l-Arginine Grafted Poly(Glycerol Sebacate) Materials: An Antimicrobial Material for Wound Dressing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
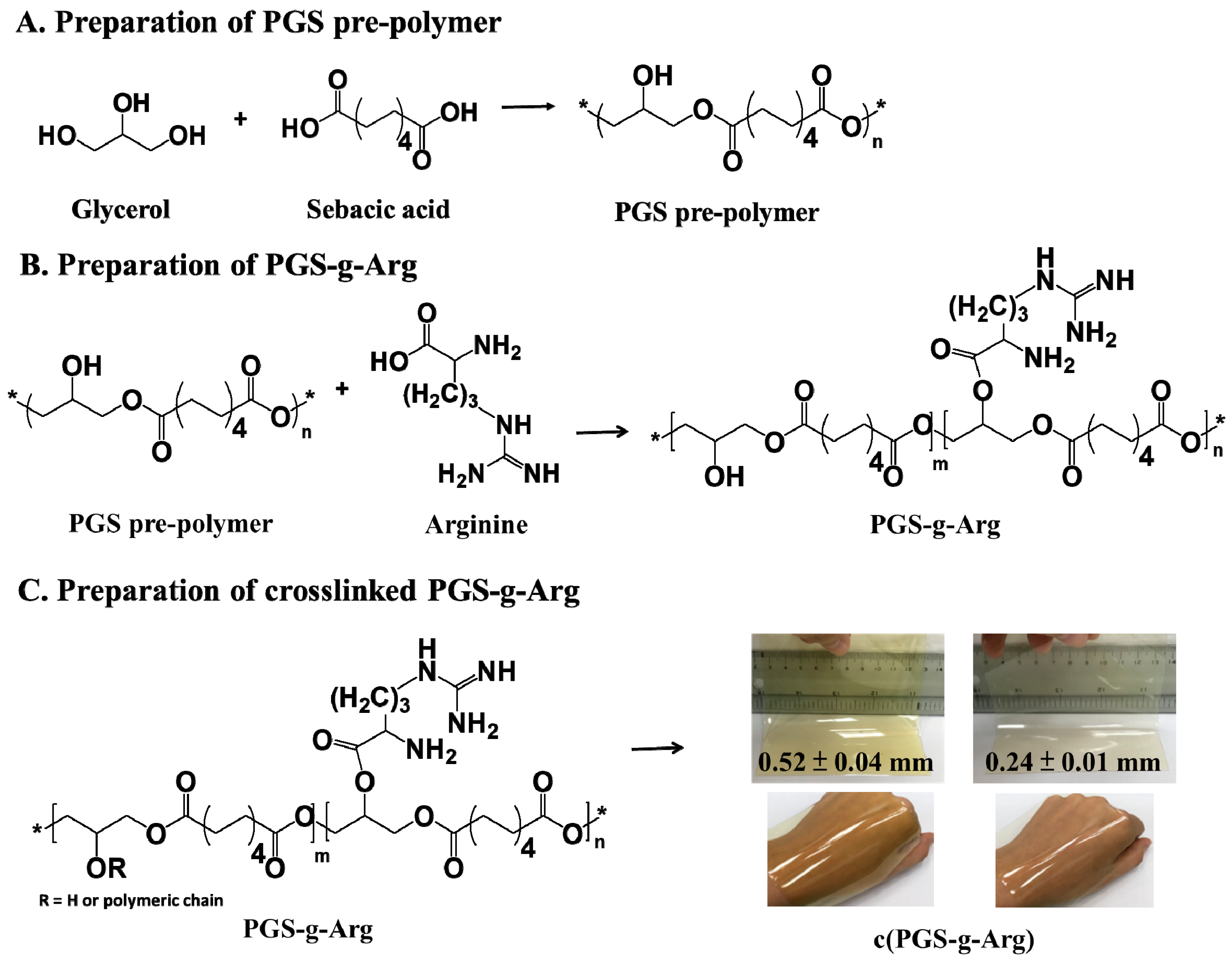
2.2. Preparation of Poly(Glycerol Sebacate) and l-arginine Modified Poly(Glycerol Sebacate) Prepolymer
2.3. Preparation of Poly(Glycerol Sebacate) and L-arginine Modified Poly(Glycerol Sebacate) Crosslinking Films
2.4. Mechanical Property Test
2.5. Swelling Ratio Test
2.6. Water Vapor Transmission Rate (WVTR) Test
2.6.1. Vial Method
2.6.2. Mocon Method
2.7. Antimicrobial Test
2.8. In-Vitro Cytocompatibility
3. Results and Discussion
3.1. Structural Analysis of Prepared Polymers
3.2. Analyses of Mechanical Property and Swelling Ratio of Crosslinking Films
3.3. Water Vapor Transmission Rate of Crosslinking Films and Commercial Products
3.4. Determination of Antimicrobial Function
3.5. Cytocompatibility Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bleasdale, B.; Finnegan, S.; Murray, K.; Kelly, S.; Percival, S.L. The use of silicone adhesives for scar reduction. Adv. Wound Care 2015, 4, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Quinn, K.J. Silicone gel in scar treatment. Burn. Incl. Therm. Inj. 1987, 13, S33–S40. [Google Scholar] [CrossRef]
- Gilman, T.H. Silicone sheet for treatment and prevention of hypertrophic scar: A new proposal for the mechanism of efficacy. Wound Repair Regen. 2003, 11, 235–236. [Google Scholar] [CrossRef]
- Jackson, B.A.; Shelton, A.J. Pilot study evaluating topical onion extract as treatment for postsurgical scars. Dermatol. Surg. 1999, 25, 267–269. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Borrego, B.; Juárez-Moreno, K.; García-García, M.; Morales, J.D.M.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicol. Lett. 2017, 276, 11–20. [Google Scholar] [CrossRef]
- Sambale, F.; Wagner, S.; Stahl, F.; Khaydarov, R.; Scheper, T.; Bahnemann, D. Investigations of the toxic effect of silver nanoparticles on mammalian cell lines. J. Nanomater. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef]
- Yu, B.; Kang, S.-Y.; Akthakul, A.; Ramadurai, N.; Pilkenton, M.; Patel, A.; Nashat, A.; Anderson, D.G.; Sakamoto, F.H.; Gilchrest, B.A. An elastic second skin. Nat. Mater. 2016, 15, 911–918. [Google Scholar] [CrossRef] [Green Version]
- Pérez, L.; Infante, M.R.; Pons, R.; Morán, C.; Vinardell, P.; Mitjans, M.; Pinazo, A. A synthetic alternative to natural lecithins with antimicrobial properties. Colloids Surf. B Biointerfaces 2004, 35, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Morán, C.; Clapés, P.; Comelles, F.; García, T.; Pérez, L.; Vinardell, P.; Mitjans, M.; Infante, M.R. Chemical structure/property relationship in single-chain arginine surfactants. Langmuir 2001, 17, 5071–5075. [Google Scholar] [CrossRef]
- Pinazo, A.; Manresa, M.; Marques, A.M.; Bustelo, M.; Espuny, M.J.; Pérez, L. Amino acid–based surfactants: New antimicrobial agents. Adv. Colloid Interface Sci. 2016, 228, 17–39. [Google Scholar] [CrossRef] [Green Version]
- FDA. Food Additive Permitted For Direct Addition To Food For Human Consumption. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=172.320 (accessed on 1 April 2020).
- ASTM, D.570-98. Standard Test Method for Water Absorption of Plastics; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Astm, S. Standard Test Methods for Water Vapor Transmission of Materials-ASTM E96-95; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Kawakami, H.; Yoshida, K.; Nishida, Y.; Kikuchi, Y.; Sato, Y. Antibacterial properties of metallic elements for alloying evaluated with application of JIS Z 2801: 2000. ISIJ Int. 2008, 48, 1299–1304. [Google Scholar] [CrossRef] [Green Version]
- Wallin, R.F.; Arscott, E. A practical guide to ISO 10993-5: Cytotoxicity. Med. Device Diagn. Ind. 1998, 20, 96–98. [Google Scholar]
- ASTM, F.895. Standard Test Method for Agar Diffusion Cell Culture Screening Cytotoxicity. In ASTM F; ASTM International: West Conshohocken, PA, USA, 2016; ASTM F895-11; pp. 895–2001. [Google Scholar]
- Lamke, L.-O.; Nilsson, G.; Reithner, H. The evaporative water loss from burns and the water-vapour permeability of grafts and artificial membranes used in the treatment of burns. Burns 1977, 3, 159–165. [Google Scholar] [CrossRef]
- Tandara, A.A.; Mustoe, T.A. The role of the epidermis in the control of scarring: Evidence for mechanism of action for silicone gel. J. Plast. Reconstr. Aesthetic Surg. 2008, 61, 1219–1225. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919. [Google Scholar]
- Andreu, D.; Merrifield, R.; Steiner, H.; Boman, H.G. N-terminal analogs of cecropin A: Synthesis, antibacterial activity, and conformational properties. Biochemistry 1985, 24, 1683–1688. [Google Scholar] [CrossRef]
- Hancock, R.E.; Chapple, D.S. Peptide antibiotics. Antimicrob. Agents Chemother. 1999, 43, 1317–1323. [Google Scholar] [CrossRef] [Green Version]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [Green Version]
- Hae Cho, C.A.; Liang, C.; Perera, J.; Liu, J.; Varnava, K.G.; Sarojini, V.; Cooney, R.P.; McGillivray, D.J.; Brimble, M.A.; Swift, S. Molecular weight and charge density effects of guanidinylated biodegradable polycarbonates on antimicrobial activity and selectivity. Biomacromolecules 2017, 19, 1389–1401. [Google Scholar] [CrossRef]
- Ushimaru, K.; Hamano, Y.; Katano, H. Antimicrobial activity of ε-poly-L-lysine after forming a water-insoluble complex with an anionic surfactant. Biomacromolecules 2017, 18, 1387–1392. [Google Scholar] [CrossRef]
- Żywicka, A.; Fijałkowski, K.; Junka, A.F.; Grzesiak, J.; El Fray, M. Modification of bacterial cellulose with quaternary ammonium compounds based on fatty acids and amino acids and the effect on antimicrobial activity. Biomacromolecules 2018, 19, 1528–1538. [Google Scholar] [CrossRef]
- Castelletto, V.; Barnes, R.H.; Karatzas, K.-A.; Edwards-Gayle, C.J.; Greco, F.; Hamley, I.W.; Rambo, R.; Seitsonen, J.; Ruokolainen, J. Arginine-containing surfactant-like peptides: Interaction with lipid membranes and antimicrobial activity. Biomacromolecules 2018, 19, 2782–2794. [Google Scholar] [CrossRef] [Green Version]
- Nichols, D. Biocides in Plastics; iSmithers Rapra Publishing: Milton Keynes, UK, 2005; Volume 15. [Google Scholar]
- Borenfreund, E.; Puerner, J.A. A simple quantitative procedure using monolayer cultures for cytotoxicity assays (HTD/NR-90). J. Tissue Cult. methods 1985, 9, 7–9. [Google Scholar] [CrossRef]






| Polymer Abbreviation | L-Arginine Content (mol%) | Molecular Weight | Solid Content in Ethanol (%) | Size of Crosslinked Films (L./W./T. in mm) |
|---|---|---|---|---|
| Mw/Mn/PDI | ||||
| c (PGS-g- 7% Arg) | 7 | 3060/1809/1.7 | 30 | 105/104/0.24 |
| 50 | 108/108/0.46 | |||
| c (PGS-g- 13% Arg) | 13 | 3668/2221/1.7 | 50 | 106/105/0.47 |
| c (PGS-g- 18% Arg) | 18 | 5001/2420/2.1 | 50 | 107/107/0.50 |
| Polymer Abbreviation | L-Arginine Content (mol%) | Mechanical Properties | ||
|---|---|---|---|---|
| Young’s Modulus (MPa) | UTS (MPa) | Elongation (%) | ||
| c (PGS-g- 7% Arg) | 7% | 1.79 ± 0.15 | 0.84 ± 0.08 | 84 ± 12 |
| c (PGS-g- 13% Arg) | 13% | 0.89 ± 0.06 | 0.59 ± 0.08 | 112 ± 11 |
| c (PGS-g- 18% Arg) | 18% | 0.37 ± 0.05 | 0.23 ± 0.02 | 129 ± 14 |
| Tested Sample | Thickness (mm) | Water Vapor Transmission Rate (g/m2/h) |
|---|---|---|
| c (PGS-g- 7% Arg) | 0.46 ± 0.05 | 6.1 ± 0.3 |
| 0.24 ± 0.01 | 10.3 ± 0.5 | |
| c (PGS-g- 13% Arg) | 0.47 ± 0.04 | 7.7 ± 0.3 |
| c (PGS-g- 18% Arg) | 0.50 ± 0.06 | 8.3 ± 0.4 |
| Cica-Care ® | 1.00 | 7.2 ± 1.0 |
| SavDerm ® | 0.50 | 7.8 ± 0.7 |
| Rystora ® | 0.30 | 12.2 ± 0.3 |
| 0% occlusive control of water | 0.00 | 174.1 ± 8.2 |
| Tested Sample (n = 3) | Reactivity Grades of Zone | Morphological Grades of Cytotoxicity |
|---|---|---|
| Positive control | 3, 3, 3 | 3, 3, 3 (54%, 50%, 48%) |
| Negative control | 0, 0, 0 | 0, 0, 0 (0%, 0%, 0%) |
| c (PGS-g- 7% Arg) | 1, 1, 1 | 1, 1, 1 (1%, 2%, 4%) |
| c (PGS-g- 13% Arg) | 1, 1, 1 | 1, 1, 1 (4%, 2%, 7%) |
| c (PGS-g- 18% Arg) | 1, 1, 1 | 1, 1, 1 (8%, 5%, 5%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-C.; Shih, T.-Y.; Hsieh, Y.-T.; Huang, J.-L.; Wang, J. l-Arginine Grafted Poly(Glycerol Sebacate) Materials: An Antimicrobial Material for Wound Dressing. Polymers 2020, 12, 1457. https://doi.org/10.3390/polym12071457
Wang C-C, Shih T-Y, Hsieh Y-T, Huang J-L, Wang J. l-Arginine Grafted Poly(Glycerol Sebacate) Materials: An Antimicrobial Material for Wound Dressing. Polymers. 2020; 12(7):1457. https://doi.org/10.3390/polym12071457
Chicago/Turabian StyleWang, Chia-Chun, Ting-Yu Shih, Yi-Ting Hsieh, Jie-Len Huang, and Jane Wang. 2020. "l-Arginine Grafted Poly(Glycerol Sebacate) Materials: An Antimicrobial Material for Wound Dressing" Polymers 12, no. 7: 1457. https://doi.org/10.3390/polym12071457
APA StyleWang, C. -C., Shih, T. -Y., Hsieh, Y. -T., Huang, J. -L., & Wang, J. (2020). l-Arginine Grafted Poly(Glycerol Sebacate) Materials: An Antimicrobial Material for Wound Dressing. Polymers, 12(7), 1457. https://doi.org/10.3390/polym12071457





