Flexible Conductive Cellulose Network-Based Composite Hydrogel for Multifunctional Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fractionation, Impregnation, and Polymerization Processes
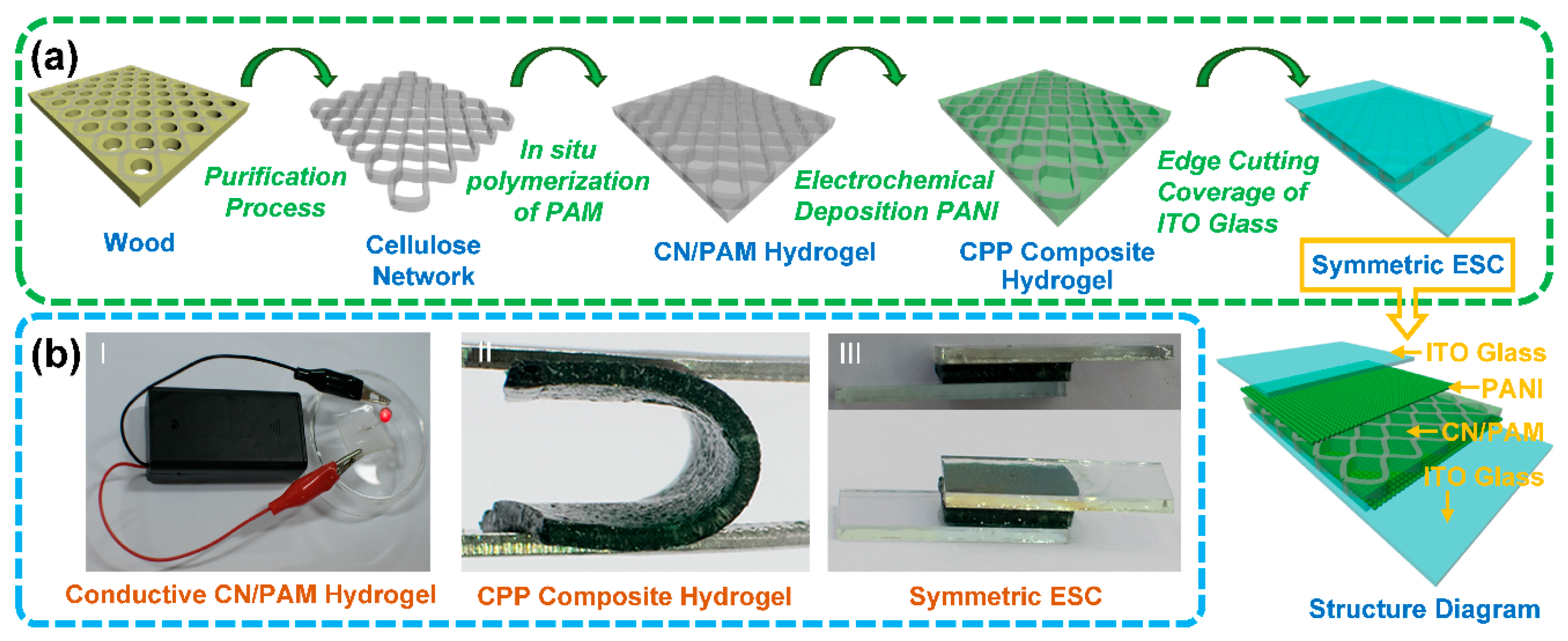
2.3. Preparation of Cellulose Network/Polyacrylamide (CN/PAM) Conductive Hydrogel
2.4. Preparation of Cellulose Network/Polyacrylamide Hydrogel/PANI (CPP) Composites
2.5. Electrochemical Test of CPP Electrode
2.6. Assembly of Symmetric ESC Based on CPP Composite Hydrogel
2.7. Characterizations
3. Results and Discussion
3.1. Preparation and Morphology of CPP Composite Hydrogel
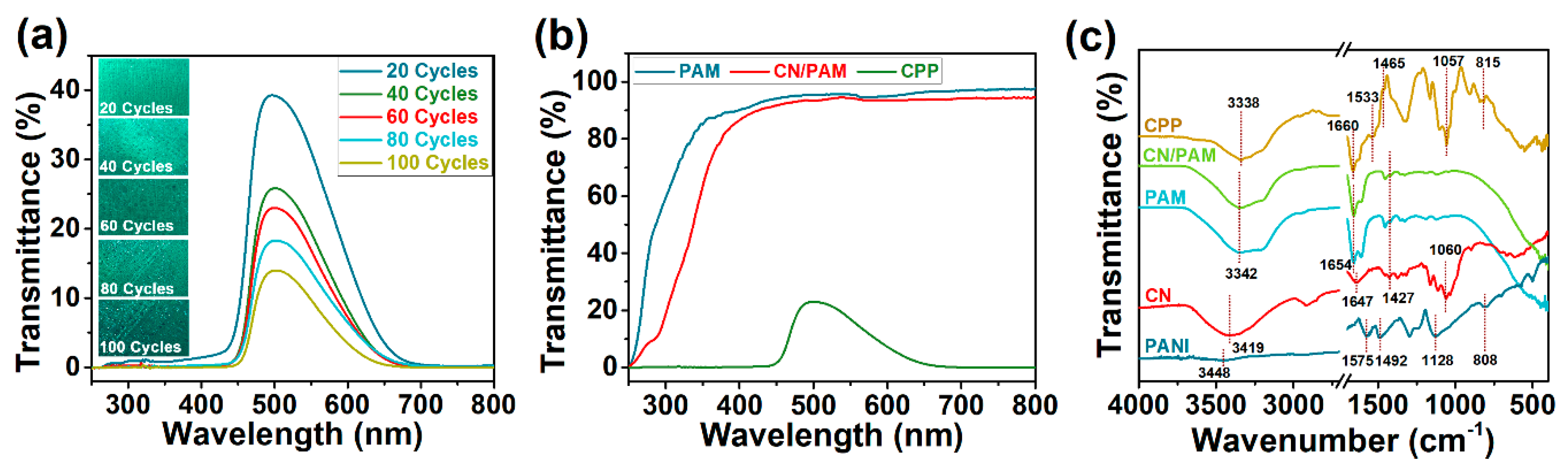
3.2. Optical Properties of CPP Composite Hydrogel
3.3. Characterizations of the CPP Composite Hydrogel
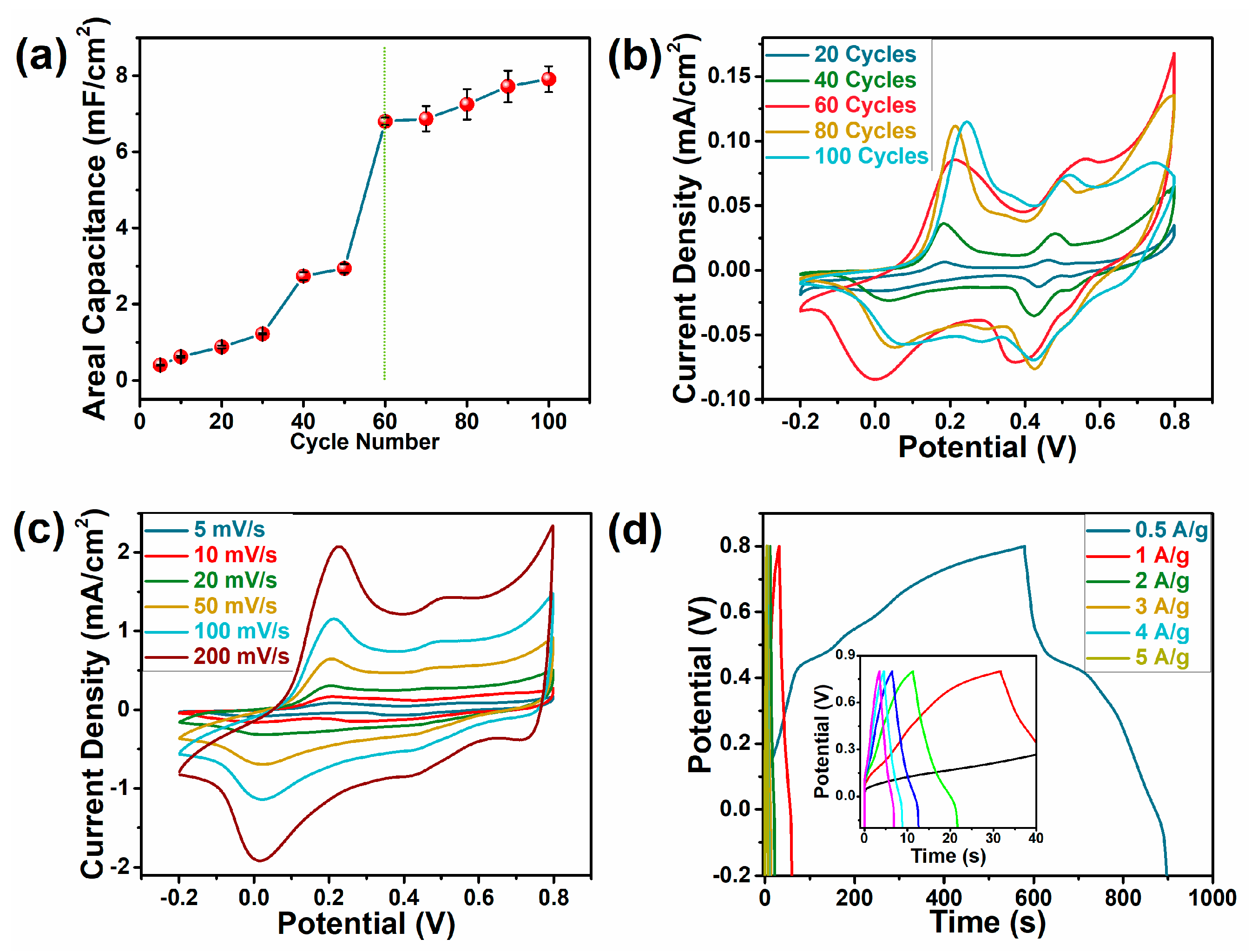
3.4. Electrochemical Properties of the CPP Composite Electrodes
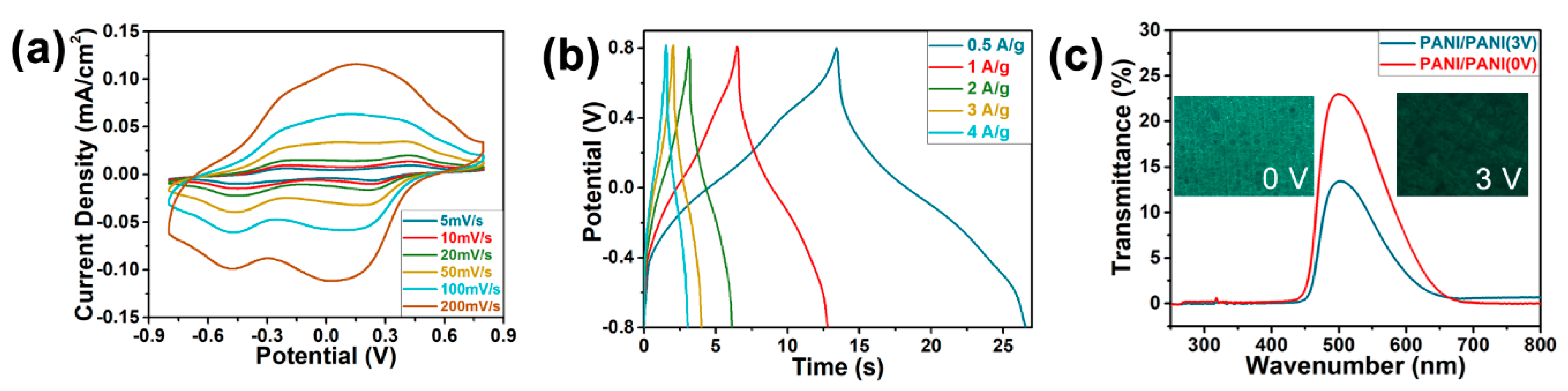
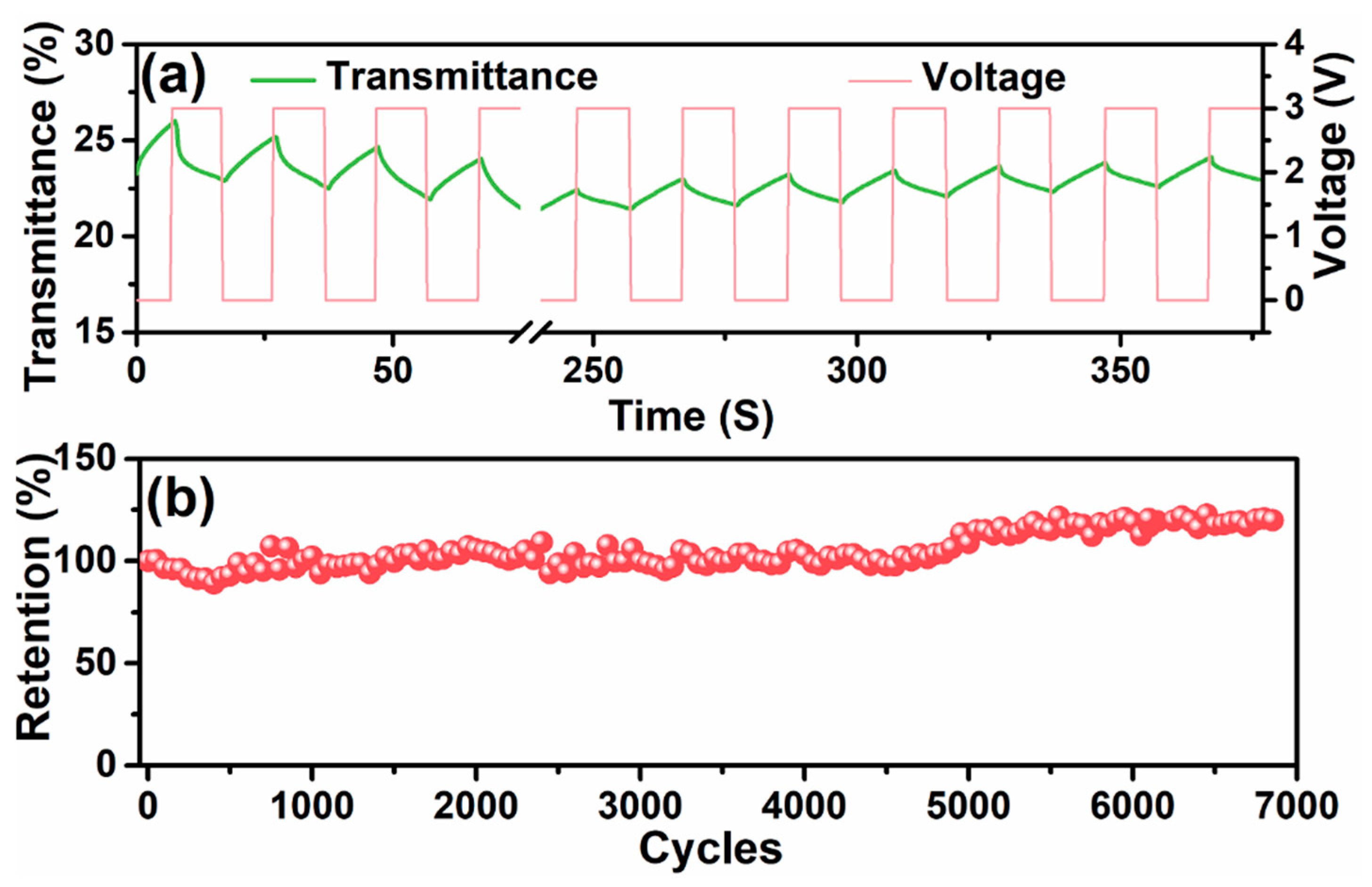
3.5. Electrochemical and Electrochromic Properties of ESCs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cai, G.; Wang, J.; Lee, P.S. Next-Generation Multifunctional Electrochromic Devices. Acc. Chem. Res. 2016, 49, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Wu, Y.; Wang, H.; Yang, G.; Li, W.; Xu, L.; Jiang, X. Preparation of hybrid polymer based on polyurethane lithium salt and polyvinylidene fluoride as electrolyte for lithium-ion batteries. Electrochim. Acta 2014, 136, 513–520. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Wu, J.; Wang, J.; Bai, H.; Li, L. Electrochemical supercapacitor with polymeric active electrolyte. J. Mater. Chem. A 2014, 2, 10526–10531. [Google Scholar] [CrossRef] [Green Version]
- Cai, G.; Eh, A.L.-S.; Ji, L.; Lee, P.S. Recent Advances in Electrochromic Smart Fenestration. Adv. Sustain. Syst. 2017, 1, 1700074. [Google Scholar] [CrossRef]
- Brezesinski, T.; Fattakhova Rohlfing, D.; Sallard, S.; Antonietti, M.; Smarsly, B.M. Highly Crystalline WO3 Thin Films with Ordered 3D Mesoporosity and Improved Electrochromic Performance. Small 2006, 2, 1203–1211. [Google Scholar] [CrossRef]
- Yuksel, R.; Ekber, A.; Turan, J.; Alpugan, E.; Hacioglu, S.O.; Toppare, L.; Cirpan, A.; Gunbas, G.; Unalan, H.E. A Novel Blue to Transparent Polymer for Electrochromic Supercapacitor Electrodes. Electroanalysis 2018, 30, 266–273. [Google Scholar] [CrossRef]
- Wu, W.; Wang, M.; Ma, J.; Cao, Y.; Deng, Y. Electrochromic Metal Oxides: Recent Progress and Prospect. Adv. Electron. Mater. 2018, 4, 1800185. [Google Scholar] [CrossRef]
- Zhong, Y.; Chai, Z.; Liang, Z.; Sun, P.; Xie, W.; Zhao, C.; Mai, W. Electrochromic Asymmetric Supercapacitor Windows Enable Direct Determination of Energy Status by the Naked Eye. ACS Appl. Mater. Interfaces 2017, 9, 34085–34092. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Reddy, B.N.; Kumar, P.N.; Deepa, M. A Poly(3,4-ethylenedioxypyrrole)-Au@WO3-Based Electrochromic Pseudocapacitor. Chem. Phys. Chem. 2015, 16, 377–389. [Google Scholar] [CrossRef]
- Chen, X.; Lin, H.; Deng, J.; Zhang, Y.; Sun, X.; Chen, P.; Fang, X.; Zhang, Z.; Guan, G.; Peng, H. Electrochromic Fiber-Shaped Supercapacitors. Adv. Mater. 2014, 26, 8126–8132. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Ouyang, T.; Zhang, K.; Nong, Y.; Mo, Y.; Mo, Q.; Wei, Y.; Cheng, F. Highly Conductive Cellulose Network/Polyaniline Composites Prepared by Wood Fractionation and In Situ Polymerization of Aniline. Macromol. Mater. Eng. 2019, 304, 1900112. [Google Scholar] [CrossRef]
- Niklasson, G.A.; Granqvist, C.G. Electrochromics for smart windows: Thin films of tungsten oxide and nickel oxide, and devices based on these. J. Mater. Chem. 2007, 17, 127–156. [Google Scholar] [CrossRef] [Green Version]
- Appetecchi, G.B.; Croce, F.; Scrosati, B. Kinetics and stability of the lithium electrode in poly(methylmethacrylate)-based gel electrolytes. Electrochim. Acta 1995, 40, 991–997. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Li, C.; Sun, X.; Meng, Q.; Ma, Y.; Wei, Z. Chemically Crosslinked Hydrogel Film Leads to Integrated Flexible Supercapacitors with Superior Performance. Adv. Mater. 2015, 27, 7451–7457. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; McMullen, L.M.; McDermott, M.T.; Deng, H.; Du, Y.; Chen, L.; Zhang, L. Stretchable, tough, self-recoverable, and cytocompatible chitosan/cellulose nanocrystals/polyacrylamide hybrid hydrogels. Carbohydr. Polym. 2019, 222, 114977. [Google Scholar] [CrossRef]
- Kong, W.; Wang, C.; Jia, C.; Kuang, Y.; Pastel, G.; Chen, C.; Chen, G.; He, S.; Huang, H.; Zhang, J.; et al. Muscle-Inspired Highly Anisotropic, Strong, Ion-Conductive Hydrogels. Adv. Mater. 2018, 30, 1801934. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Liu, Z.; Tang, Z.; Liang, G.; Mo, F.; Yang, Q.; Ma, L.; Zhi, C. A Nanofibrillated Cellulose/Polyacrylamide Electrolyte-Based Flexible and Sewable High-Performance Zn-MnO2 Battery with Superior Shear Resistance. Small 2018, 14, 1803978. [Google Scholar] [CrossRef]
- Shah, A.-U.-H.A.; Khan, M.O.; Bilal, S.; Rahman, G.; Hoang, H.V. Electrochemical co-deposition and characterization of polyaniline and manganese oxide nanofibrous composites for energy storage properties. Adv. Polym. Tech. 2018, 37, 2230–2237. [Google Scholar] [CrossRef]
- Marrinan, H.J.; Mann, J. Infrared spectra of the crystalline modifications of cellulose. J. Polym. Sci. 1956, 21, 301–311. [Google Scholar] [CrossRef]
- Rezaei, S.J.T.; Bide, Y.; Nabid, M.R. A new approach for the synthesis of polyaniline microstructures with a unique tetragonal star-like morphology. Synth. Met. 2011, 161, 1414–1419. [Google Scholar] [CrossRef]
- Yu, H.Y.; Wang, C.S.; Zhang, J.H.; Li, H.S.; Liu, S.X.; Ran, Y.; Xia, H.B. Cyclodextrin-assisted synthesis of water-dispersible polyaniline nanofibers by controlling secondary growth. Mater. Chem. Phys. 2012, 133, 459–464. [Google Scholar] [CrossRef]
- He, W.; Li, J.P.; Tian, J.X.; Jing, H.; Li, Y.J. Characteristics and Properties of Wood/Polyaniline Electromagnetic Shielding Composites Synthesized via In Situ Polymerization. Polym. Compos. 2018, 39, 537–543. [Google Scholar] [CrossRef]
- Xu, D.; Xiao, X.; Cai, J.; Zhou, J.; Zhang, L. Highly rate and cycling stable electrode materials constructed from polyaniline/cellulose nanoporous microspheres. J. Mater. Chem. A 2015, 3, 16424–16429. [Google Scholar] [CrossRef]
- Megha, R.; Ravikiran, Y.T.; Kotresh, S.; Kumari, S.C.V.; Prakash, H.G.R.; Thomas, S. Carboxymethyl cellulose: An efficient material in enhancing alternating current conductivity of HCl doped polyaniline. Cellulose 2018, 25, 1147–1158. [Google Scholar] [CrossRef]
- Matveeva, E.S. Could the acid doping of polyaniline represent the charge transfer interaction? Synth. Met. 1996, 83, 89–96. [Google Scholar] [CrossRef]
- Shaikh, S.F.; Lim, J.-Y.; Mane, R.S.; Zate, M.K.; Han, S.-H.; Joo, O.-S. Template-free electrochemical synthesis and electrochemical supercapacitors application of polyaniline nanobuds. J. Appl. Polym. Sci. 2013, 128, 3660–3664. [Google Scholar] [CrossRef]
- Sumboja, A.; Tefashe, U.M.; Wittstock, G.; Lee, P.S. Investigation of Charge Transfer Kinetics of Polyaniline Supercapacitor Electrodes by Scanning Electrochemical Microscopy. Adv. Mater. Interfaces 2015, 2, 1400154. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, X.; Li, Z.; Wang, D.; Nie, G. A novel solid-state electrochromic supercapacitor with high energy storage capacity and cycle stability based on poly(5-formylindole)/WO3 honeycombed porous nanocomposites. Chem. Eng. J. 2020, 384, 123370. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Li, L.; Li, C.; Zhong, W.; Zhang, H. Construction of Hierarchically One-Dimensional Core-Shell CNT@Microporous Carbon by Covalent Bond-Induced Surface-Confined Cross-Linking for High-Performance Supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 15557–15565. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, H.; Jiu, J.; Liu, J.; Yan, H.; Suganuma, K. Polyaniline films with modified nanostructure for bifunctional flexible multicolor electrochromic and supercapacitor applications. Chem. Eng. J. 2018, 345, 290–299. [Google Scholar] [CrossRef]

| Sample | Wavenumber (cm−1) | Assignment |
|---|---|---|
| Cellulose Network | 3342 | -OH stretching vibration |
| 1647 | C=O stretching vibration | |
| 1427 | -CH2 bending vibration | |
| 1060 | -OCH stretching vibration; C-O-C stretching vibration | |
| PANI | 3448 | -OH stretching vibration |
| 1575 | C=C stretching vibration of the quinone ring | |
| 1492 | C=C stretching vibration of the benzene ring | |
| 1128 | C-H in-plane bending vibration | |
| 808 | C-H out-of-plane bending vibration | |
| PAM | 3342 | -OH stretching vibration |
| 1654 | C=O vibration | |
| CPP | 1533 | C=C stretching vibration of the quinone ring |
| 1465 | C=C stretching vibration of the benzene ring |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ke, S.; Wang, Z.; Zhang, K.; Cheng, F.; Sun, J.; Wang, N.; Zhu, Y. Flexible Conductive Cellulose Network-Based Composite Hydrogel for Multifunctional Supercapacitors. Polymers 2020, 12, 1369. https://doi.org/10.3390/polym12061369
Ke S, Wang Z, Zhang K, Cheng F, Sun J, Wang N, Zhu Y. Flexible Conductive Cellulose Network-Based Composite Hydrogel for Multifunctional Supercapacitors. Polymers. 2020; 12(6):1369. https://doi.org/10.3390/polym12061369
Chicago/Turabian StyleKe, Shaoqiu, Zhiqi Wang, Kai Zhang, Fangchao Cheng, Jianping Sun, Nannan Wang, and Yanqiu Zhu. 2020. "Flexible Conductive Cellulose Network-Based Composite Hydrogel for Multifunctional Supercapacitors" Polymers 12, no. 6: 1369. https://doi.org/10.3390/polym12061369






