Particle Size Related Effects of Multi-Component Flame-Retardant Systems in poly(butadiene terephthalate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Characterization and Testing
2.2.1. Fire Testing
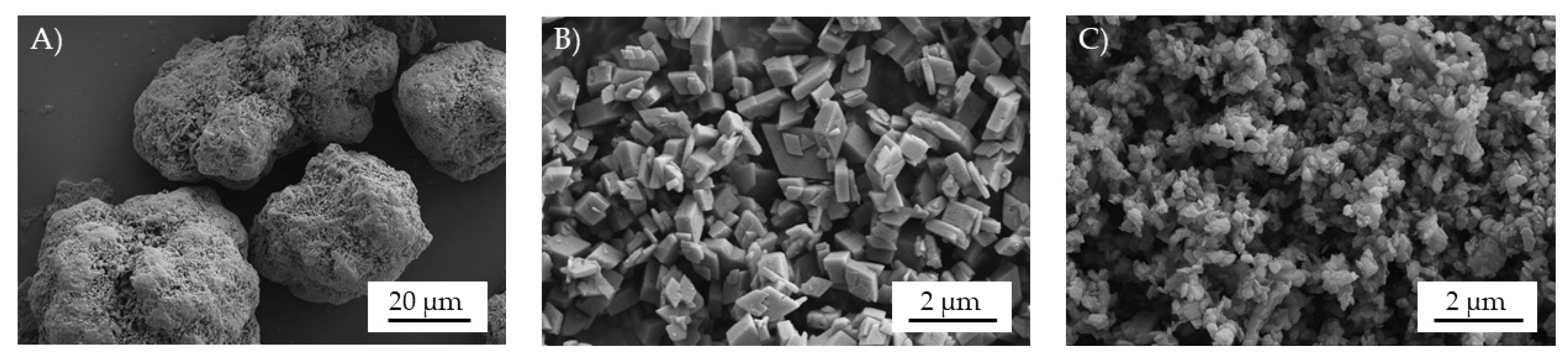
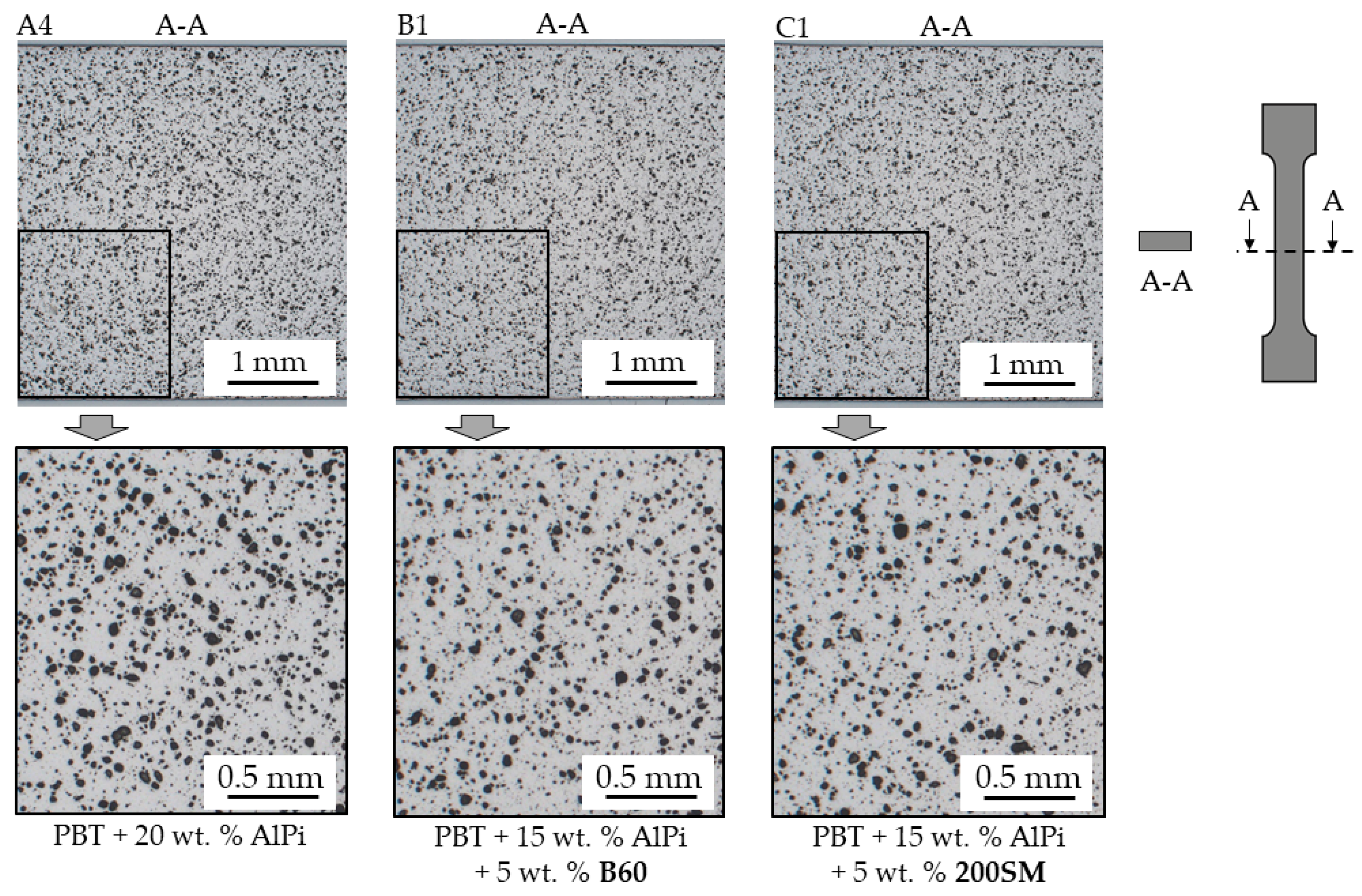
2.2.2. Microscopy
2.2.3. Thermal Analysis and Evolved Gas Analysis
2.2.4. Mechanical Testing
3. Results
3.1. Microscopy—Particle Size and Particle Distribution
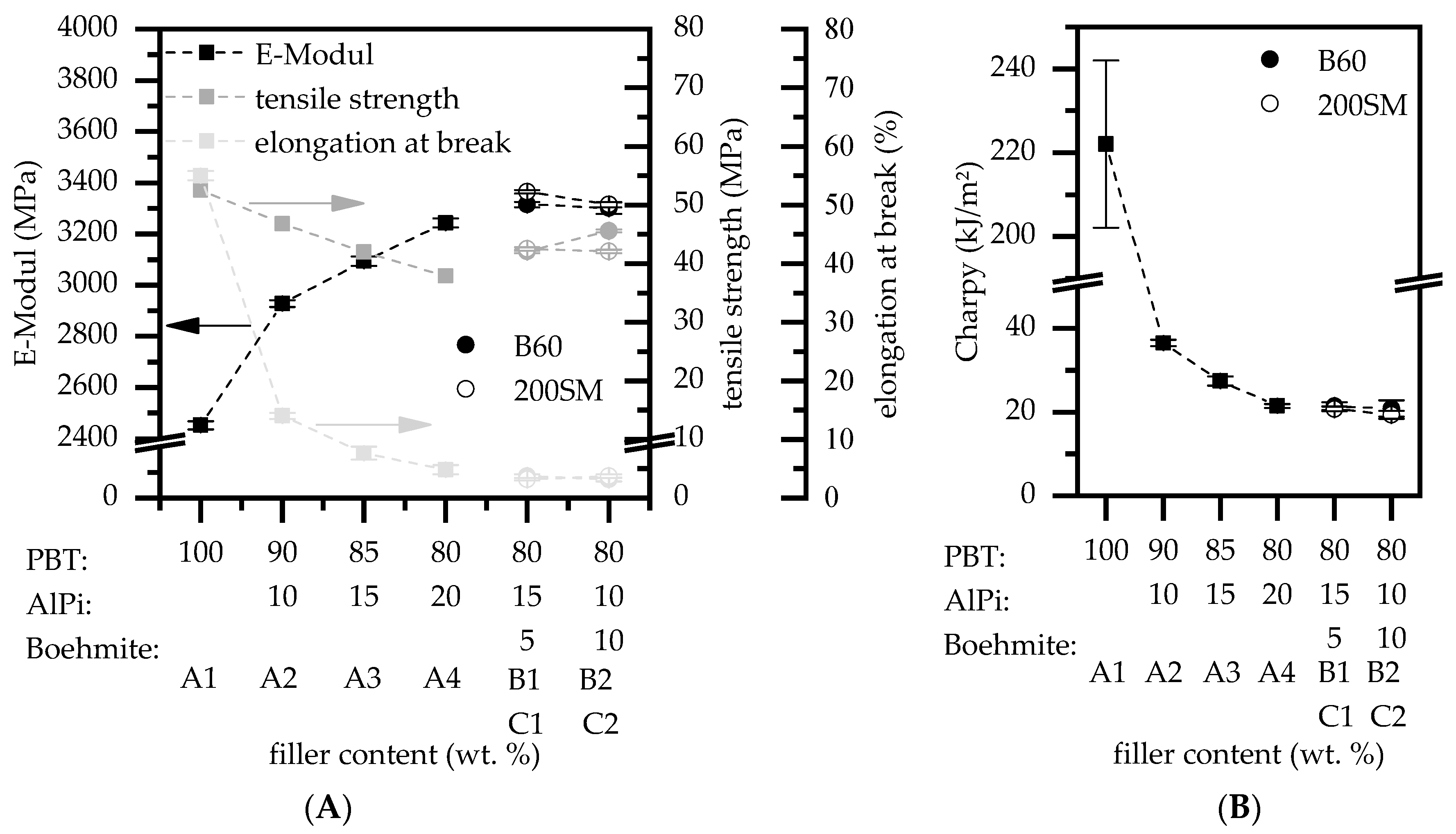
3.1.1. Mechanical Testing
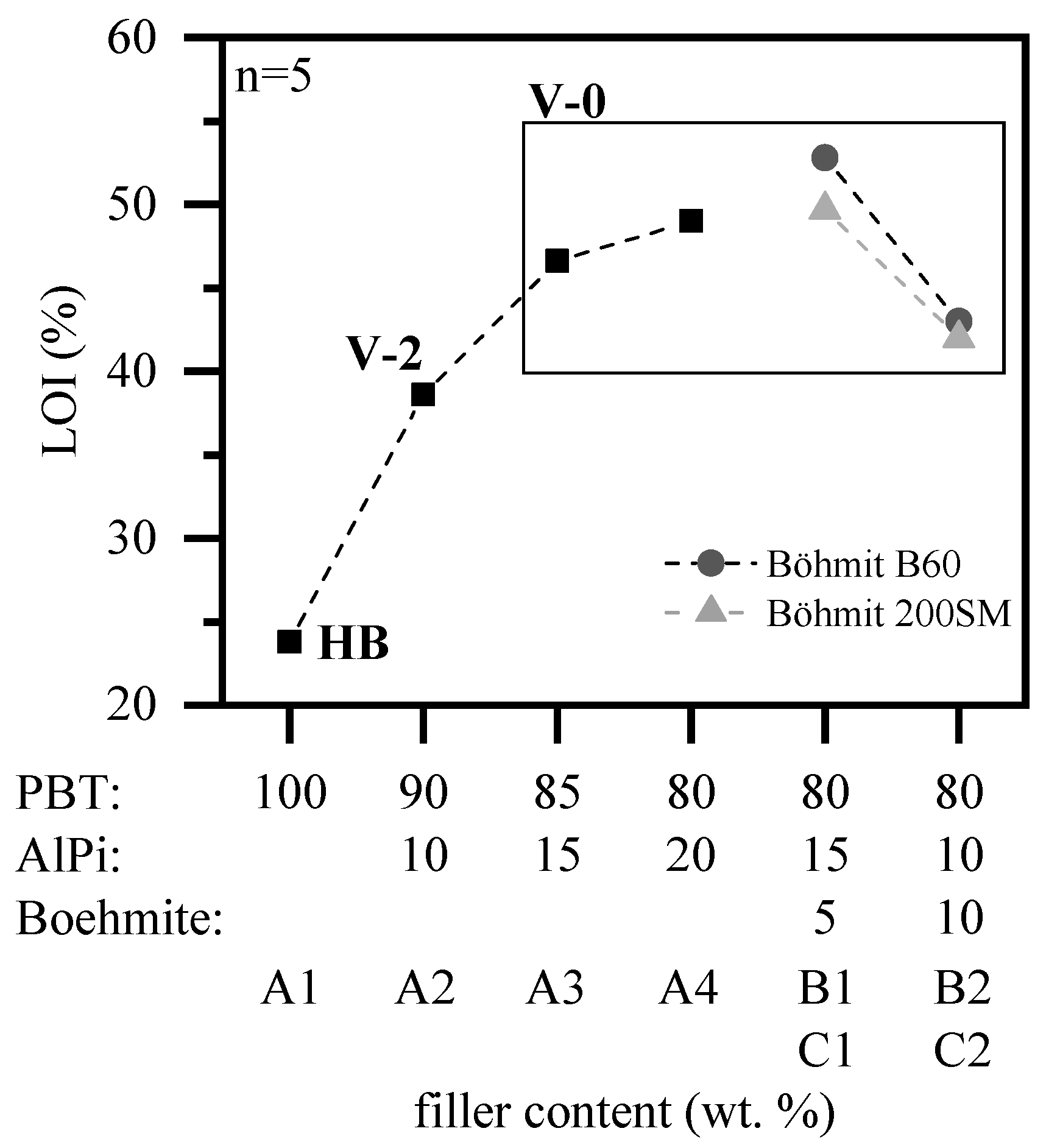
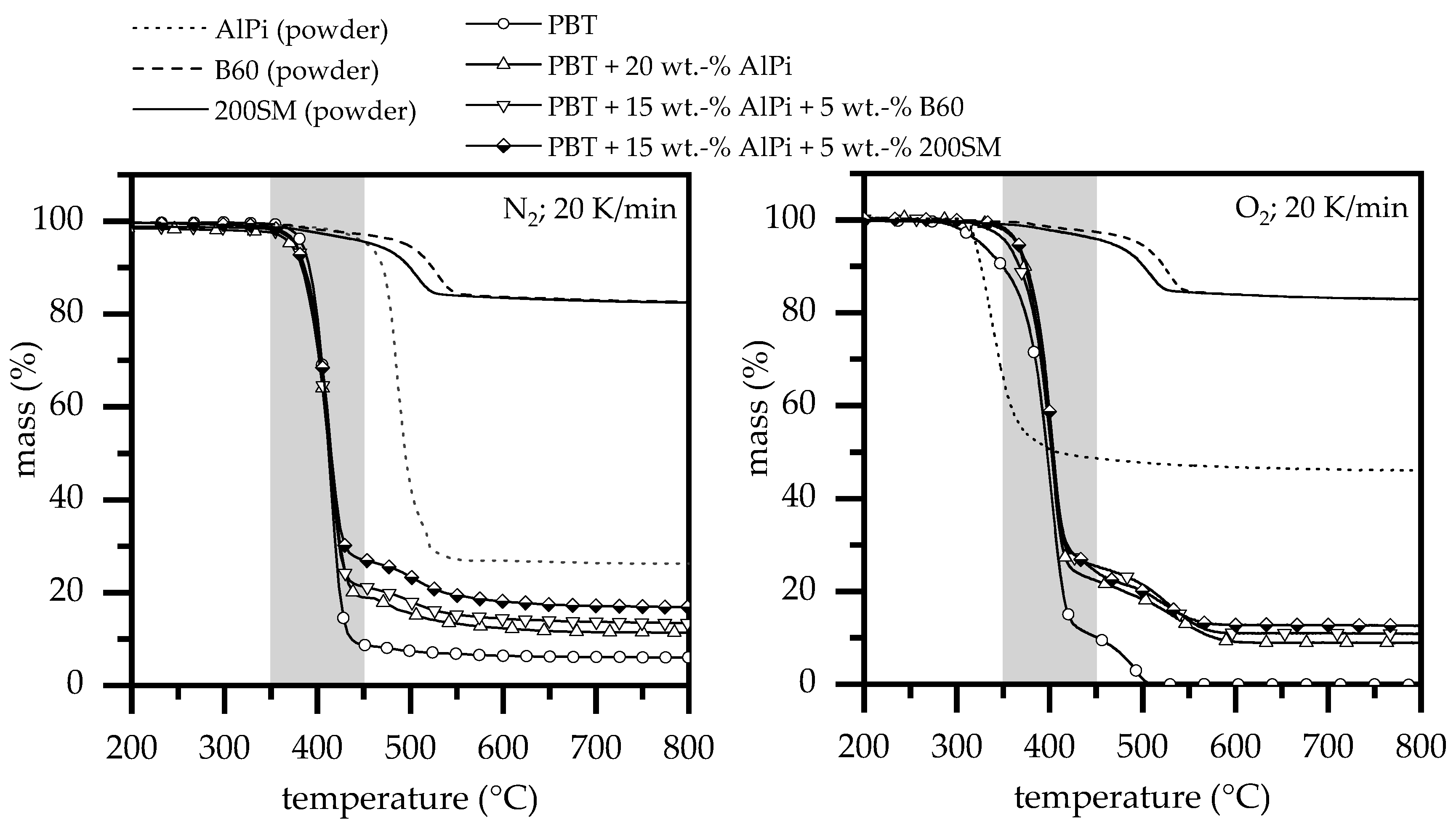
3.1.2. Fire Testing
3.1.3. Thermal Analysis
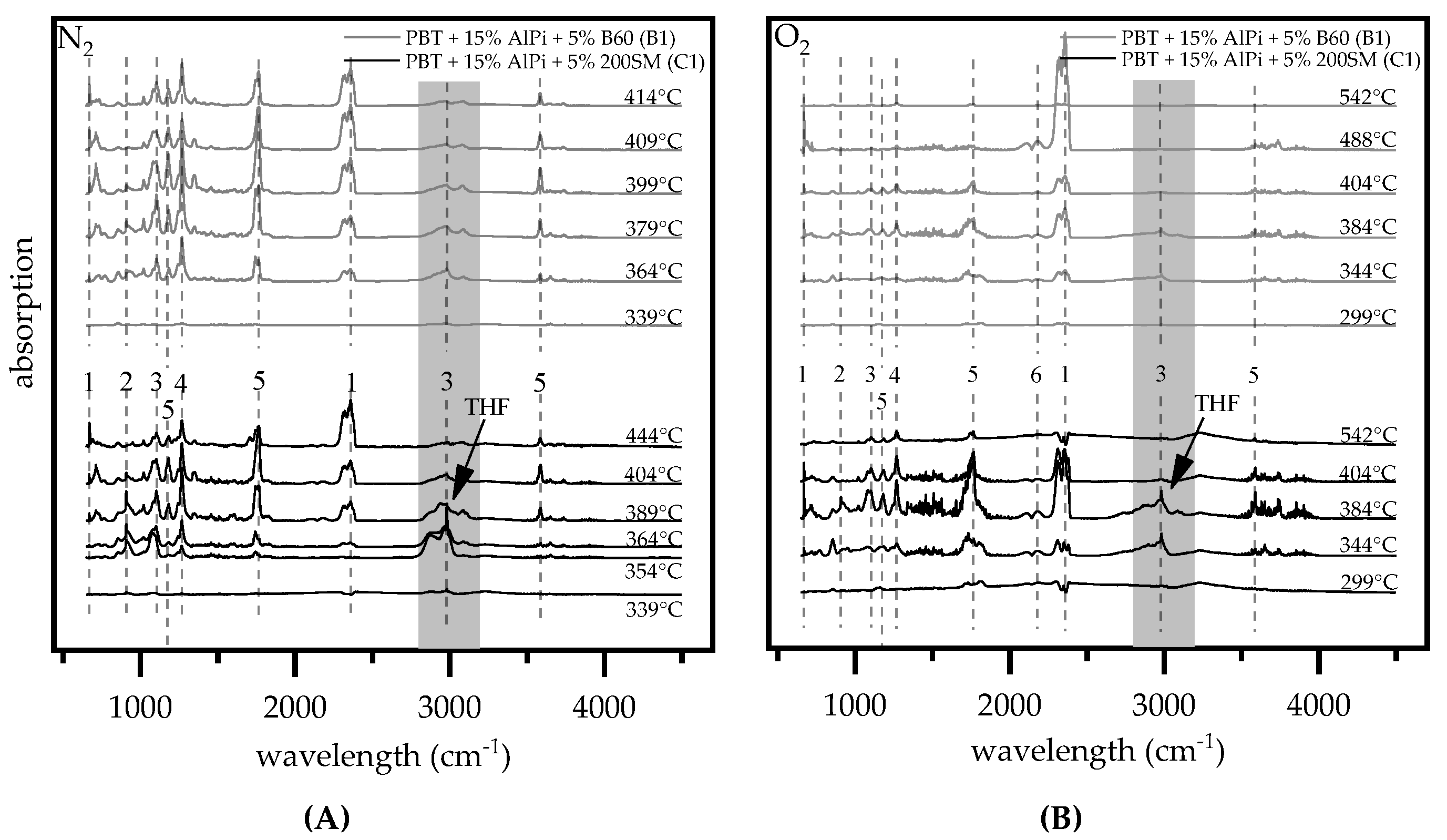
3.1.4. Gas Analysis
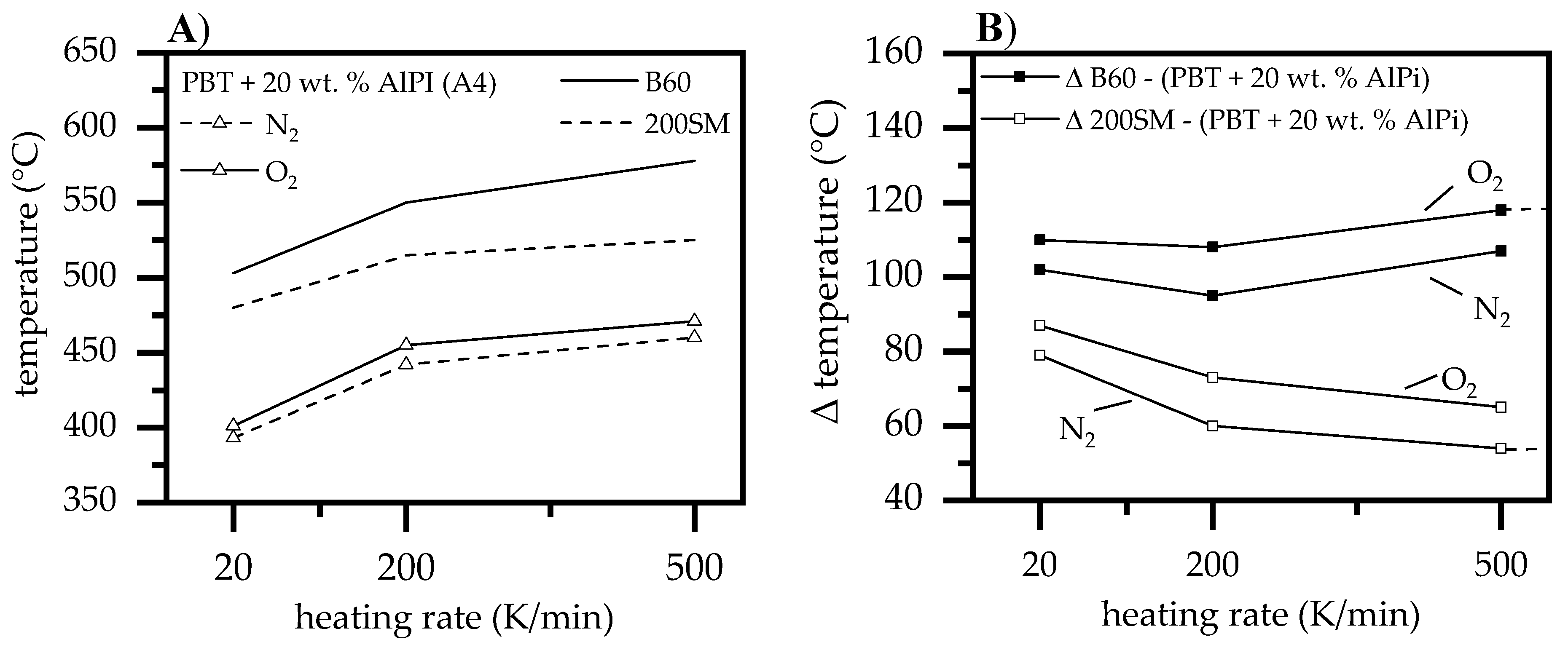
3.1.5. Heating Rates
3.1.6. Residue Structure
4. Conclusions
- Partial substitution of AlPi by boehmite increases the tensile strength as well as the e-module and reduces the elongation at break. We conclude that this effect is caused by higher surface-volume ratios comparing of boehmite and AlPi particles, resulting in better polymer-particle bonding.
- PBT/AlPi/boehmite mixtures show synergism in LOI replacing 5 wt.% of AlPi with boehmite. We conclude that two effects cause the synergism. The efficiency of AlPi progressively decreases with concentration higher than 12–15 wt.% offering synergistic mixtures just by replacing less efficient AlPi by a second flame retardant. The clear outperforming of PBT/ AlPi only for adding a certain amount evidences that an additional synergistic interaction occurs.
- PBT/AlPi/boehmite mixtures containing larger as compared to smaller boehmite particles, increased the LOI value by 4%. We conclude that this effect is caused by an earlier water release of smaller boehmite particles changing the decomposition path, in particular during the beginning of pyrolysis. This leads to a quantitatively higher formation of highly flammable tetrahydrofuran due to hydrolysis.
- At higher heating rates, inertial effects of larger boehmite particles (B60) show a much greater shift in water release towards higher temperatures than smaller boehmite particles (200SM). Thus, an earlier water release of smaller boehmite particles in PBT/AlPi/boehmite mixtures lead to higher water concentrations being available at lower temperatures. We conclude that higher heating rates promote a higher THF production due to hydrolysis.
- The decomposition of PBT/AlPi/boehmite mixtures resulted in higher amount of residue. The residue amount increases with applying smaller boehmite particles, most probably due to the higher activity of the formed metal oxides catalyzing some carbonaceous charring. What is more, the morphology of fire residues strongly changed. Adding mixtures of AlPi and boehmite yields the formation of residual protective layers. We conclude that the combination of flame-retardant modes of action in the gas phase, flame inhibition due to AlPi, and the flame-retardant modes of action in the condensed phase, charring, and protective layer formation, causes the pronounced synergy.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Troitzsch, J. Flammschutz. In Handbuch Kunststoff, 4th ed.; Maier, R.D., Schiller, M., Eds.; Carl Hanser Verlag: Munich, Germany, 2016; pp. 1015–1068. [Google Scholar]
- Schartel, B. Phosphorus-Based Flame Retardancy Mechanisms—Old Hat or a Starting Point for Future Development? Materials 2010, 10, 4710–4745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornsby, P.R.; Rothon, R.N. Fire Retardant Fillers for Polymers. In Polymer Green Flame Retardants, 1st ed.; Papaspyrides, C.D., Kiliaris, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 289–321. [Google Scholar]
- Watson, A.V.D.; Schiraldi, D.A. Biomolecules as Flame Retardant Additives for Polymers: A Review. Polymers 2020, 4, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Cao, Y.; Qian, L.; Chen, Y.; Qiu, Y.; Xu, B.; Xin, F. Synergistic Charring Flame-Retardant Behavior of Polyimide and Melamine Polyphosphate in Glass Fiber-Reinforced Polyamide 66. Polymers 2019, 11, 1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cárdenas, M.A.; García-López, D.; Gobernado-Mitre, I.; Merino, J.C.; Pastor, J.M.; Martínez, J.D.D.; Barbeta, J.; Calveras, D. Mechanical and fire retardant properties of EVA/clay/ATH nanocomposites—Effect of particle size and surface treatment of ATH filler. Polym. Degrad. Stab. 2008, 11, 2032–2037. [Google Scholar]
- Hughes, P.V.; Jackson, G.; Rothon, R.N. Particle morphology effects on the performance of PMMA filled with aluminium hydroxide in a variety of fire tests. Makromol. Chem. Macromol. Symp. 1993, 1, 179–183. [Google Scholar] [CrossRef]
- Atay, H.Y.; Çelik, E. Use of Turkish huntite/hydromagnesite mineral in plastic materials as a flame retardant. Polym. Compos. 2010, 10, 1692–1700. [Google Scholar] [CrossRef]
- DeArmitt, C. Composites Using Nano-Fillers. In Particulate-Filled Polymer Composites, 2nd ed.; Rothon, R.N., Ed.; RAPRA: Shropshire, UK, 2003; pp. 489–514. [Google Scholar]
- Rothon, R.N. Mineral Fillers in Thermoplastics: Filler Manufacture and Characterisation. In Mineral Fillers in Thermoplastics I, 139th ed.; Jancar, J., Fekete, E., Hornsby, P.R., Jancar, J., Pukánszky, B., Rothon, R.N., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 67–107. [Google Scholar]
- Braun, U.; Bahr, H.; Sturm, H.; Schartel, B. Flame retardancy mechanisms of metal phosphinates and metal phosphinates in combination with melamine cyanurate in glass-fiber reinforced poly (1,4-butylene terephthalate). Polym. Adv. Technol. 2008, 6, 680–692. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B. Flame Retardancy Mechanisms of Aluminium Phosphinate in Combination with Melamine Cyanurate in Glass-Fibre-Reinforced Poly (1,4-butylene terephthalate). Macromol. Mater. Eng. 2008, 3, 206–217. [Google Scholar] [CrossRef]
- Brehme, S.; Köppl, T.; Schartel, B.; Altstädt, V. Competition in aluminium phosphinate-based halogen-free flame retardancy of poly (butylene terephthalate) and its glass-fibre composites. e-Polymers 2014, 3, 193–208. [Google Scholar] [CrossRef]
- Brehme, S.; Köppl, T.; Schartel, B.; Fischer, O.; Altstädt, V.; Pospiech, D.; Döring, M. Phosphorus Polyester—An Alternative to Low-Molecular-Weight Flame Retardants in Poly (Butylene Terephthalate)? Macromol. Chem. Phys. 2012, 22, 2386–2397. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, C.; Zhang, Y.; Guo, W. Preparation and Characterization of Flame-Retarded Poly(butylene terephthalate)/Poly(ethylene terephthalate) Blends: Effect of Content and Type of Flame Retardant. Polymers 2019, 11, 1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, E.; Schartel, B.; Braun, U.; Russo, P.; Acierno, D. Fire retardant synergisms between nanometric Fe2O3 and aluminum phosphinate in poly(butylene terephthalate). Polym. Adv. Technol. 2011, 12, 2382–2391. [Google Scholar] [CrossRef]
- Duquesne, S.; Fontaine, G.; Cérin-Delaval, O.; Gardelle, B.; Tricot, G.; Bourbigot, S. Study of the thermal degradation of an aluminium phosphinate–aluminium trihydrate combination. Thermochim. Acta. 2013, 551, 175–183. [Google Scholar] [CrossRef]
- Hull, T.R.; Witkowski, A.; Hollingbery, L. Fire retardant action of mineral fillers. Polym. Degrad. Stab. 2011, 8, 1462–1469. [Google Scholar] [CrossRef] [Green Version]
- Pawlowski, K.H.; Schartel, B. Flame retardancy mechanisms of aryl phosphates in combination with boehmite in bisphenol A polycarbonate/acrylonitrile–butadiene–styrene blends. Polym. Degrad. Stab. 2008, 3, 657–667. [Google Scholar] [CrossRef]
- Greiner, L.; Kukla, P.; Eibl, S.; Döring, M. Phosphorus Containing Polyacrylamides as Flame Retardants for Epoxy-Based Composites in Aviation. Polymers 2019, 2, 284. [Google Scholar] [CrossRef] [Green Version]
- Clariant, A.G. Particle size distribution of neat phosphinates. Unpublished work.
- Nabaltec, A.G. Mineralischer Flammschutz mit Metallhydraten. Unpublished work.
- Langfeld, K.; Wilke, A.; Sut, A.; Greiser, S.; Ulmer, B.; Andrievici, V.; Limbach, P.; Bastian, M.; Schartel, B. Halogen-free fire retardant styrene–ethylene–butylene–styrene-based thermoplastic elastomers using synergistic aluminum diethylphosphinate–based compositions. J. Fire Sci. 2015, 2, 157–177. [Google Scholar] [CrossRef]
- Schartel, B.; Wilkie, C.A.; Camino, G. Recommendations on the scientific approach to polymer flame retardancy: Part 2—Concepts. J. Fire Sci. 2017, 1, 3–20. [Google Scholar] [CrossRef]
- Samyn, F.; Bourbigot, S. Thermal decomposition of flame retarded formulations PA6/aluminum phosphinate/melamine polyphosphate/organomodified clay. Polym. Degrad. Stab. 2012, 11, 2217–2230. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B.; Fichera, M.A.; Jäger, C. Flame retardancy mechanisms of aluminium phosphinate in combination with melamine polyphosphate and zinc borate in glass-fibre reinforced polyamide 6,6. Polym. Degrad. Stab. 2007, 8, 1528–1545. [Google Scholar] [CrossRef]
- Hornsby, P.R. The Application of Fire-Retardant Fillers for Use in Textile Barrier Materials. In Multifunctional Barriers for Flexible Structure: Textile, Leather and Paper, 1st ed.; Duquesne, S., Magniez, C., Camino, G., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2007; pp. 3–22. [Google Scholar]
- Lucchesi, C.A.; Lewis, W.T. Latent heat of sublimation of terephthalic acid from differential thermal analysis data. J. Chem. Eng. Data 1968, 3, 389–391. [Google Scholar] [CrossRef]
- Ramani, A.; Dahoe, A.E. On the performance and mechanism of brominated and halogen free flame retardants in formulations of glass fibre reinforced poly(butylene terephthalate). Polym. Degrad. Stab. 2014, 71–86. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. A review on thermal decomposition and combustion of thermoplastic polyesters. Polym. Adv. Technol. 2004, 12, 691–700. [Google Scholar] [CrossRef]
- Gallo, E.; Braun, U.; Schartel, B.; Russo, P.; Acierno, D. Halogen-free flame retarded poly(butylene terephthalate) (PBT) using metal oxides/PBT nanocomposites in combination with aluminium phosphinate. Polym. Degrad. Stab. 2009, 8, 1245–1253. [Google Scholar] [CrossRef]
- Lum, R.M. Thermal decomposition of poly(butylene terephthalate). J. Polym. Sci. Polym. Chem. Ed. 1979, 1, 203–213. [Google Scholar] [CrossRef]
- Kumagai, S.; Morohoshi, Y.; Grause, G.; Kameda, T.; Yoshioka, T. Pyrolysis versus hydrolysis behavior during steam decomposition of polyesters using 18 O-labeled steam. RSC Adv. 2015, 67, 61828–61837. [Google Scholar] [CrossRef]
- Wilkie, C.A.; Morgan, A.B. Fire Retardancy of Polymeric Materials; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Khanna, Y.P.; Pearce, E.M. Synergism and Flame Retardancy. In Flame—Retardant Polymeric Materials, 2nd ed.; Menachem, L., Atlas, S., Pearce, E.P., Eds.; Springer: Boston, MA, USA, 1978; pp. 43–61. [Google Scholar]
- Camino, B.; Camino, G. The chemical kinetics of the polymer combustion allows for inherent fire retardant synergism. Polym. Degrad. Stab 2019, 160, 142–147. [Google Scholar] [CrossRef]



| Symbol | PBT (wt.%) | Ratio AlPi/Boehmite | AlPi (Exolit 1240) (wt.%) | Boemite (AlOOH) B60; d90 < 5 μm (wt.%) | Boemite (AlOOH) 200SM; d90 < 0.6 μm (wt.%) |
|---|---|---|---|---|---|
| A1 | 100 | ||||
| A2 | 90 | 10 | |||
| A3 | 85 | 15 | |||
| A4 | 80 | 20 | |||
| B1 | 80 | 3:1 | 15 | 5 | |
| B2 | 80 | 1:1 | 10 | 10 | |
| C1 | 80 | 3:1 | 15 | 5 | |
| C2 | 80 | 1:1 | 10 | 10 |
| Symbol | Materials (wt.%) | TGA- Standard | TGA-High Speed | TGA & FTIR | Test Atmosphere |
|---|---|---|---|---|---|
| A1 | PBT | 20 K/min | N2, O2 | ||
| - | AlPi (powder) | 20 K/min | N2, O2 | ||
| - | B60 (powder) | 20 K/min | 200, 500 K/min | N2, O2 | |
| - | 200SM (powder) | 20 K/min | 200, 500 K/min | N2, O2 | |
| A2 | PBT + 10% AlPi | 20 K/min | N2, O2 | ||
| A3 | PBT + 15% AlPi | 20 K/min | N2, O2 | ||
| A4 | PBT + 20% AlPi | 20 K/min | 200, 500 K/min | N2, O2 | |
| B1 | PBT + 15% AlPi + 5% B60 | 20 K/min | 5 K/min | N2, O2 | |
| C1 | PBT + 15% AlPi + 5% 200SM | 20 K/min | 5 K/min | N2, O2 |
| N2 | O2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Material Compositions (wt.%) | T95 (°C) | T50 (°C) | Char Residue (%) | Char Residue Calc (%) | T95 (°C) | T50 (°C) | Char Residue (%) | Char Residue Calc. (%) | |
| A1 | PBT | 380 | 412 | ≈6% | - | 325 | 397 | ≈0% | - |
| - | AlPi (powder) | 452 | 493 | ≈26% | - | 319 | 408 | ≈46% | - |
| - | B60 (powder) | 495 | - | ≈82% | - | 495 | - | ≈82% | - |
| - | 200SM (powder) | 460 | - | ≈82% | - | 460 | - | ≈82% | - |
| A4 | PBT + 20% | 370 | 412 | ≈11% | 10% | 364 | 401 | ≈9% | 14% |
| B1 | PBT + 15% + 5% B60 | 370 | 412 | ≈13% | 13% | 365 | 404 | ≈11% | 16% |
| C1 | PBT + 15% + 5% 200SM | 370 | 412 | ≈17% | 13% | 354 | 401 | ≈13% | 16% |
| Literature Values (cm−1) | ||||
|---|---|---|---|---|
| Molecule | Characteristic Wavelength(cm−1) | PBT/PBT + AlPi [12] | PBT [31] | PBT/PBT + AlPi [18] |
| 1,3 butadien | 908 | 908 | 905 | 908 |
| therephthalic esters | 1743, 1267, 1100 | 1743, 1267, 1100 | 1737, 1265, 1100 | 1743, 1267, 1100 |
| benzoic acid | 3578, 1758, 1178 | 3580, 1760, 1177 | 3580, 1760, 1177 | |
| CO | 2110, 2181 | |||
| CO2 | 2450–2300, 668 | 2354, 2334, 667 | 3580. 1760, 669 | 2354, 667 |
| tetrahydrofuran (THF) | 2978, 1083 | 2980, 1083 | 2993, 2871, 1079 | 2980, 1083 |
| 1,4-butanediol | 2982, 2987, 1072 | |||
| benzene | 672 | |||
| diethylphosphinic acid | 708, 850, 1018 | 1017, 853, 773, 650 | 3650, 850, 773 | |
| ethene | 950 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomiak, F.; Schartel, B.; Wolf, M.; Drummer, D. Particle Size Related Effects of Multi-Component Flame-Retardant Systems in poly(butadiene terephthalate). Polymers 2020, 12, 1315. https://doi.org/10.3390/polym12061315
Tomiak F, Schartel B, Wolf M, Drummer D. Particle Size Related Effects of Multi-Component Flame-Retardant Systems in poly(butadiene terephthalate). Polymers. 2020; 12(6):1315. https://doi.org/10.3390/polym12061315
Chicago/Turabian StyleTomiak, Florian, Bernhard Schartel, Michael Wolf, and Dietmar Drummer. 2020. "Particle Size Related Effects of Multi-Component Flame-Retardant Systems in poly(butadiene terephthalate)" Polymers 12, no. 6: 1315. https://doi.org/10.3390/polym12061315
APA StyleTomiak, F., Schartel, B., Wolf, M., & Drummer, D. (2020). Particle Size Related Effects of Multi-Component Flame-Retardant Systems in poly(butadiene terephthalate). Polymers, 12(6), 1315. https://doi.org/10.3390/polym12061315






