Luminescent Properties of Lanthanoid-Poly(Sodium Acrylate) Composites: Insights on the Interaction Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Eu3+/PSA and Tb3+/PSA Composite Solutions
2.3. Preparation of Metal-Organic Gels
2.4. Apparatus and Characterization Methods of Eu(III)/PSA and Tb(III)/PSA
3. Results
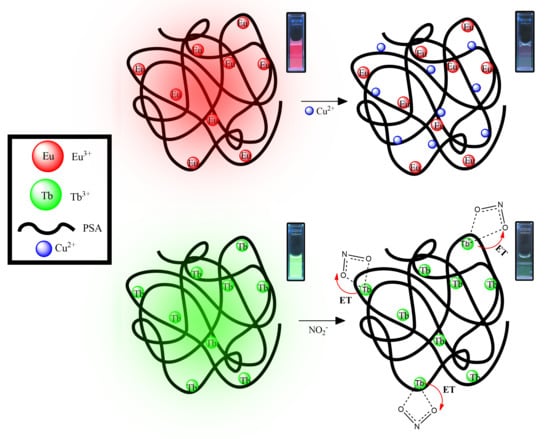
3.1. Lanthanoid-PSA Interactions
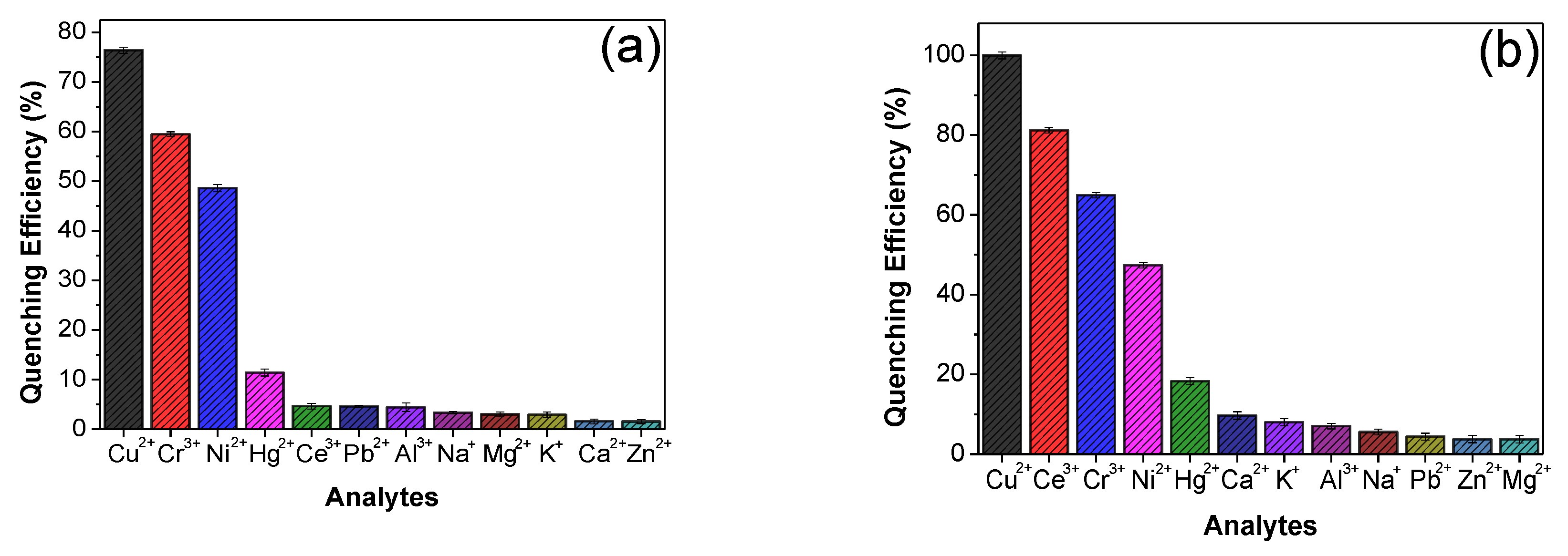
3.2. Effect of Cations on the Luminescence Properties Lanthanoid/PSA Complexes
3.3. Effect of Anions on Lanthanoid/PSA-Based Complex Properties
3.4. On the Interaction Mechanism Between Cu(II) and Lanthanoid/PSA-Based Complexes
3.5. Effect of pH and Quenching Rate
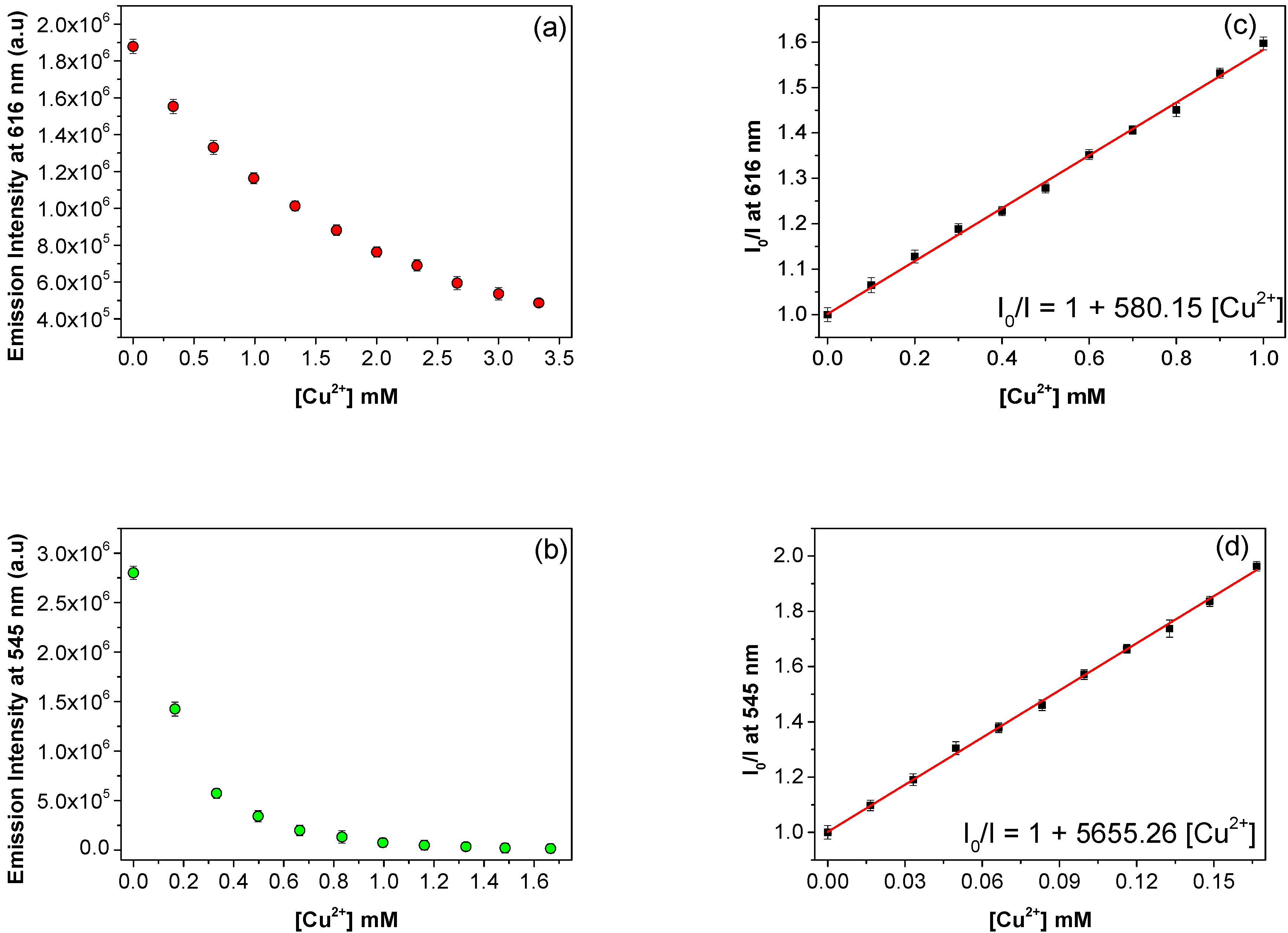
3.6. Sensitivity of Eu3+/PSA and Tb3+/PSA to Cu2+
3.7. Characterization of Solid Lanthanoid/PSA Composites Containing Cu2+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, G.; Nie, P.; Dou, H.; Ding, B.; Li, L.; Zhang, X. Exploring metal organic frameworks for energy storage in batteries and supercapacitors. Mater. Today 2017, 20, 191–209. [Google Scholar] [CrossRef]
- Yao, C.-X.; Zhao, N.; Liu, J.-C.; Chen, L.-J.; Liu, J.-M.; Fang, G.; Wang, S. Recent Progress on Luminescent Metal-Organic Framework-Involved Hybrid Materials for Rapid Determination of Contaminants in Environment and Food. Polymers 2020, 12, 691. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.-Q.; Jiang, H.-L.; Sun, L.-B. Metal–Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Peng, Z.W.; Jiang, Z.W.; Tang, X.Q.; Huang, C.; Li, Y.F. Novel Iron(III)-Based Metal–Organic Gels with Superior Catalytic Performance toward Luminol Chemiluminescence. ACS Appl. Mater. Interfaces 2017, 9, 31834–31840. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, H.; Gascon, J.; Schins, J.M.; Siebbeles, L.D.A.; Kapteijn, F.; Kapteijn, F. Unraveling the Optoelectronic and Photochemical Behavior of Zn4O-Based Metal Organic Frameworks. J. Phys. Chem. C 2011, 115, 12487–12493. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, Y.D.; Jiang, Z.W.; Peng, Z.W.; Huang, C.; Li, Y.F. Tb-containing metal-organic gel with high stability for visual sensing of nitrite. Mater. Lett. 2018, 211, 157–160. [Google Scholar] [CrossRef]
- Lloyd, G.O.; Steed, J.W. Anion-tuning of supramolecular gel properties. Nat. Chem. 2009, 1, 437–442. [Google Scholar] [CrossRef]
- Sutar, P.; Maji, T.K. Coordination polymer gels: Soft metal–organic supramolecular materials and versatile applications. Chem. Commun. 2016, 52, 8055–8074. [Google Scholar] [CrossRef]
- Zhang, J.; Su, C.-Y. Metal-organic gels: From discrete metallogelators to coordination polymers. Coord. Chem. Rev. 2013, 257, 1373–1408. [Google Scholar] [CrossRef]
- Tian, D.; Li, Y.; Chen, R.-Y.; Chang, Z.; Wang, G.; Bu, X.-H. A luminescent metal–organic framework demonstrating ideal detection ability for nitroaromatic explosives. J. Mater. Chem. A 2014, 2, 1465–1470. [Google Scholar] [CrossRef]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Springer US: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Liu, J.; Zuo, W.; Zhang, W.; Liu, J.; Wang, Z.; Yang, Z.; Wang, B. Europium(III) complex-functionalized magnetic nanoparticle as a chemosensor for ultrasensitive detection and removal of copper(II) from aqueous solution. Nanoscale 2014, 6, 11473–11478. [Google Scholar] [CrossRef] [PubMed]
- Aksuner, N.; Henden, E.; Yilmaz, I.; Çukurovalı, A.; Yılmaz, I. A highly sensitive and selective fluorescent sensor for the determination of copper(II) based on a schiff base. Dye. Pigment. 2009, 83, 211–217. [Google Scholar] [CrossRef]
- Kluczka, J. Removal of Boron and Manganese Ions from Wet-Flue Gas Desulfurization Wastewater by Hybrid Chitosan-Zirconium Sorbent. Polymers 2020, 12, 635. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Reddyprasad, P.; Imae, T. Selective detection of copper ion in water by tetradentate ligand sensor. J. Taiwan Inst. Chem. Eng. 2017, 72, 194–199. [Google Scholar] [CrossRef]
- Ghaedi, M.; Shokrollahi, A.; Kianfar, A.; Mirsadeghi, A.; Pourfarokhi, A.; Soylak, M. The determination of some heavy metals in food samples by flame atomic absorption spectrometry after their separation-preconcentration on bis salicyl aldehyde, 1,3 propan diimine (BSPDI) loaded on activated carbon. J. Hazard. Mater. 2008, 154, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Tashkhourian, J.; Montazerozohori, M.; Biyareh, M.N.; Sadeghian, B. Highly selective and sensitive determination of copper ion by two novel optical sensors. Arab. J. Chem. 2017, 10, S2319–S2326. [Google Scholar] [CrossRef]
- Gómez, M.R.; Cerutti, S.; Sombra, L.L.; Silva, M.F.; Martínez, L.D. Determination of heavy metals for the quality control in argentinian herbal medicines by ETAAS and ICP-OES. Food Chem. Toxicol. 2007, 45, 1060–1064. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, K.; Qu, Y.; Li, K.; Zhang, Y.; Fu, E. Hairy Fluorescent Nanospheres Based on Polyelectrolyte Brush for Highly Sensitive Determination of Cu(II). Polymers 2020, 12, 577. [Google Scholar] [CrossRef]
- Zeng, C.-H.; Meng, X.-T.; Xu, S.-S.; Han, L.-J.; Zhong, S.; Jia, M.-Y. A polymorphic lanthanide complex as selective Co2+ sensor and luminescent timer. Sens. Actuators B Chem. 2015, 221, 127–135. [Google Scholar] [CrossRef]
- Du, J.-L.; Zhang, X.-Y.; Li, C.-P.; Gao, J.-P.; Hou, J.-X.; Jing, X.; Mu, Y.-J.; Li, L.-J. A bi-functional luminescent Zn(II)-MOF for detection of nitroaromatic explosives and Fe3+ ions. Sens. Actuators B Chem. 2018, 257, 207–213. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kumar, S.; Umar, A.; Singh, J.; Rawat, M.; Mehta, S. Europium-doped gadolinium oxide nanoparticles: A potential photoluminescencent probe for highly selective and sensitive detection of Fe3+ and Cr3+ ions. Sens. Actuators B Chem. 2017, 243, 579–588. [Google Scholar] [CrossRef]
- Matsushita, A.F.; Filho, C.M.; Piñeiro, M.; Pais, A.A.C.C.; Valente, A.J.M. Effect of Eu(III) and Tb(III) chloride on the gelification behavior of poly(sodium acrylate). J. Mol. Liq. 2018, 264, 205–214. [Google Scholar] [CrossRef]
- Qi, X.; Wang, Z.; Ma, S.; Wu, L.; Yang, S.; Xu, J. Complexation behavior of poly(acrylic acid) and lanthanide ions. Polymers 2014, 55, 1183–1189. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, X.; Gao, P.; Hu, M. A water stable europium coordination polymer as fluorescent sensor for detecting Fe3+, CrO42−, and Cr2O72− ions. J. Solid State Chem. 2018, 258, 86–92. [Google Scholar] [CrossRef]
- Tang, K.; Ma, Q.; Zhan, Q.; Wang, Q. An intelligent copper(II) luminescent sensor using europium narrow emissions based on titania hybrid material. Opt. Mater. 2014, 36, 1520–1524. [Google Scholar] [CrossRef]
- Bogale, R.F.; Chen, Y.; Ye, J.; Yang, Y.; Rauf, A.; Duan, L.; Tian, P.; Ning, G. Highly selective and sensitive detection of 4-nitrophenol and Fe3+ ion based on a luminescent layered terbium (III) coordination polymer. Sens. Actuators B Chem. 2017, 245, 171–178. [Google Scholar] [CrossRef]
- Yang, W.; Feng, J.; Zhang, H. Facile and rapid fabrication of nanostructured lanthanide coordination polymers as selective luminescent probes in aqueous solution. J. Mater. Chem. 2012, 22, 6819. [Google Scholar] [CrossRef]
- Costa, D.; Burrows, H.D.; Miguel, M. Changes in Hydration of Lanthanide Ions on Binding to DNA in Aqueous Solution. Langmuir 2005, 21, 10492–10496. [Google Scholar] [CrossRef]
- Horrocks, W.D.; Sudnick, D.R. Lanthanide ion probes of structure in biology. Laser-induced luminescence decay constants provide a direct measure of the number of metal-coordinated water molecules. J. Am. Chem. Soc. 1979, 101, 334–340. [Google Scholar] [CrossRef]
- Horrocks, W.D.; Schmidt, G.F.; Sudnick, D.R.; Kittrell, C.; Bernheim, R.A. Laser-induced lanthanide ion luminescence lifetime measurements by direct excitation of metal ion levels. A new class of structural probe for calcium-binding proteins and nucleic acids. J. Am. Chem. Soc. 1977, 99, 2378–2380. [Google Scholar] [CrossRef] [PubMed]
- Tapia, M.J.; Burrows, H.D. Cation Polyelectrolyte Interactions in Aqueous Sodium Poly(vinyl sulfonate) as Seen by Ce3+ to Tb3+ Energy Transfer. Langmuir 2002, 18, 1872–1876. [Google Scholar] [CrossRef]
- Tapia, M.J.; Burrows, H.D.; Azenha, M.; Miguel, M.; Pais, A.A.C.C.; Sarraguca, J. Cation Association with Sodium Dodecyl Sulfate Micelles As Seen by Lanthanide Luminescence. J. Phys. Chem. B 2002, 106, 6966–6972. [Google Scholar] [CrossRef]
- Wang, Y.; Xin, X.; Li, W.; Jia, C.; Wang, L.; Shen, J.; Xu, G.-Y. Studies on the gel behavior and luminescence properties of biological surfactant sodium deoxycholate/rare-earth salts mixed systems. J. Colloid Interface Sci. 2014, 431, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, J.; Wang, Y.; Ren, S. Synthesis and humidity controlling properties of halloysite/poly(sodium acrylate-acrylamide) composite. J. Mater. Chem. 2012, 22, 11093. [Google Scholar] [CrossRef]
- Adachi, T.; Shirotani, I.; Hayashi, J.; Shimomura, O. Phase transitions of lanthanide monophosphides with NaCl-type structure at high pressures. Phys. Lett. A 1998, 250, 389–393. [Google Scholar] [CrossRef]
- Jin, H.; Huang, Y.; Jian, J. Plate-like Cr2O3 for highly selective sensing of nitric oxide. Sens. Actuators B Chem. 2015, 206, 107–110. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Chu, T.; Li, W.; Yang, Y. A facile fabrication of electrodeposited luminescent MOF thin film for selective and recyclable sensing nitroaromatic explosives. Analyst 2016, 141, 4502–4510. [Google Scholar] [CrossRef]
- Aulsebrook, M.L.; Graham, A.P.B.; Grace, M.R.; Tuck, K.L. Lanthanide complexes for luminescence-based sensing of low molecular weight analytes. Coord. Chem. Rev. 2018, 375, 191–220. [Google Scholar] [CrossRef]
- Hao, Z.; Song, X.; Zhu, M.; Meng, X.; Zhao, S.; Su, S.; Yang, W.; Song, S.; Zhang, H. One-dimensional channel-structured Eu-MOF for sensing small organic molecules and Cu2+ ion. J. Mater. Chem. A 2013, 1, 11043. [Google Scholar] [CrossRef]
- Hao, Z.; Yang, G.; Song, X.; Zhu, M.; Meng, X.; Zhao, S.; Song, S.; Zhang, H. A europium(iii) based metal–organic framework: Bifunctional properties related to sensing and electronic conductivity. J. Mater. Chem. A 2014, 2, 237–244. [Google Scholar] [CrossRef]
- Xiao, Y.; Cui, Y.; Zheng, Q.; Xiang, S. A microporous luminescent metal–organic framework for highly selective and sensitive sensing of Cu2+ in aqueous solution. ChemComm 2010, 56, 5503–5505. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Gao, J.; Deng, S.; Zhang, R.; Zheng, Y. Dual-target optical sensors assembled by lanthanide complex incorporated sol–gel-derived polymeric films. J. Sol Gel Sci. Technol. 2016, 78, 606–612. [Google Scholar] [CrossRef]
- Bogale, R.F.; Ye, J.; Sun, Y.; Sun, T.; Zhang, S.; Rauf, A.; Hang, C.; Tian, P.; Ning, G. Highly selective and sensitive detection of metal ions and nitroaromatic compounds by an anionic europium(III) coordination polymer. Dalton Trans. 2016, 45, 11137–11144. [Google Scholar] [CrossRef]
- Bogale, R.F.; Chen, Y.; Ye, J.; Zhang, S.; Li, Y.; Liu, X.; Zheng, T.; Rauf, A.; Ning, G. A terbium(III)-based coordination polymer for selective and sensitive sensing of nitroaromatics and ferric ion: Synthesis, crystal structure and photoluminescence properties. New J. Chem. 2017, 41, 12713–12720. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Song, L.; Silver, M.A.; Xie, J.; Zhang, L.; Chen, L.; Diwu, J.; Chai, Z.; Wang, S. Efficient and selective sensing of Cu2+ and UO22+ by a europium metal-organic framework. Talanta 2019, 196, 515–522. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, Y.; Chen, Y. Detection of mercury ions (Hg2+) in urine using a terbium chelate fluorescent probe. Sens. Actuators B Chem. 2011, 156, 120–125. [Google Scholar] [CrossRef]
- Chen, B.; Wang, L.; Xiao, Y.; Fronczek, F.R.; Xue, M.; Cui, Y.; Qian, G. A Luminescent Metal-Organic Framework with Lewis Basic Pyridyl Sites for the Sensing of Metal Ions. Angew. Chem. Int. Ed. 2009, 48, 500–503. [Google Scholar] [CrossRef]
- Du, P.-Y.; Gu, W.; Liu, X. Multifunctional Three-Dimensional Europium Metal–Organic Framework for Luminescence Sensing of Benzaldehyde and Cu2+ and Selective Capture of Dye Molecules. Inorg. Chem. 2016, 55, 7826–7828. [Google Scholar] [CrossRef]
- Sun, Z.; Li, H.; Sun, G.; Guo, J.; Ma, Y.; Li, L. Design and construction of lanthanide metal-organic frameworks through mixed-ligand strategy: Sensing property of acetone and Cu2+. Inorg. Chim. Acta 2018, 469, 51–56. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Wang, S.; Rao, Z.; Yang, Y. A luminescent Terbium-Succinate MOF thin film fabricated by electrodeposition for sensing of Cu2+ in aqueous environment. Sens. Actuators B Chem. 2015, 220, 779–787. [Google Scholar] [CrossRef]






| Sample | Solvent | τ (μs) | k (ms−1) | n (H2O Coordinated) |
|---|---|---|---|---|
| EuCl3 | H2O | 112 | 8.92 | 9 |
| EuCl3 | D2O | 1775 | 0.56 | |
| Eu3+/PSA | H2O | 274 | 3.65 | 3 |
| Eu3+/PSA | D2O | 1602 | 0.62 | |
| TbCl3 | H2O | 431 | 2.32 | 9 |
| TbCl3 | D2O | 3565 | 0.28 | |
| Tb3+/PSA | H2O | 861 | 1.16 | 3 |
| Tb3+/PSA | D2O | 3002 | 0.33 |
| Luminescent Material | Ksv (M−1) | Reference |
|---|---|---|
| [Eu(pdc)1.5(dmf)]·(DMF)0.5(H2O)0.5 | 89 | [49] |
| Eu3+/PSA | 580 | This Work |
| {[Eu2(abtc)1.5(H2O)3(DMA)]·H2O·DMA}n | 529 | [50] |
| {[Eu(HL)(L)(H2O)2]_2H2O}n | 116 | [43] |
| {[Eu(L)(ox)0.5(H2O)2]_H2O}n | 2074 | [51] |
| Tb3+/PSA | 5655 | This work |
| Tb-SA | 6298 | [52] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsushita, A.F.Y.; Tapia, M.J.; Pais, A.A.C.C.; Valente, A.J.M. Luminescent Properties of Lanthanoid-Poly(Sodium Acrylate) Composites: Insights on the Interaction Mechanism. Polymers 2020, 12, 1314. https://doi.org/10.3390/polym12061314
Matsushita AFY, Tapia MJ, Pais AACC, Valente AJM. Luminescent Properties of Lanthanoid-Poly(Sodium Acrylate) Composites: Insights on the Interaction Mechanism. Polymers. 2020; 12(6):1314. https://doi.org/10.3390/polym12061314
Chicago/Turabian StyleMatsushita, Alan F. Y., María José Tapia, Alberto A. C. C. Pais, and Artur J. M. Valente. 2020. "Luminescent Properties of Lanthanoid-Poly(Sodium Acrylate) Composites: Insights on the Interaction Mechanism" Polymers 12, no. 6: 1314. https://doi.org/10.3390/polym12061314
APA StyleMatsushita, A. F. Y., Tapia, M. J., Pais, A. A. C. C., & Valente, A. J. M. (2020). Luminescent Properties of Lanthanoid-Poly(Sodium Acrylate) Composites: Insights on the Interaction Mechanism. Polymers, 12(6), 1314. https://doi.org/10.3390/polym12061314