ‘Seen’ by X-rays. Polymers 2020, 12, 342. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Zhang, L. Rapid Dissolution of Cellulose in LiOH/Urea and NaOH/Urea Aqueous Solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef]
- Wang, S.; Sun, P.; Liu, M.; Lu, A.; Zhang, L. Weak interactions and their impact on cellulose dissolution in an alkali/urea aqueous system. Phys. Chem. Chem. Phys. 2017, 19, 17909–17917. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Heiskanen, J.P. Room-temperature dissolution and chemical modification of cellulose in aqueous tetraethylammonium hydroxide-carbamide solutions. Cellulose 2020, 27, 1933–1950. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Meng, F.; Cui, Y.; Jiang, M.; Zhou, Z. Room temperature dissolution of cellulose in tetra-butylammonium hydroxide aqueous solvent through adjustment of solvent amphiphilicity. Cellulose 2017, 24, 49–59. [Google Scholar] [CrossRef]
- Lilienfeld, L. Cellulose Solutions and Process for Their Production. U.S. Patent 17,714,62A, 29 July 1930. [Google Scholar]
- Gubitosi, M.; Nosrati, P.; Koder, H.M.; Kuczera, S.; Behrens, M.A.; Johansson, E.G.; Olsson, U. Stable, metastable and unstable cellulose solutions. R. Soc. Open Sci. 2017, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.; Wang, C.; Huang, F.; Jia, H.; Wei, P. Wheat straw cellulose dissolution and isolation by tetra-n-butylammonium hydroxide. Carbohydr. Polym. 2013, 94, 38–45. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, C.; Wang, F.; Jia, H.; Wei, P.; Zhao, Y. Application of tetra-n-methylammonium hydroxide on cellulose dissolution and isolation from sugarcane bagasse. Carbohydr. Polym. 2016, 136, 979–987. [Google Scholar] [CrossRef]
- Kostag, M.; Jedvert, K.; Achtel, C.; Heinze, T.; El Seoud, O.A. Recent Advances in Solvents for the Dissolution; Shaping and Derivatization of Cellulose: Quaternary Ammonium Electrolytes and their Solutions in Water and Molecular Solvents. Molecules 2018, 23, 511. [Google Scholar] [CrossRef] [Green Version]
- Blokzijl, W.; Engberts, J.B.F.N. Hydrophobic Effects. Opinions and Facts. Angew. Chem. Int. Ed. Engl. 1993, 32, 1545–1579. [Google Scholar] [CrossRef]
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Lindman, B.; Karlström, G.; Stigsson, L. On the mechanism of dissolution of cellulose. J. Mol. Liq. 2010, 156, 76–81. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Chen, P.; Zhang, L.; Lu, A. Cationic hydrophobicity promotes dissolution of cellulose in aqueous basic solution by freezing–thawing. Phys. Chem. Chem. Phys. 2018, 20, 14223–14233. [Google Scholar] [CrossRef] [PubMed]
- Swensson, B.; Larsson, A.; Hasani, M. Dissolution of cellulose using a combination of hydroxide bases in aqueous solution. Cellulose 2020, 27, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Powers, D.H.; Bock, L.H. Cellulose Solutions. U.S. Patent 2009015A, 23 July 1935. [Google Scholar]
- Bialik, E.; Stenqvist, B.; Fang, Y.; Östlund, Å.; Furó, I.; Lindman, B.; Bernin, D. Ionization of Cellobiose in Aqueous Alkali and the Mechanism of Cellulose Dissolution. J. Phys. Chem. Lett. 2016, 7, 5044–5048. [Google Scholar] [CrossRef] [PubMed]
- Kamlet, M.J.; Abboud, J.L.; Taft, R.W. The solvatochromic comparison method. 6. The .pi.* scale of solvent polarities. J. Am. Chem. Soc. 1977, 99, 6027–6038. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Taft, R.W. The solvatochromic comparison method. I. The .beta.-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Taft, R.W.; Kamlet, M.J. The solvatochromic comparison method. 2. The .alpha.-scale of solvent hydrogen-bond donor (HBD) acidities. J. Am. Chem. Soc. 1976, 98, 2886–2894. [Google Scholar] [CrossRef]
- Pereira, A.; Duarte, H.; Nosrati, P.; Gubitosi, M.; Gentile, L.; Romano, A.; Olsson, U. Cellulose gelation in NaOH solutions is due to cellulose crystallization. Cellulose 2018, 25, 3205–3210. [Google Scholar] [CrossRef]
- Ab Rani, M.A.; Brant, A.; Crowhurst, L.; Dolan, A.; Lui, M.; Hassan, N.H.; Schrems, M. Understanding the polarity of ionic liquids. Phys. Chem. Chem. Phys. 2011, 13, 16831–16840. [Google Scholar] [CrossRef]
- Zhong, C.; Cheng, F.; Zhu, Y.; Gao, Z.; Jia, H.; Wei, P. Dissolution mechanism of cellulose in quaternary ammonium hydroxide: Revisiting through molecular interactions. Carbohydr. Polym. 2017, 174, 400–408. [Google Scholar] [CrossRef]
- Lee, C.; McCammon, J.A.; Rossky, P.J. The structure of liquid water at an extended hydrophobic surface. J. Chem. Phys. 1984, 80, 4448–4455. [Google Scholar] [CrossRef]
- Schmidt, D.A.; Miki, K. Structural Correlations in Liquid Water: A New Interpretation of IR Spectroscopy. J. Phys. Chem. A 2007, 111, 10119–10122. [Google Scholar] [CrossRef]
- Fischer, W.B.; Fedorowicz, A.; Koll, A. Structured water around ions—FTIR difference spectroscopy and quantum-mechanical calculations. Phys. Chem. Chem. Phys. 2001, 3, 4228–4234. [Google Scholar] [CrossRef]


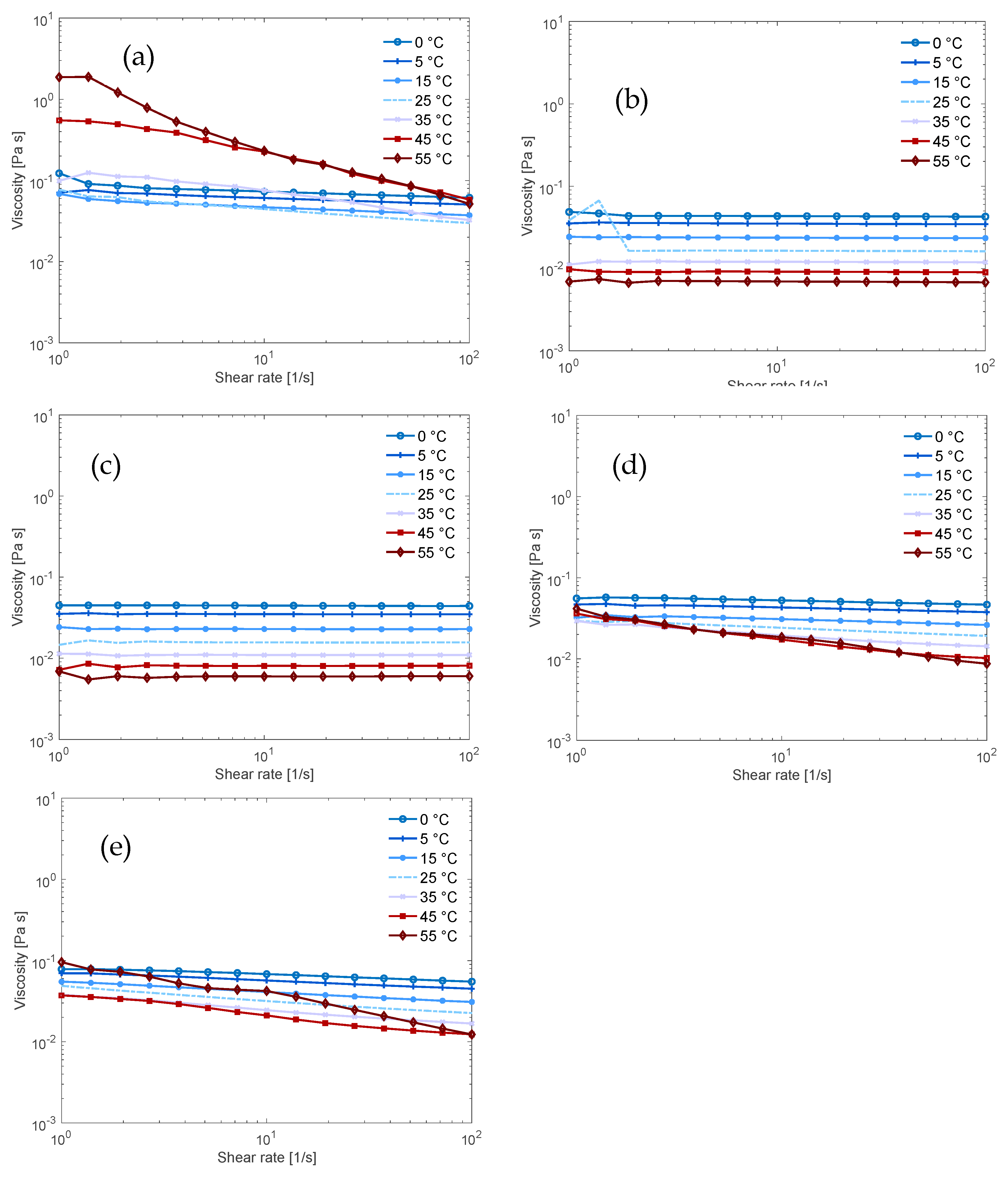
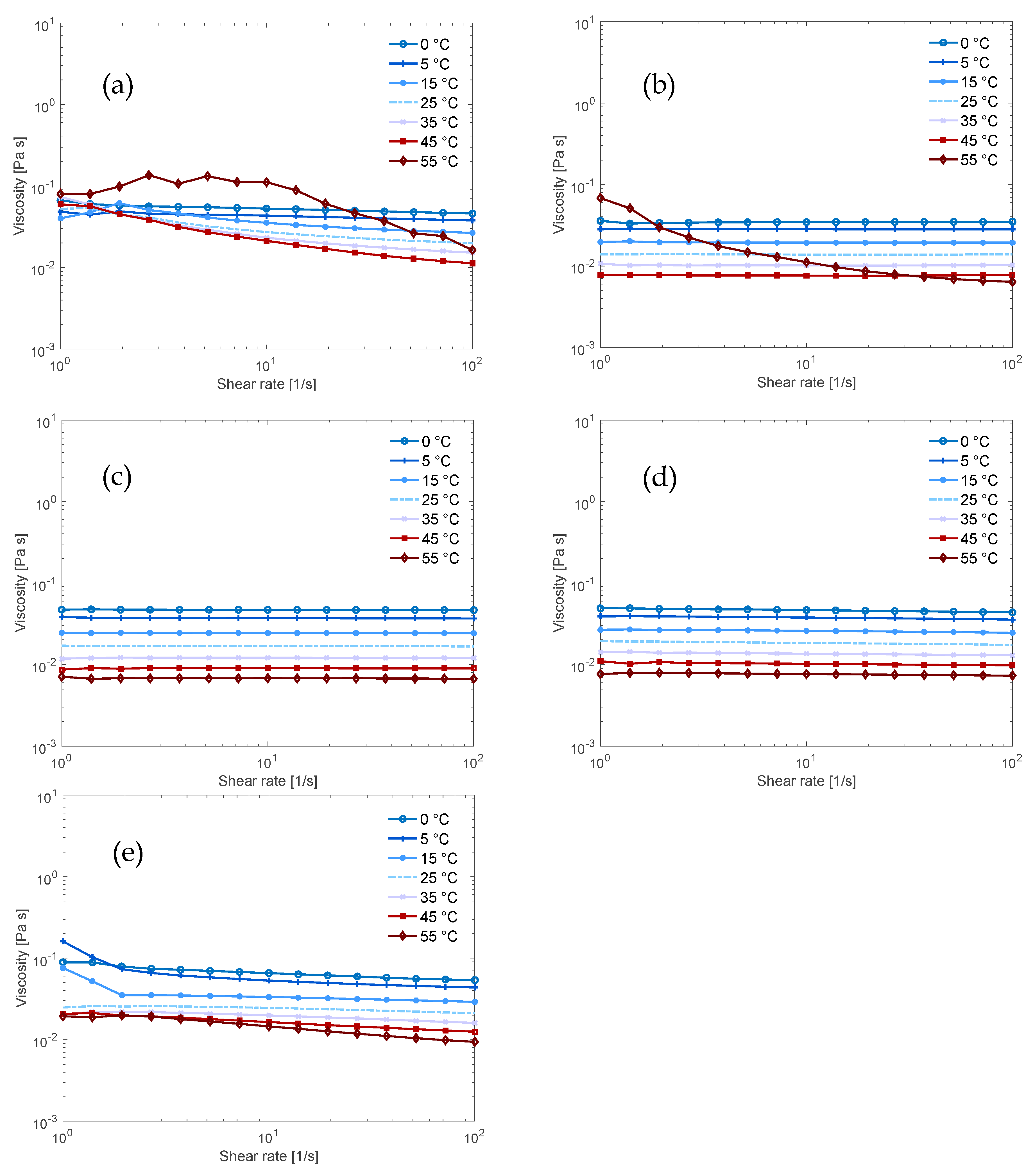

| Solvent (aq) | Dissolution Capacity [wt.%] | Dissolution Capacity [mol AGU/L] | Mol Base per mol AGU at the Dissolution Limit |
|---|---|---|---|
| 4 mol% NaOH | 2.1 ± 0.4 | 0.14 ± 0.03 | 15.9 |
| 4 mol% TMAH | 2.7 ± 0.1 | 0.16 ± 0.01 | 11.2 |
| 4 mol% NaOH + Triton B (50/50) | 2.9 ± 0.2 | 0.19 ± 0.01 | 9.9 |
| 4 mol% NaOH + urea | 3.4 ± 0.1 | 0.24 ± 0.01 | 8.4 |
| 4 mol% NaOH + TMAH (50/50) | 4.0 ± 0.3 | 0.26 ± 0.02 | 7.8 |
| 4 mol% NaOH + Triton B (50/50) + urea | 4.0 ± 0.3 | 0.26 ± 0.02 | 6.5 |
| 4 mol% TMAH + urea | 4.9 ± 0.1 | 0.32 ± 0.00 | 5.3 |
| 4 mol% NaOH + TMAH (50/50) + urea | 6.0 ± 0.3 | 0.41 ± 0.02 | 4.4 |
| 4 mol% Triton B | 6.5 ± 0.2 | 0.42 ± 0.01 | 3.9 |
| 4 mol% Triton B + urea | 6.9 ± 0.2 | 0.47 ± 0.01 | 3.3 |
| 4 mol% (aq) Solvent at RT | Δπ1,urea | Δπ2,urea | ||
|---|---|---|---|---|
| H2O | 1.06 | 1.31 | 0.10 | 0.07 |
| NaOH | 1.12 | 1.36 | 0.11 | 0.05 |
| TMAH | 1.08 | 1.30 | 0.07 | 0.03 |
| Triton B | 1.22 | 1.39 | 0.02 | 0.01 |
| 50/50 NaOH/TMAH | 1.08 | 1.35 | 0.11 | 0.02 |
| 50/50 NaOH/Triton B | 1.14 | 1.40 | 0.04 | 0.01 |
| 4 mol% (aq) Solvent at RT | β ± 0.02 | Δβurea |
|---|---|---|
| H2O | 0.13 | 0.04 |
| NaOH | 0.20 | 0.01 |
| TMAH | 0.31 | −0.02 |
| Triton B | 0.38 | −0.01 |
| 50/50 NaOH/TMAH | 0.24 | 0.00 |
| 50/50 NaOH/Triton B | 0.31 | −0.01 |
| 4 mol% (aq) Solvent | α ± 0.02 | Δαurea |
|---|---|---|
| H2O | 1.05 | −0.10 |
| NaOH | 0.24 | −0.11 |
| TMAH | 0.28 | −0.08 |
| Triton B | 0.55 | 0.03 |
| 5050 NaOH TMAH | 0.26 | −0.05 |
| 5050 NaOH Triton B | 0.69 | −0.04 |
| Solvent at RT | β ± 0.02 | α ± 0.02 | ||
|---|---|---|---|---|
| 4 mol% NaOH | 1.11 | 1.36 | 0.20 | 0.24 * |
| 8 mol% NaOH | 1.22 | 1.29 | 0.39 | 0.04 |
| 4 mol% TMAH | 1.06 | 1.30 | 0.31 | 0.28 * |
| 6.2 mol% TMAH | 1.06 | 1.29 | 0.40 | 0.17 |
| 4 mol% Triton B | 1.20 | 1.39 | 0.38 | 0.55 * |
| 6.7 mol% Triton B | 1.24 | 1.37 | 0.47 | 0.57 |
| 4 mol% 50/50 NaOH/TMAH | 1.15 | 1.35 | 0.24 | 0.26 * |
| 8 mol% 50/50 NaOH/TMAH | 1.09 | 1.30 | 0.40 | 0.09 |
| 4 mol% 50/50 NaOH/Triton B | 1.16 | 1.40 | 0.31 | 0.69 * |
| 8 mol% 50/50 NaOH/Triton B | 1.23 | 1.37 | 0.47 | 0.61 |
| Solvent | Tm,1 °C | ΔH1 J/g Sample | Tm,2 °C | ΔH2 J/g Sample | Tm,3 °C | ΔH3 J/g Sample |
|---|---|---|---|---|---|---|
| NaOH * | −33.7 | 95.0 | −9.7 | 170.6 | – | – |
| TMAH * | −26.3 | 68.0 | −17.3 | 27.8 | – | – |
| Triton B | −21.2 | 88.5 | – | – | – | – |
| 50/50 NaOH/Triton B | −16.3 | 38.5 | – | – | – | – |
| 50/50 NaOH/TMAH * | −27.8 | 15.3 | −25.1 | 72.2 | −14.7 | 42.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swensson, B.; Larsson, A.; Hasani, M. Probing Interactions in Combined Hydroxide Base Solvents for Improving Dissolution of Cellulose. Polymers 2020, 12, 1310. https://doi.org/10.3390/polym12061310
Swensson B, Larsson A, Hasani M. Probing Interactions in Combined Hydroxide Base Solvents for Improving Dissolution of Cellulose. Polymers. 2020; 12(6):1310. https://doi.org/10.3390/polym12061310
Chicago/Turabian StyleSwensson, Beatrice, Anette Larsson, and Merima Hasani. 2020. "Probing Interactions in Combined Hydroxide Base Solvents for Improving Dissolution of Cellulose" Polymers 12, no. 6: 1310. https://doi.org/10.3390/polym12061310
APA StyleSwensson, B., Larsson, A., & Hasani, M. (2020). Probing Interactions in Combined Hydroxide Base Solvents for Improving Dissolution of Cellulose. Polymers, 12(6), 1310. https://doi.org/10.3390/polym12061310






