Hyper-Crosslinked Polymer Nanocomposites Containing Mesoporous Silica Nanoparticles with Enhanced Adsorption Towards Polar Dyes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Mesoporous Silica Nanoparticles
2.3. Synthesis of Hyper-Crosslinked Resins and Nanocomposites
2.4. Characterization
3. Results
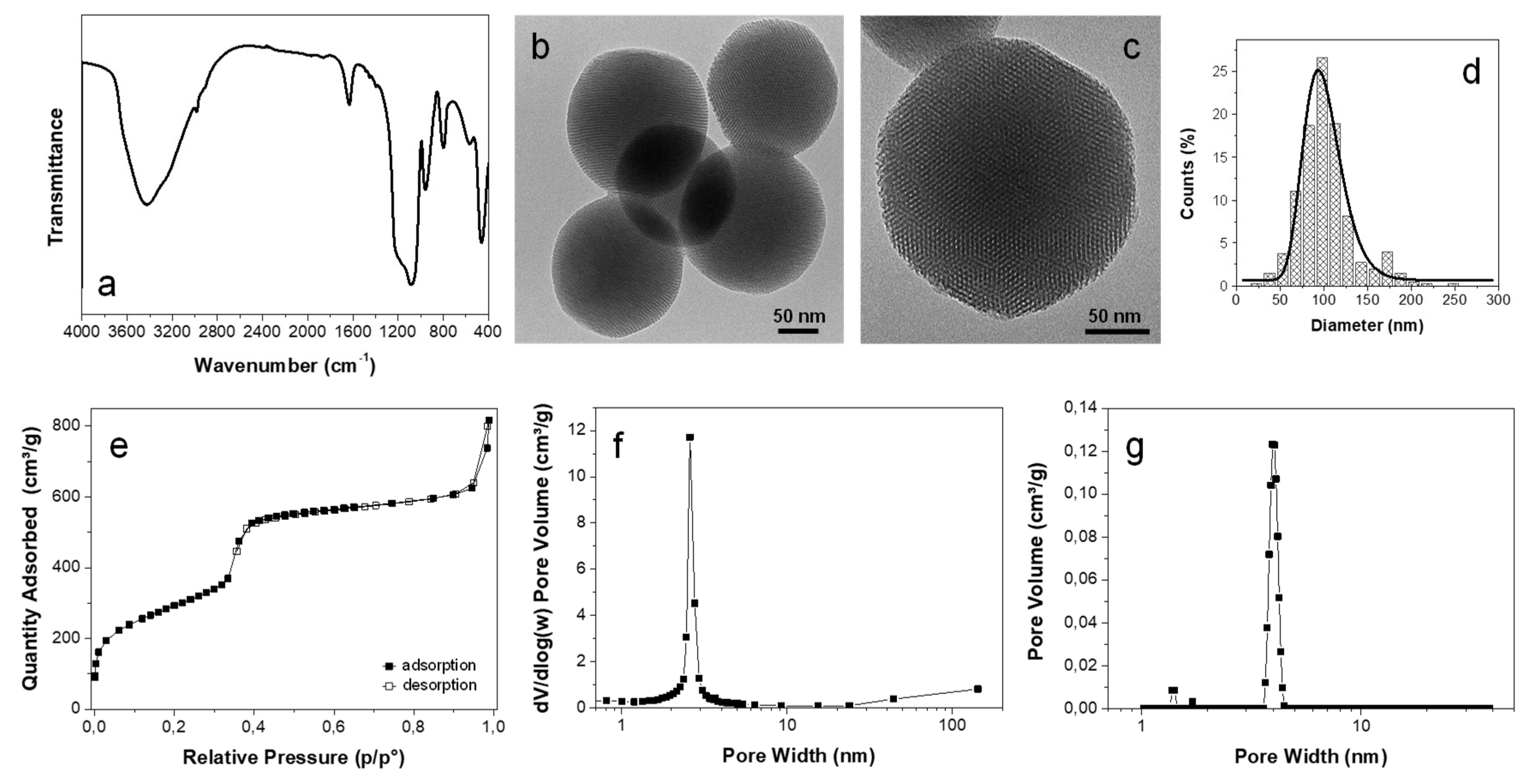
3.1. Mesoporous Silica Nanoparticles
3.2. Hyper-Crosslinked Resins and Nanocomposites
3.3. Equilibrium Adsorption Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forgacs, E.; Cserhati, T.; Oros, G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Shao, Y.; Ren, B.; Jiang, H.; Zhou, B.; Liping, L.V.; Ren, J.; Dong, L.; Liu, Z.; Jing, L. Dual-porosity Mn2O3 cubes for highly efficient dye adsorption. J. Hazard. Mater. 2017, 333, 222–231. [Google Scholar] [CrossRef]
- Hao, Y.; Fei, T.; Gu, W.; Liu, Z.; Zhao, Y.; An, Z.; Zhe, L.; Teng, Y. A simple post-synthesis conversion approach to Zn(OH)F and the effects of fluorine and hydroxyl on the photodegradation properties of dye wastewater. J. Hazard. Mater. 2017, 333, 250–258. [Google Scholar]
- Sun, W.; Zhang, C.; Chen, J.; Zhang, B.; Zhang, H.; Zhang, Y.; Chen, L. Accelerating biodegradation of a monoazo dye Acid Orange 7 by using its endogenous electron donors. J. Hazard. Mater. 2016, 324, 739–743. [Google Scholar] [CrossRef]
- Bassyouni, D.; Hamad, H.; El-Ashtoukhy, E.; Amin, N.; El-Latif, M.A. Comparative performance of anodic oxidation and electrocoagulation as clean processes for electrocatalytic degradation of diazo dye Acid Brown 14 in aqueous medium. J. Hazard. Mater. 2017, 335, 178–187. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, H.; Jiang, Z.; Cai, T.; Li, H.; Li, H.; Li, A.; Cheng, R. Flocculation of both anionic and cationic dyes in aqueous solutions by the amphoteric grafting flocculant carboxymethyl chitosan-graft-polyacrylamide. J. Hazard. Mater. 2013, 254, 36–45. [Google Scholar] [CrossRef]
- Xin, S.; Yang, N.; Gao, F.; Zhao, J.; Li, L.; Teng, C. Three-dimensional polypyrrole-derived carbon nanotube framework for dye adsorption and electrochemical supercapacitor. Appl. Surf. Sci. 2017, 414, 218–223. [Google Scholar] [CrossRef]
- Bonilla-Petriciolet, A.; Mendoza-Castillo, D.I.; Reynel-Ávila, H.E. Adsorption Processes for Water Treatment and Purification; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Ahn, J.H.; Jang, J.E.; Oh, C.G.; Ihm, S.K.; Cortez, J.; Sherrington, D.C. Rapid generation and control of microporosity, bimodal pore size distribution, and surface area in Davankov-type hyper-cross-linked resins. Macromolecules 2006, 39, 627–632. [Google Scholar] [CrossRef]
- Castaldo, R.; Ambrogi, V.; Avolio, R.; Cocca, M.; Gentile, G.; Errico, M.E.; Avella, M. Functional hyper-crosslinked resins with tailored adsorption properties for environmental applications. Chem. Eng. 2019, 362, 497–503. [Google Scholar] [CrossRef]
- Wang, X.; He, J.; Huang, J. Amino-modified hyper-cross-linked polymers with hierarchical porosity for adsorption of salicylic acid from aqueous solution. J. Chem. Thermodyn. 2019, 131, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Ou, H.; Huang, J. One-pot synthesis of hyper-cross-linked polymers chemically modified with pyrrole, furan, and thiophene for phenol adsorption from aqueous solution. J. Colloid Interfaces Sci. 2019, 538, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, X.; Huang, J. Hierarchical porous hyper-cross-linked polymers modified with phenolic hydroxyl groups and their efficient adsorption of aniline from aqueous solution. Colloid Surface A 2018, 558, 80–87. [Google Scholar] [CrossRef]
- Castaldo, R.; Avolio, R.; Cocca, M.; Gentile, G.; Errico, M.E.; Avella, M.; Carfagna, C.; Ambrogi, V. A Versatile Synthetic Approach toward Hyper-Cross-Linked Styrene-Based Polymers and Nanocomposites. Macromolecules 2017, 50, 4132–4143. [Google Scholar] [CrossRef] [Green Version]
- Castaldo, R.; Gentile, G.; Avella, M.; Carfagna, C.; Ambrogi, V. Microporous hyper-crosslinked polystyrenes and nanocomposites with high adsorption properties: A review. Polymers 2017, 9, 651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castaldo, R.; Avolio, R.; Cocca, M.; Gentile, G.; Errico, M.E.; Avella, M.; Carfagna, C.; Ambrogi, V. Synthesis and adsorption study of hyper-crosslinked styrene-based nanocomposites containing multi-walled carbon nanotubes. RSC Adv. 2017, 7, 6865–6874. [Google Scholar] [CrossRef] [Green Version]
- Castaldo, R.; Iuliano, M.; Cocca, M.; Ambrogi, V.; Gentile, G.; Sarno, M. A New Route for Low Pressure and Temperature CWAO: A PtRu/MoS2_Hyper-Crosslinked Nanocomposite. Nanomaterials 2019, 9, 1477. [Google Scholar] [CrossRef] [Green Version]
- Stepacheva, A.A.; Markova, M.E.; Manaenkov, O.V.; Gavrilenko, A.V.; Sidorov, A.I.; Sulman, M.G.; Kosivtsov, Y.Y.; Matveeva, V.G.; Sulmana, E.M. Modification of the hypercrosslinked polystyrene surface. New approaches to the synthesis of polymer-stabilized catalysts. Russ. Chem. Bull. 2020, 69, 721–730. [Google Scholar] [CrossRef]
- Fu, Z.; Li, H.; Yang, L.; Yuan, H.; Jiao, Z.; Chen, L.; Huang, J.; Liu, Y.N. Magnetic polar post-cross-linked resin and its adsorption towards salicylic acid from aqueous solution. Chem. Eng. 2015, 273, 240–246. [Google Scholar] [CrossRef]
- Sannino, F.; Costantini, A.; Ruffo, F.; Aronne, A.; Venezia, V.; Califano, V. Covalent immobilization of β-Glucosidase into mesoporous silica nanoparticles from anhydrous acetone enhances its catalytic performance. Nanomaterials 2020, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Zhang, L.; Xing, F.; Lin, H. Controlled synthesis of monodispersed mesoporous silica nanoparticles: Particle size tuning and formation mechanism investigation. Micropor. Mesopor. Mat. 2016, 225, 238–244. [Google Scholar] [CrossRef]
- Gibson, L.T. Mesosilica materials and organic pollutant adsorption: Part B removal from aqueous solution. Chem. Soc. Rev. 2014, 43, 5173–5182. [Google Scholar] [CrossRef]
- Branda, F.; Silvestri, B.; Costantini, A.; Luciani, G. Effect of exposure to growth media on size and surface charge of silica based Stöber nanoparticles: A DLS and ζ-potential study. J. Sol Gel Sci. Technol. 2015, 73, 54–61. [Google Scholar] [CrossRef]
- Castaldo, R.; de Luna, M.S.; Siviello, C.; Gentile, G.; Lavorgna, M.; Amendola, E.; Cocca, M. On the acid-responsive release of benzotriazole from engineered mesoporous silica nanoparticles for corrosion protection of metal surfaces. J. Cult. Herit. 2020, in press. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed.-Nanotechnol. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Branda, F.; Malucelli, G.; Durante, M.; Piccolo, A.; Mazzei, P.; Costantini, A.; Silvestri, B.; Pennetta, M.; Bifulco, A. Silica treatments: A fire retardant strategy for hemp fabric/epoxy composites. Polymers 2016, 8, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, P.; Venezia, V.; Tescione, F.; Avossa, J.; Luciani, G.; Silvestri, B.; Costantini, A. Improving Interaction at Polymer–Filler Interface: The Efficacy of Wrinkle Texture. Nanomaterials 2020, 10, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cashin, V.B.; Eldridge, D.S.; Yu, A.; Zhao, D. Surface functionalization and manipulation of mesoporous silica adsorbents for improved removal of pollutants: A review. Environ. Sci. Water Res. Technol. 2018, 4, 110–128. [Google Scholar] [CrossRef]
- Tunc, Ö.; Tanacı, H.; Aksu, Z. Potential use of cotton plant wastes for the removal of Remazol Black B reactive dye. J. Hazard. Mater. 2009, 163, 187–198. [Google Scholar] [CrossRef]
- Radu, D.R.; Lai, C.Y.; Jeftinija, K.; Rowe, E.W.; Jeftinija, S.; Lin, V. A Polyamidoamine Dendrimer-Capped Mesoporous Silica Nanosphere-Based Gene Transfection Reagent. J. Am. Chem. Soc. 2004, 126, 13216–13217. [Google Scholar] [CrossRef]
- Qin, P.; Yang, Y.; Zhang, X.; Niu, J.; Yang, H.; Tian, S.; Zhu, J.; Lu, M. Highly Efficient, Rapid, and Simultaneous Removal of Cationic Dyes from Aqueous Solution Using Monodispersed Mesoporous Silica Nanoparticles as the Adsorbent. Nanomaterials 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Avolio, R.; Gentile, G.; Avella, M.; Capitani, D.; Errico, M.E. Synthesis and characterization of poly (methylmethacrylate)/silica nanocomposites: Study of the interphase by solid-state NMR and structure/properties relationships. J. Polym. Sci. Pol. Chem. 2010, 48, 5618–5629. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, V.; Rodriguez-Reinoso, J.P.; Rouquerol, F.; Sing, J. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Sotomayor, F.; Cychoszv, K.; Thommes, M. Characterization of Micro/Mesoporous Materials by Physisorption: Concepts and Case Studies. Acc. Mater. Surf. Res. 2018, 3, 34–50. [Google Scholar]
- Kareem, S.H.; Inaam, H.A.; Jalhoom, M.G. Synthesis and characterization of organic functionalized mesoporous silica and evaluate their adsorptive behavior for removal of methylene blue from aqueous solution. Am. J. Environ. Sci. 2014, 10, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Li, A.; Zhang, Q.; Zhang, G.; Chen, J.; Feif, Z.; Li, L. Adsorption of phenolic compounds from aqueous solutions by a water-compatible hypercrosslinked polymeric adsorbent. Chemosphere 2002, 47, 981–989. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Alrozi, R. Optimization of preparation conditions for mangosteen peel-based activated carbons for the removal of Remazol Brilliant Blue R using response surface methodology. Chem. Eng. J. 2010, 165, 883–890. [Google Scholar] [CrossRef]
- Zhong, Z.Y.; Yang, Q.; Li, X.M.; Luo, K.; Liu, Y.; Zeng, G.M. Preparation of peanut hull-based activated carbon by microwave-induced phosphoric acid activation and its application in Remazol Brilliant Blue R adsorption. Ind. Crops Prod. 2012, 37, 178–185. [Google Scholar] [CrossRef]
- Silva, T.L.; Ronix, A.; Pezoti, O.; Souza, L.S.; Leandro, P.K.; Bedin, K.C.; Beltramea, K.K.; Cazetta, A.L.; Almeida, V.C. Mesoporous activated carbon from industrial laundry sewage sludge: Adsorption studies of reactive dye Remazol Brilliant Blue R. Chem. Eng. J. 2016, 303, 467–476. [Google Scholar] [CrossRef]



| Sample | BET SSA (m2/g) | Total Pore Volume (cm3/g) | Micropore Fraction (%) |
|---|---|---|---|
| XDV | 1928 ± 6 | 1.36 | 31 |
| XDV-5MSN | 1824 ± 23 | 1.14 | 39 |
| XDV-15MSN | 1644 ± 20 | 1.08 | 36 |
| Sample | n | KF [(mg/g)/(mg/L)1/n] | R2 |
|---|---|---|---|
| XDV | 3.6 | 28 | 0.99 |
| XDV-5MSN | 2.6 | 24.5 | 0.99 |
| XDV-15MSN | 3.3 | 30.1 | 0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerritore, M.; Castaldo, R.; Silvestri, B.; Avolio, R.; Cocca, M.; Errico, M.E.; Avella, M.; Gentile, G.; Ambrogi, V. Hyper-Crosslinked Polymer Nanocomposites Containing Mesoporous Silica Nanoparticles with Enhanced Adsorption Towards Polar Dyes. Polymers 2020, 12, 1388. https://doi.org/10.3390/polym12061388
Guerritore M, Castaldo R, Silvestri B, Avolio R, Cocca M, Errico ME, Avella M, Gentile G, Ambrogi V. Hyper-Crosslinked Polymer Nanocomposites Containing Mesoporous Silica Nanoparticles with Enhanced Adsorption Towards Polar Dyes. Polymers. 2020; 12(6):1388. https://doi.org/10.3390/polym12061388
Chicago/Turabian StyleGuerritore, Marco, Rachele Castaldo, Brigida Silvestri, Roberto Avolio, Mariacristina Cocca, Maria Emanuela Errico, Maurizio Avella, Gennaro Gentile, and Veronica Ambrogi. 2020. "Hyper-Crosslinked Polymer Nanocomposites Containing Mesoporous Silica Nanoparticles with Enhanced Adsorption Towards Polar Dyes" Polymers 12, no. 6: 1388. https://doi.org/10.3390/polym12061388
APA StyleGuerritore, M., Castaldo, R., Silvestri, B., Avolio, R., Cocca, M., Errico, M. E., Avella, M., Gentile, G., & Ambrogi, V. (2020). Hyper-Crosslinked Polymer Nanocomposites Containing Mesoporous Silica Nanoparticles with Enhanced Adsorption Towards Polar Dyes. Polymers, 12(6), 1388. https://doi.org/10.3390/polym12061388












