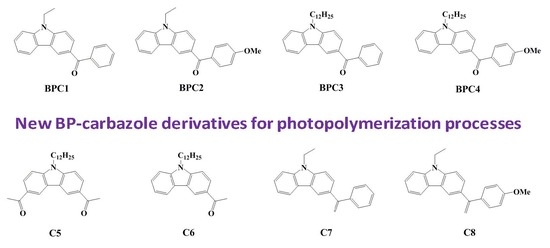
Monocomponent Photoinitiators based on Benzophenone-Carbazole Structure for LED Photoinitiating Systems and Application on 3D Printing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. UV-Vis Absorption Spectra
2.3. Computational Procedure
2.4. Cationic Photopolymerization and Free Radical Photopolymerization
2.5. 3D Printing Experiments
2.6. Fluorescence Experiments
2.7. Electron Spin Resonance - Spin Trapping (ESR-ST)
3. Results
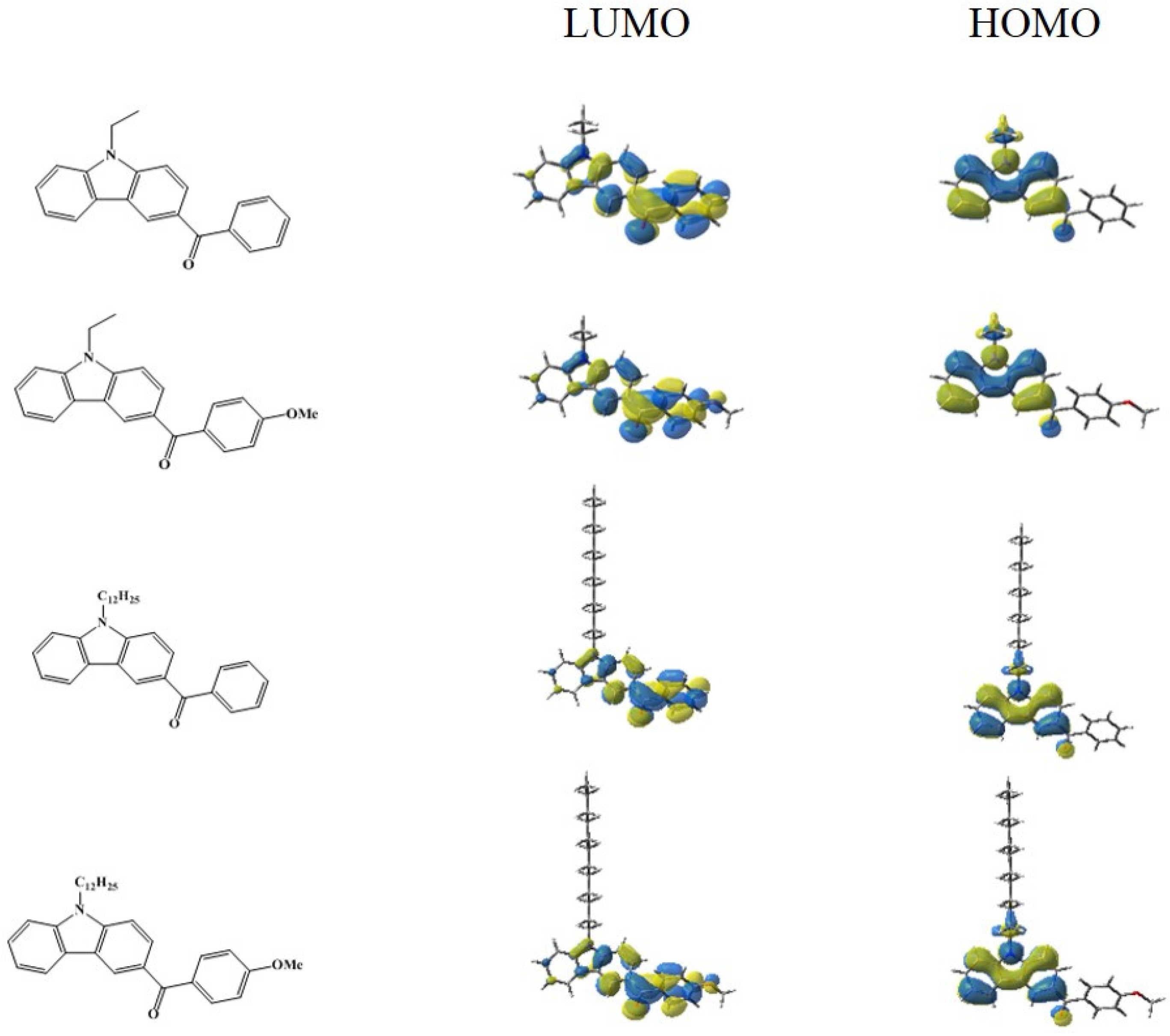
3.1. Light Absorption Properties and Molecular Orbital Calculations on the Optimized Geometries of the Carbazole Derivatives
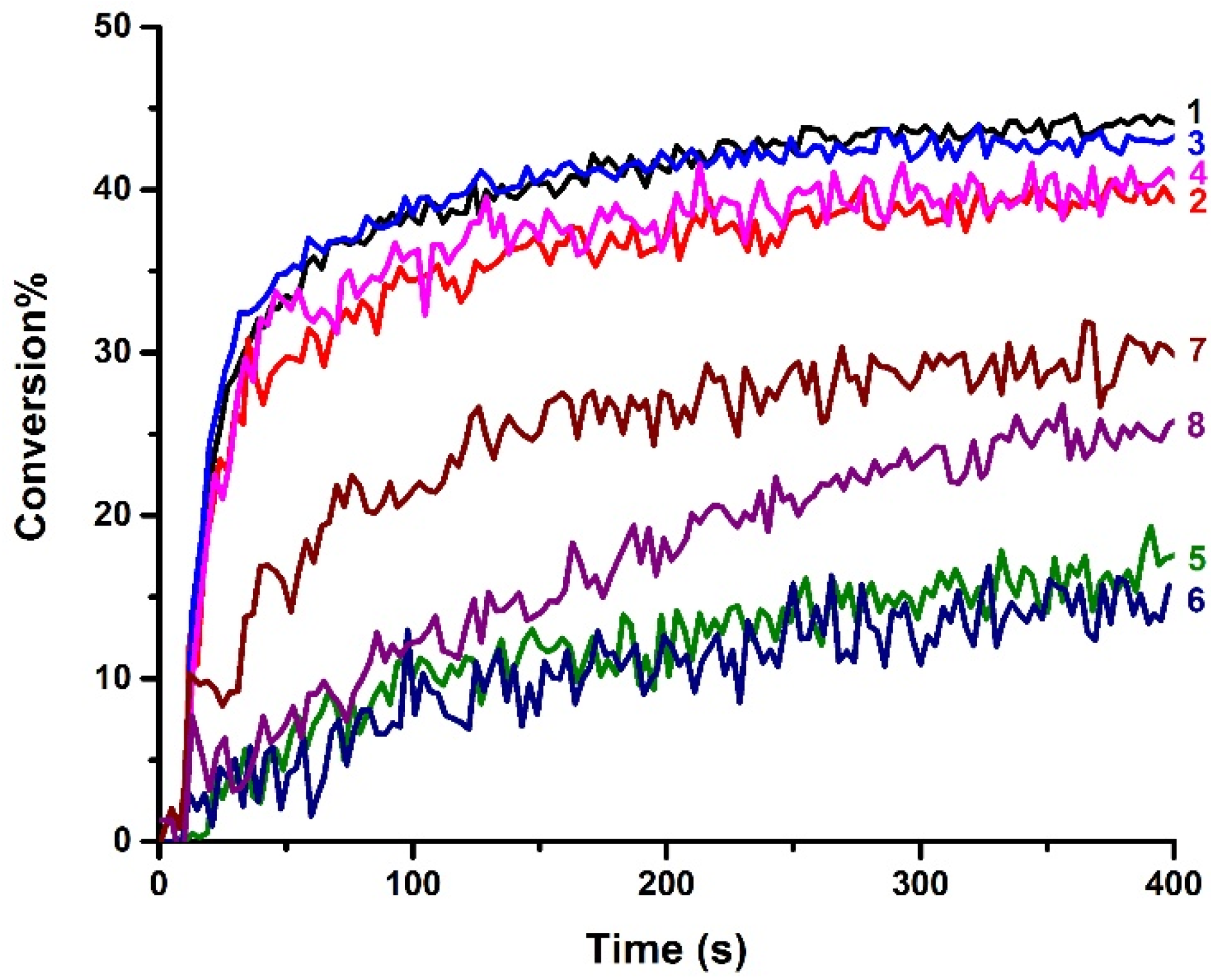
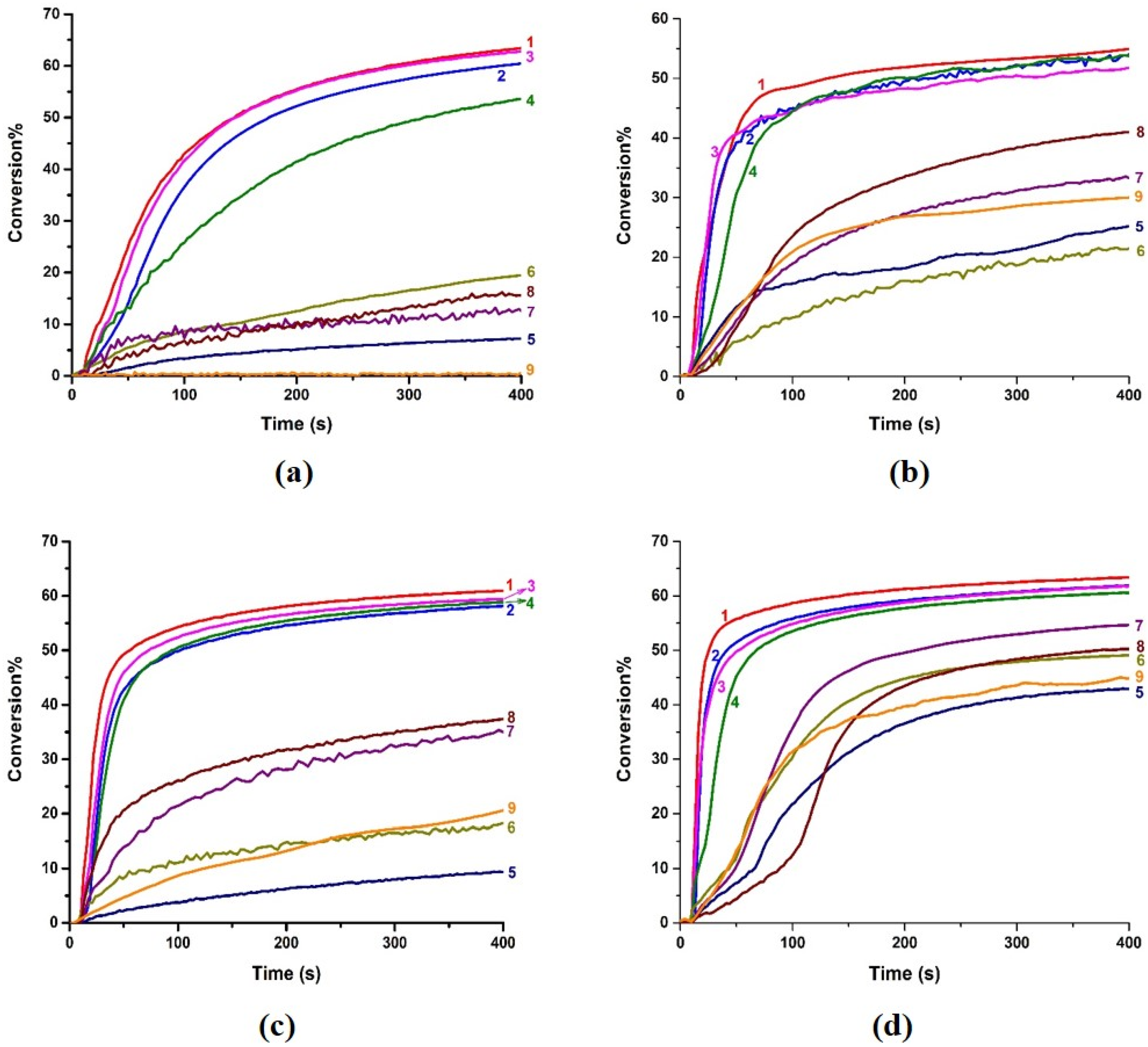
3.2. Cationic Photopolymerization and Free Radical Photopolymerization
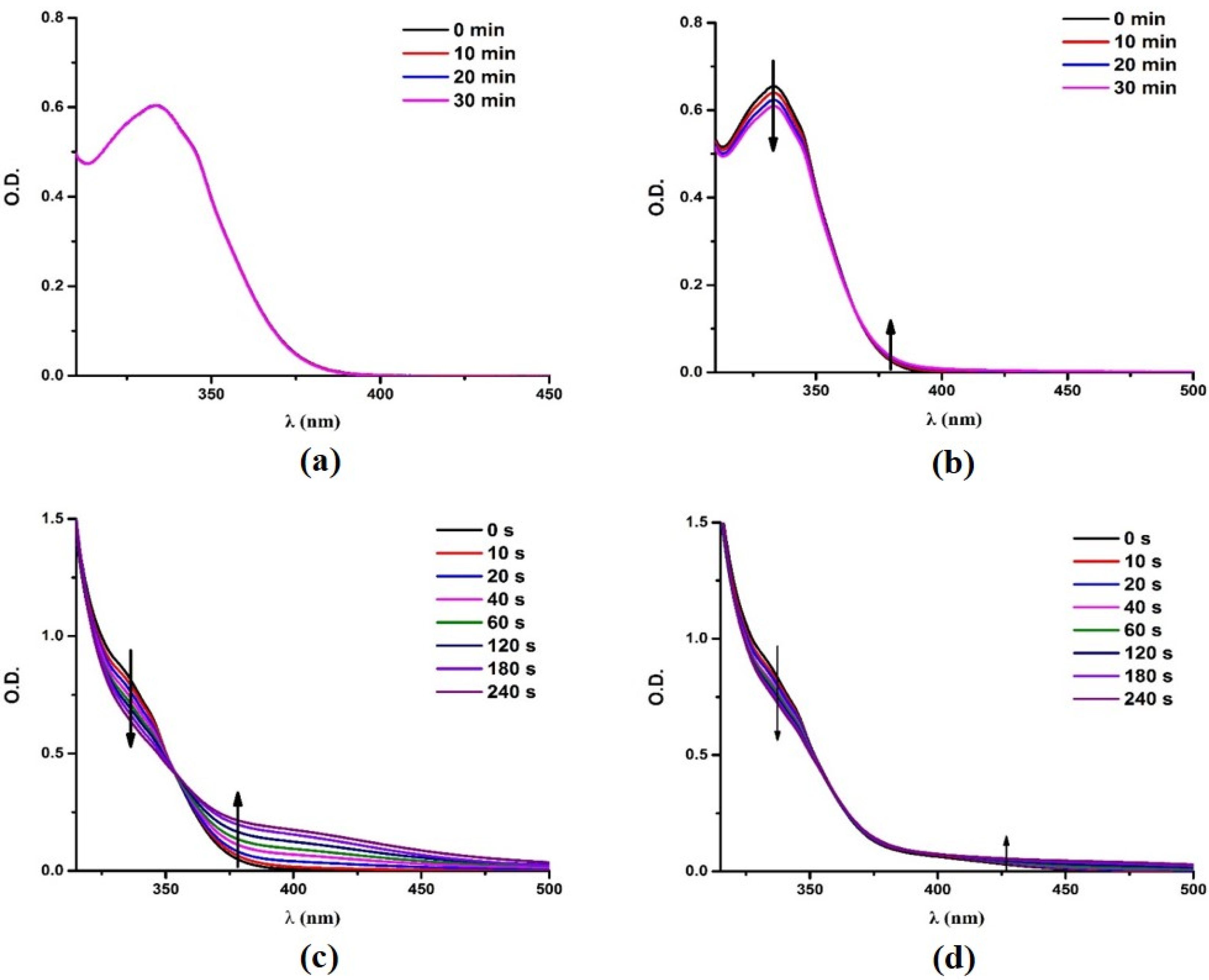
3.3. Steady State Photolysis
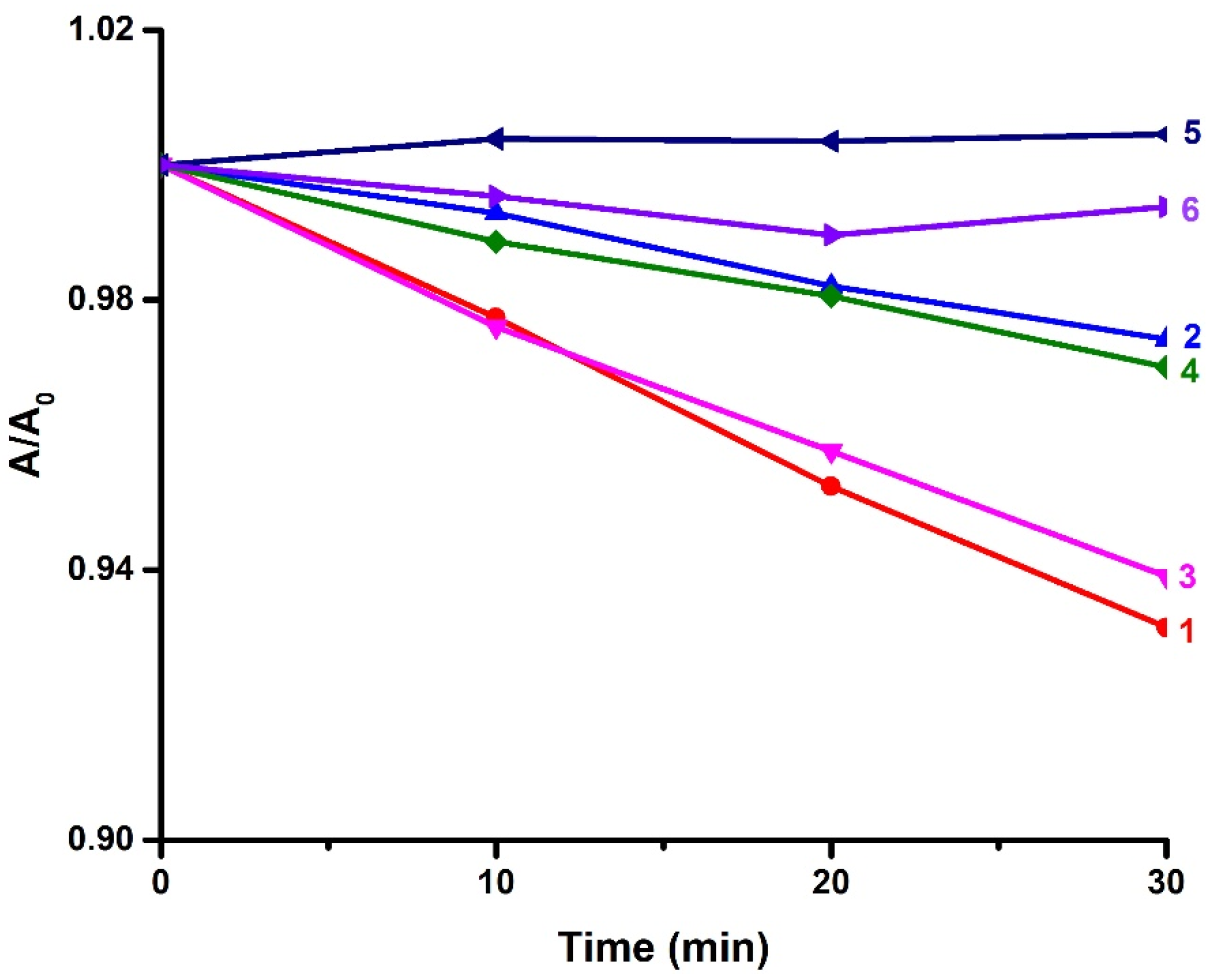
3.4. Fluorescence Quenching Experiments
4. Discussion
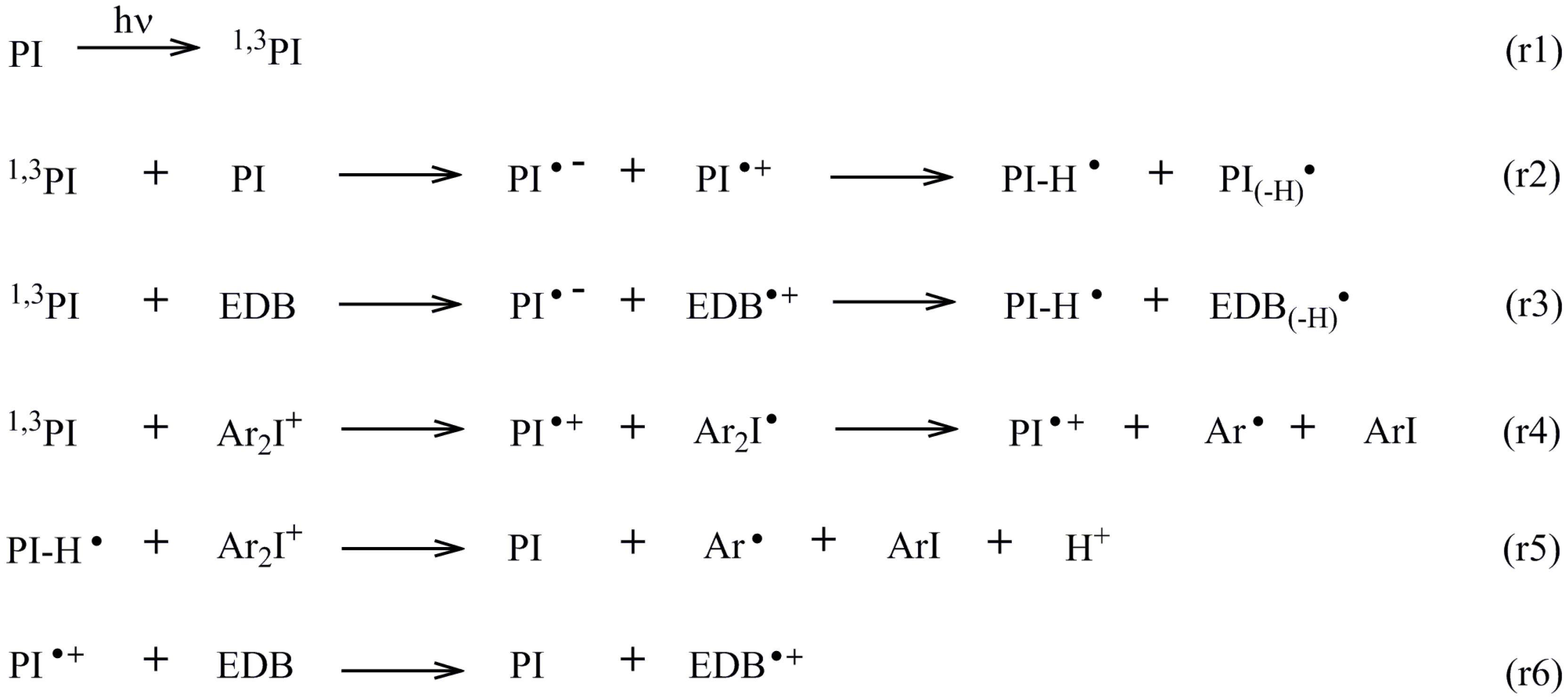
4.1. Proposed Chemical Mechanisms
4.2. Structure/Reactivity/Efficiency Relationship
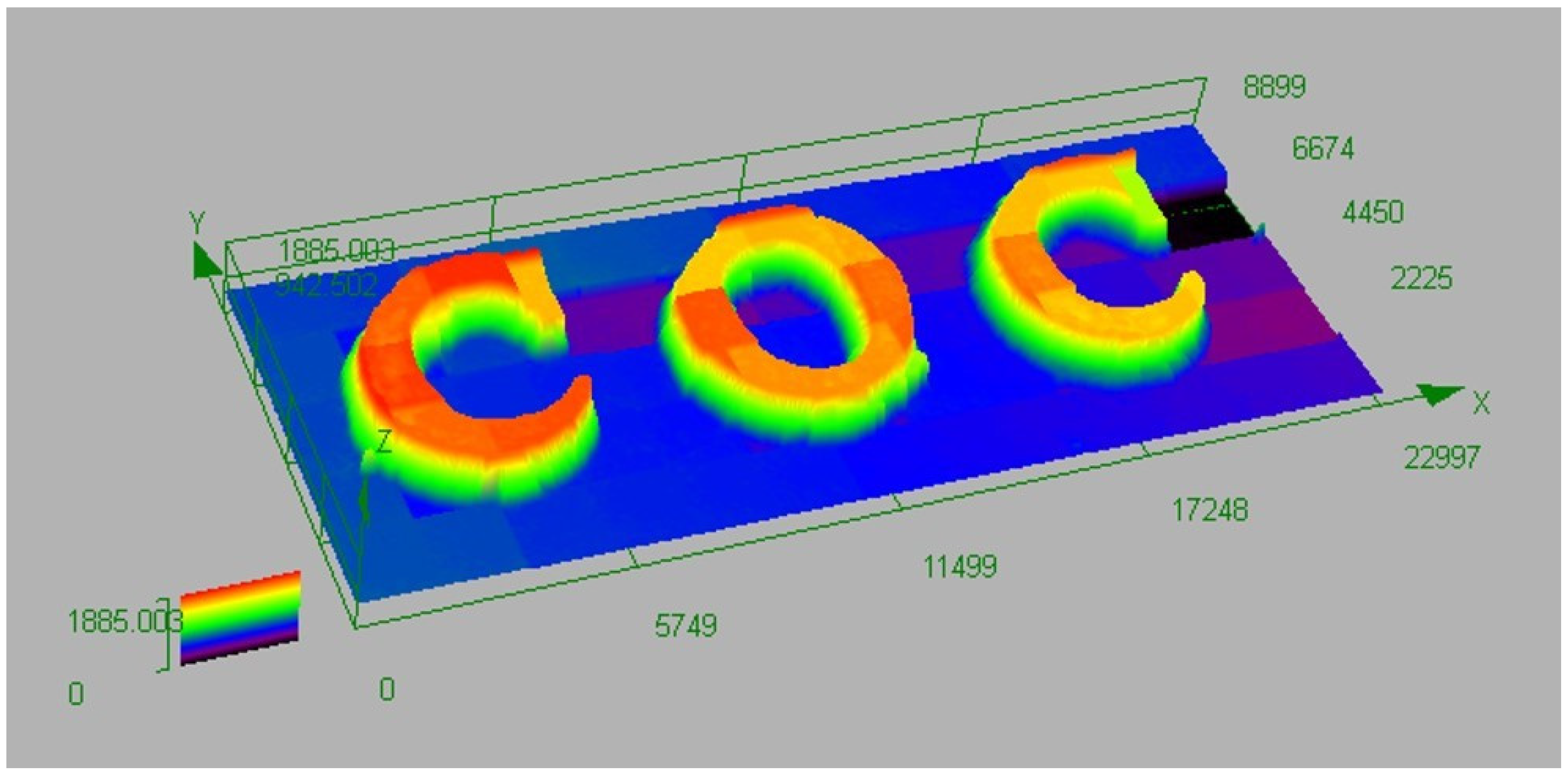
4.3. 3D Printing Experiment Based on BPC1/EDB/Iod System
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tehfe, M.-A.; Lalevée, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P. A Breakthrough toward Long Wavelength Cationic Photopolymerization: Initiating Systems Based on Violanthrone Derivatives and Silyl Radicals. Macromolecules 2011, 44, 8374–8379. [Google Scholar] [CrossRef]
- Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Organic Electronics: An El Dorado in the Quest of New Photocatalysts for Polymerization Reactions. Acc. Chem. Res. 2016, 49, 1980–1989. [Google Scholar] [CrossRef] [PubMed]
- Ganster, B.; Fischer, U.K.; Moszner, N.; Liska, R. New Photocleavable Structures, 4. Macromol. Rapid Commun. 2008, 29, 57–62. [Google Scholar] [CrossRef]
- Hikmet, R.A.M. Piezoelectric networks obtained by photopolymerization of liquid crystal molecules. Macromolecules 1992, 25, 5759–5764. [Google Scholar] [CrossRef]
- Cabral, J.T.; Hudson, S.D.; Harrison, C.; Douglas, J.F. Frontal Photopolymerization for Microfluidic Applications. Langmuir 2004, 20, 10020–10029. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Y.; Nie, J.; He, Y. UV-cured organic–inorganic hybrid moisture barrier materials based on polybutadiene dimethacrylate. J. Coat. Technol. Res. 2019, 16, 429–437. [Google Scholar] [CrossRef]
- Zhang, J.; Lalevée, J.; Mou, X.; Morlet-Savary, F.; Graff, B.; Xiao, P. N-Phenylglycine as a Versatile Photoinitiator under Near-UV LED. Macromolecules 2018, 51, 3767–3773. [Google Scholar] [CrossRef]
- Sun, K.; Xu, Y.; Dumur, F.; Morlet-Savary, F.; Chen, H.; Dietlin, C.; Graff, B.; Lalevée, J.; Xiao, P. In silico rational design by molecular modeling of new ketones as photoinitiators in three-component photoinitiating systems: application in 3D printing. Polymer Chem. 2020, 11, 2230–2242. [Google Scholar] [CrossRef]
- Schmitz, C.; Pang, Y.; Gülz, A.; Gläser, M.; Horst, J.; Jäger, M.; Strehmel, B. New High-Power LEDs Open Photochemistry for Near-Infrared-Sensitized Radical and Cationic Photopolymerization. Angew. Chem. Int. Ed. 2019, 58, 4400–4404. [Google Scholar] [CrossRef]
- Abdallah, M.; Bui, T.-T.; Goubard, F.; Theodosopoulou, D.; Dumur, F.; Hijazi, A.; Fouassier, J.-P.; Lalevée, J. Phenothiazine derivatives as photoredox catalysts for cationic and radical photosensitive resins for 3D printing technology and photocomposite synthesis. Polym. Chem. 2019, 10, 6145–6156. [Google Scholar] [CrossRef]
- Zuo, X.; Morlet-Savary, F.; Graff, B.; Blanchard, N.; Goddard, J.-P.; Lalevée, J. Fluorescent Brighteners as Visible LED-Light Sensitive Photoinitiators for Free Radical Photopolymerizations. Macromol. Rapid Commun. 2016, 37, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Shi, Y.; Xue, T.; Wang, T. Synthesis and electrochemical, linear and third-order nonlinear optical properties of ferrocene-based D-π-A dyes as novel photoredox catalysts in photopolymerization under visible LED irradiations. Dyes Pigment. 2019, 166, 140–148. [Google Scholar] [CrossRef]
- Layani, M.; Wang, X.; Magdassi, S. Novel Materials for 3D Printing by Photopolymerization. Adv. Mater. 2018, 30, 1706344. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jambou, C.; Sun, K.; Lalevée, J.; Simon-Masseron, A.; Xiao, P. Effect of Zeolite Fillers on the Photopolymerization Kinetics for Photocomposites and Lithography. ACS Appl. Polym. Mater. 2019, 1, 2854–2861. [Google Scholar] [CrossRef]
- Herzberger, J.; Sirrine, J.M.; Williams, C.B.; Long, T.E. Polymer Design for 3D Printing Elastomers: Recent Advances in Structure, Properties, and Printing. Progress Polym. Sci. 2019, 97, 101144. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef] [Green Version]
- Garra, P.; Dumur, F.; Gigmes, D.; Al Mousawi, A.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lalevée, J. Copper (Photo)redox Catalyst for Radical Photopolymerization in Shadowed Areas and Access to Thick and Filled Samples. Macromolecules 2017, 50, 3761–3771. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced Electron Transfer Reactions for Macromolecular Syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Redox two-component initiated free radical and cationic polymerizations: Concepts, reactions and applications. Progress Polym. Sci. 2019, 94, 33–56. [Google Scholar] [CrossRef]
- Han, W.; Fu, H.; Xue, T.; Liu, T.; Wang, Y.; Wang, T. Facilely prepared blue-green light sensitive curcuminoids with excellent bleaching properties as high performance photosensitizers in cationic and free radical photopolymerization. Polym. Chem. 2018, 9, 1787–1798. [Google Scholar] [CrossRef]
- Jia, X.; Han, W.; Xue, T.; Zhao, D.; Li, X.; Nie, J.; Wang, T. Diphenyl sulfone-based A–π-D–π-A dyes as efficient initiators for one-photon and two-photon initiated polymerization. Polym. Chem. 2019, 10, 2152–2161. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Push–pull (thio)barbituric acid derivatives in dye photosensitized radical and cationic polymerization reactions under 457/473 nm laser beams or blue LEDs. Polym. Chem. 2013, 4, 3866–3875. [Google Scholar] [CrossRef]
- Koyuncu, S.; Hu, P.; Li, Z.; Liu, R.; Bilgili, H.; Yagci, Y. Fluorene–Carbazole-Based Porous Polymers by Photoinduced Electron Transfer Reactions. Macromolecules 2020, 53, 1645–1651. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on carbazole-based photoinitiators of polymerization. Eur. Polym. J. 2020, 125, 109503. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Carbazole Scaffold Based Photoinitiator/Photoredox Catalysts: Toward New High Performance Photoinitiating Systems and Application in LED Projector 3D Printing Resins. Macromolecules 2017, 50, 2747–2758. [Google Scholar] [CrossRef]
- Zhou, J.; Allonas, X.; Ibrahim, A.; Liu, X. Progress in the development of polymeric and multifunctional photoinitiators. Progress Polym. Sci. 2019, 99, 101165. [Google Scholar] [CrossRef]
- Yang, J.; Xu, F.; Shi, S.; Nie, J. Influence of structure of benzodioxole derivatives on photoinitiation efficiency of benzophenone. Photochem. Photobiol. Sci. 2012, 11, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Allushi, A.; Kutahya, C.; Aydogan, C.; Kreutzer, J.; Yilmaz, G.; Yagci, Y. Conventional Type II photoinitiators as activators for photoinduced metal-free atom transfer radical polymerization. Polym. Chem. 2017, 8, 1972–1977. [Google Scholar] [CrossRef]
- Wang, K.; Yang, K.; Yu, Q. Novel polymeric photoinitiators with side-chain benzophenone: Facile synthesis and photopolymerization properties without coinitiator. Progress Organ. Coat. 2014, 77, 1929–1934. [Google Scholar] [CrossRef]
- Karaca Balta, D.; Karahan, Ö.; Avci, D.; Arsu, N. Synthesis, photophysical and photochemical studies of benzophenone based novel monomeric and polymeric photoinitiators. Progress Organ. Coat. 2015, 78, 200–207. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Gigmes, D.; Graff, B.; Fouassier, J.P.; Lalevée, J. New functionalized aromatic ketones as photoinitiating systems for near visible and visible light induced polymerizations. Polymer 2013, 54, 2857–2864. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Xiao, P.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. A benzophenone-naphthalimide derivative as versatile photoinitiator of polymerization under near UV and visible lights. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 445–451. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Variations on the Benzophenone Skeleton: Novel High Performance Blue Light Sensitive Photoinitiating Systems. Macromolecules 2013, 46, 7661–7667. [Google Scholar] [CrossRef]
- Tunc, D.; Yagci, Y. Thioxanthone-ethylcarbazole as a soluble visible light photoinitiator for free radical and free radical promoted cationic polymerizations. Polym. Chem. 2011, 2, 2557–2563. [Google Scholar] [CrossRef]
- Liu, S.; Brunel, D.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. A monocomponent bifunctional benzophenone–carbazole type II photoinitiator for LED photoinitiating systems. Polym. Chem. 2020. [Google Scholar] [CrossRef]
- Xiao, P.; Zhang, J.; Dumur, F.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Visible light sensitive photoinitiating systems: Recent progress in cationic and radical photopolymerization reactions under soft conditions. Progress Polymer Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Kim, D.; Scranton, A.B.; Stansbury, J.W. Analysis of association constant for ground-state dye-electron acceptor complex of photoinitiator systems and the association constant effect on the kinetics of visible-light-induced polymerizations. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1429–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousawi, A.A.; Dietlin, C.; Graff, B.; Morlet-Savary, F.; Toufaily, J.; Hamieh, T.; Fouassier, J.P.; Chachaj-Brekiesz, A.; Ortyl, J.; Lalevée, J. Meta-Terphenyl Derivative/Iodonium Salt/9H-Carbazole-9-ethanol Photoinitiating Systems for Free Radical Promoted Cationic Polymerization upon Visible Lights. Macromol. Chem. Phys. 2016, 217, 1955–1965. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Lalevée, J.; Morlet-Savary, F.; Lam, E.S.H.; Graff, B.; Liu, J.; Xing, F.; Xiao, P. 1,2-Diketones as photoinitiators of both cationic and free-radical photopolymerization under UV (392 nm) or Blue (455 nm) LEDs. J. Polym. Sci. 2020, 58, 792–802. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Lara, D.M.; Noirbent, G.; Dumur, F.; Toufaily, J.; Hamieh, T.; Bui, T.-T.; Goubard, F.; Graff, B.; Gigmes, D.; et al. Carbazole Derivatives with Thermally Activated Delayed Fluorescence Property as Photoinitiators/Photoredox Catalysts for LED 3D Printing Technology. Macromolecules 2017, 50, 4913–4926. [Google Scholar] [CrossRef]
- Lin, J. Kinetics of enhancement for corneal cross-linking: proposed model for a two-initiator system. Ophthalmol. Res. 2019, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, M.; Hijazi, A.; Lin, J.; Graff, B.; Dumur, F.; Lalevée, J. Coumarin Derivatives as Photoinitiators in Photo-oxidation and Photo-reduction Processes and Kinetic Model for Simulations of the Associated Polymerization Profiles. ACS Appl. Polym. Mater. 2020, in press. [Google Scholar] [CrossRef]




| PI | λmax/nm | εmax/M−1cm−1 | ε365 nm/M−1cm−1 |
|---|---|---|---|
| BPC1 | 334 | 13,910 | 3270 |
| BPC2 | 325 | 13,900 | 2210 |
| BPC3 | 334 | 13,350 | 3460 |
| BPC4 | 325 | 12,400 | 2170 |
| C5 | 332 | 8590 | 70 |
| C6 | 318 | 7880 | 130 |
| C7 | 339 | 3320 | 500 |
| C8 | 337 | 3540 | 540 |
| PISs | EPOX/% | TMPTA/% | |||
|---|---|---|---|---|---|
| PI/Iod | PI | PI/EDB | PI/Iod | PI/EDB/Iod | |
| BPC1 | 44 | 64 | 55 | 61 | 63 |
| BPC2 | 40 | 60 | 54 | 58 | 62 |
| BPC3 | 43 | 63 | 52 | 59 | 62 |
| BPC4 | 41 | 54 | 54 | 59 | 61 |
| C5 | 18 | 7 | 25 | 9 | 43 |
| C6 | 16 | 20 | 21 | 18 | 49 |
| C7 | 29 | 12 | 33 | 35 | 55 |
| C8 | 25 | 16 | 41 | 37 | 50 |
| BP | — | 0 | 30 | 21 | 45 |
| BPC1 | BPC2 | BPC3 | BPC4 | |
|---|---|---|---|---|
| KSV (M−1) | 157 | 176 | 147 | 152 |
| Φet(s1) a | 0.76 | 0.78 | 0.75 | 0.76 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Chen, H.; Zhang, Y.; Sun, K.; Xu, Y.; Morlet-Savary, F.; Graff, B.; Noirbent, G.; Pigot, C.; Brunel, D.; et al. Monocomponent Photoinitiators based on Benzophenone-Carbazole Structure for LED Photoinitiating Systems and Application on 3D Printing. Polymers 2020, 12, 1394. https://doi.org/10.3390/polym12061394
Liu S, Chen H, Zhang Y, Sun K, Xu Y, Morlet-Savary F, Graff B, Noirbent G, Pigot C, Brunel D, et al. Monocomponent Photoinitiators based on Benzophenone-Carbazole Structure for LED Photoinitiating Systems and Application on 3D Printing. Polymers. 2020; 12(6):1394. https://doi.org/10.3390/polym12061394
Chicago/Turabian StyleLiu, Shaohui, Hong Chen, Yijun Zhang, Ke Sun, Yangyang Xu, Fabrice Morlet-Savary, Bernadette Graff, Guillaume Noirbent, Corentin Pigot, Damien Brunel, and et al. 2020. "Monocomponent Photoinitiators based on Benzophenone-Carbazole Structure for LED Photoinitiating Systems and Application on 3D Printing" Polymers 12, no. 6: 1394. https://doi.org/10.3390/polym12061394
APA StyleLiu, S., Chen, H., Zhang, Y., Sun, K., Xu, Y., Morlet-Savary, F., Graff, B., Noirbent, G., Pigot, C., Brunel, D., Nechab, M., Gigmes, D., Xiao, P., Dumur, F., & Lalevée, J. (2020). Monocomponent Photoinitiators based on Benzophenone-Carbazole Structure for LED Photoinitiating Systems and Application on 3D Printing. Polymers, 12(6), 1394. https://doi.org/10.3390/polym12061394