Carbonization of Polydopamine-Coating Layers on Boron Nitride for Thermal Conductivity Enhancement in Hybrid Polyvinyl Alcohol (PVA) Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
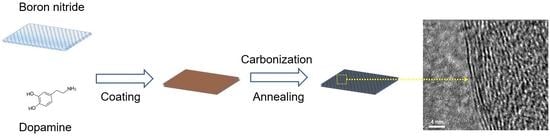
2.2. Preparation of BNPDA
2.3. Fabrication of BNPDA/PVA Composite Film
2.4. Characterization
2.4.1. Electron Microscopy
2.4.2. Raman Spectroscopy
2.4.3. X-Ray Photoelectron Spectroscopy (XPS)
2.4.4. X-Ray Diffraction (XRD)
2.4.5. UV–Vis Spectroscopy
2.4.6. Thermal Properties
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postma, A.; Yan, Y.; Wang, Y.; Zelikin, A.N.; Tjipto, E.; Caruso, F. Self-polymerization of dopamine as a versatile and robust technique to prepare polymer capsules. Chem. Mater. 2009, 21, 3042–3044. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Y.; Feng, J. Polydopamine as an efficient and robust platform to functionalize carbon fiber for high-performance polymer composites. ACS Appl. Mater. Interfaces 2014, 6, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Malollari, K.G.; Delparastan, P.; Sobek, C.; Vachhani, S.J.; Fink, T.D.; Zha, R.H.; Messersmith, P.B. Mechanical Enhancement of Bioinspired Polydopamine Nanocoatings. ACS Appl. Mater. Interfaces 2019, 11, 43599–43607. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Lin, M.-F.; Tan, E.J.; Lee, P.S. Green aqueous modification of fluoropolymers for energy storage applications. J. Mater. Chem. 2012, 22, 5951–5959. [Google Scholar] [CrossRef]
- Thakur, V.K.; Yan, J.; Lin, M.-F.; Zhi, C.; Golberg, D.; Bando, Y.; Sim, R.; Lee, P.S. Novel polymer nanocomposites from bioinspired green aqueous functionalization of BNNTs. Polym. Chem. 2012, 3, 962–969. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, K.; Luo, F.; Lu, M.; Xiao, F.; Du, X.; Zhang, S.; Liang, L.; Lu, M. Significantly enhanced thermal conductivity in polyvinyl alcohol composites enabled by dopamine modified graphene nanoplatelets. Compos. Part A 2019, 117, 134–143. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, Y.; Li, T.; Ma, P.; Zhang, S.; Du, M.; Chen, M.; Dong, W.; Ming, W. Highly thermal conductive and electrically insulating polymer composites based on polydopamine-coated copper nanowire. Compos. Sci. Technol. 2018, 164, 153–159. [Google Scholar] [CrossRef]
- Mondin, G.; Haft, M.; Wisser, F.M.; Leifert, A.; Mohamed-Noriega, N.; Dörfler, S.; Hampel, S.; Grothe, J.; Kaskel, S. Investigations of mussel-inspired polydopamine deposition on WC and Al2O3 particles: The influence of particle size and material. Mater. Chem. Phys. 2014, 148, 624–630. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, H.; Ma, P.; Bai, H.; Chen, M.; Dong, W.; Xie, Y.; Deshmukh, Y.S. Superior performance of artificial nacre based on graphene oxide nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 4215–4222. [Google Scholar] [CrossRef]
- Yu, X.; Fan, H.; Liu, Y.; Shi, Z.; Jin, Z. Characterization of carbonized polydopamine nanoparticles suggests ordered supramolecular structure of polydopamine. Langmuir 2014, 30, 5497–5505. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Chou, J.B.; Lee, K.; Lee, D.; Hong, S.H.; Zhao, R.; Lee, H.; Kim, S.G. Direct Insulation-to-Conduction Transformation of Adhesive Catecholamine for Simultaneous Increases of Electrical Conductivity and Mechanical Strength of CNT Fibers. Adv. Mater. 2015, 27, 3250–3255. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Aulin, Y.V.; Frazer, L.; Borguet, E.; Kakodkar, R.; Feser, J.; Chen, Y.; An, K.; Dikin, D.A.; Ren, F. Structure evolution and thermoelectric properties of carbonized polydopamine thin films. ACS Appl. Mater. Interfaces 2017, 9, 6655–6660. [Google Scholar] [CrossRef]
- Ma, T.; Gao, H.L.; Cong, H.P.; Yao, H.B.; Wu, L.; Yu, Z.Y.; Chen, S.M.; Yu, S.H. A Bioinspired Interface Design for Improving the Strength and Electrical Conductivity of Graphene-Based Fibers. Adv. Mater. 2018, 30, 1706435. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fan, S.; Li, Y.; Guo, Y.; Li, Y.; Ruan, K.; Zhang, S.; Zhang, J.; Kong, J.; Gu, J. Synchronously improved electromagnetic interference shielding and thermal conductivity for epoxy nanocomposites by constructing 3D copper nanowires/thermally annealed graphene aerogel framework. Compos. Part A 2020, 128, 105670. [Google Scholar] [CrossRef]
- Moore, A.L.; Shi, L. Emerging challenges and materials for thermal management of electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
- Shtein, M.; Nadiv, R.; Buzaglo, M.; Kahil, K.; Regev, O. Thermally conductive graphene-polymer composites: Size, percolation, and synergy effects. Chem. Mater. 2015, 27, 2100–2106. [Google Scholar] [CrossRef]
- Zhao, Y.; Nakamura, R.; Kamiya, K.; Nakanishi, S.; Hashimoto, K. Nitrogen-doped carbon nanomaterials as non-metal electrocatalysts for water oxidation. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Oh, H.; Kim, J. Fabrication of polymethyl methacrylate composites with silanized boron nitride by in-situ polymerization for high thermal conductivity. Compos. Sci. Technol. 2019, 172, 153–162. [Google Scholar] [CrossRef]
- Li, Y.Q.; Yu, T.; Yang, T.Y.; Zheng, L.X.; Liao, K. Bio-inspired nacre-like composite films based on graphene with superior mechanical, electrical, and biocompatible properties. Adv. Mater. 2012, 24, 3426–3431. [Google Scholar] [CrossRef]
- Shen, H.; Guo, J.; Wang, H.; Zhao, N.; Xu, J. Bioinspired modification of h-BN for high thermal conductive composite films with aligned structure. ACS Appl. Mater. Interfaces 2015, 7, 5701–5708. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, Z.; Yin, J. Boron nitride nanosheets: Large-scale exfoliation in methanesulfonic acid and their composites with polybenzimidazole. J. Mater. Chem. 2011, 21, 11371–11377. [Google Scholar] [CrossRef]
- Kong, J.; Yee, W.A.; Yang, L.; Wei, Y.; Phua, S.L.; Ong, H.G.; Ang, J.M.; Li, X.; Lu, X. Highly electrically conductive layered carbon derived from polydopamine and its functions in SnO 2-based lithium ion battery anodes. Chem. Commun. 2012, 48, 10316–10318. [Google Scholar] [CrossRef]
- Ai, K.; Liu, Y.; Ruan, C.; Lu, L.; Lu, G. Sp2 C-dominant N-doped carbon sub-micrometer spheres with a tunable size: A versatile platform for highly efficient oxygen-reduction catalysts. Adv. Mater. 2013, 25, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Raman spectroscopy of amorphous, nanostructured, diamond–like carbon, and nanodiamond. Philos. Trans. R. Soc. A 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Jan, R.; Habib, A.; Akram, M.A.; Khan, A.N. Uniaxial drawing of graphene-PVA nanocomposites: Improvement in mechanical characteristics via strain-induced exfoliation of graphene. Nanoscale Res. Lett. 2016, 11, 377. [Google Scholar] [CrossRef] [Green Version]
- Nomura, T.; Minami, E.; Kawamoto, H. Carbonization of cellulose cell wall evaluated with ultraviolet microscopy. RSC Adv. 2020, 10, 7460–7467. [Google Scholar] [CrossRef]
- Wang, X.; Wu, P. Highly Thermally conductive fluorinated graphene films with superior electrical insulation and mechanical flexibility. ACS Appl. Mater. Interfaces 2019, 11, 21946–21954. [Google Scholar] [CrossRef]
- Barani, Z.; Mohammadzadeh, A.; Geremew, A.; Huang, C.Y.; Coleman, D.; Mangolini, L.; Kargar, F.; Balandin, A.A. Thermal properties of the binary-filler hybrid composites with graphene and copper nanoparticles. Adv. Funct. Mater. 2020, 30, 1904008. [Google Scholar] [CrossRef]
- Luo, T.; Lloyd, J.R. Enhancement of thermal energy transport across graphene/graphite and polymer interfaces: A molecular dynamics study. Adv. Funct. Mater. 2012, 22, 2495–2502. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Yang, X.; He, G.; Yang, Z.; Li, J. The combustion synthesis of highly crystalline boron nitride nanosheets and their application in thermoconductive polymeric composites. CrystEngComm 2019, 21, 5461–5469. [Google Scholar] [CrossRef]
- Kim, K.; Wie, J.; Kim, J. Synergistic interaction of P and N co-doping EDTA with controllable active EDTA-cobalt sites as efficient electrocatalyst for oxygen reduction reaction. J. Ind. Eng. Chem. 2020, 83, 252–259. [Google Scholar] [CrossRef]







| Sample | Atomic Ratio (%) | |||
|---|---|---|---|---|
| O1s | B1s | C1s | N1s | |
| BNPDA | 7.15 | 33.45 | 24.33 | 35.07 |
| BNPDA-400 | 9.14 | 25.44 | 37.88 | 27.54 |
| BNPDA-800 | 6.26 | 7.46 | 76.33 | 9.95 |
| BNPDA-1000 | 5.06 | 7.7 | 78.55 | 8.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, J. Carbonization of Polydopamine-Coating Layers on Boron Nitride for Thermal Conductivity Enhancement in Hybrid Polyvinyl Alcohol (PVA) Composites. Polymers 2020, 12, 1410. https://doi.org/10.3390/polym12061410
Kim Y, Kim J. Carbonization of Polydopamine-Coating Layers on Boron Nitride for Thermal Conductivity Enhancement in Hybrid Polyvinyl Alcohol (PVA) Composites. Polymers. 2020; 12(6):1410. https://doi.org/10.3390/polym12061410
Chicago/Turabian StyleKim, Youjin, and Jooheon Kim. 2020. "Carbonization of Polydopamine-Coating Layers on Boron Nitride for Thermal Conductivity Enhancement in Hybrid Polyvinyl Alcohol (PVA) Composites" Polymers 12, no. 6: 1410. https://doi.org/10.3390/polym12061410
APA StyleKim, Y., & Kim, J. (2020). Carbonization of Polydopamine-Coating Layers on Boron Nitride for Thermal Conductivity Enhancement in Hybrid Polyvinyl Alcohol (PVA) Composites. Polymers, 12(6), 1410. https://doi.org/10.3390/polym12061410