Applications of Long-Length Carbon Nano-Tube (L-CNT) as Conductive Materials in High Energy Density Pouch Type Lithium Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Characteristics of Long-Length CNT (L-CNT)
2.2. Analysis and Dispersion of L-CNT
2.3. Manufacture of Working Electrode
2.4. Electrochemical Property Tests of the Cells
3. Results and Discussion
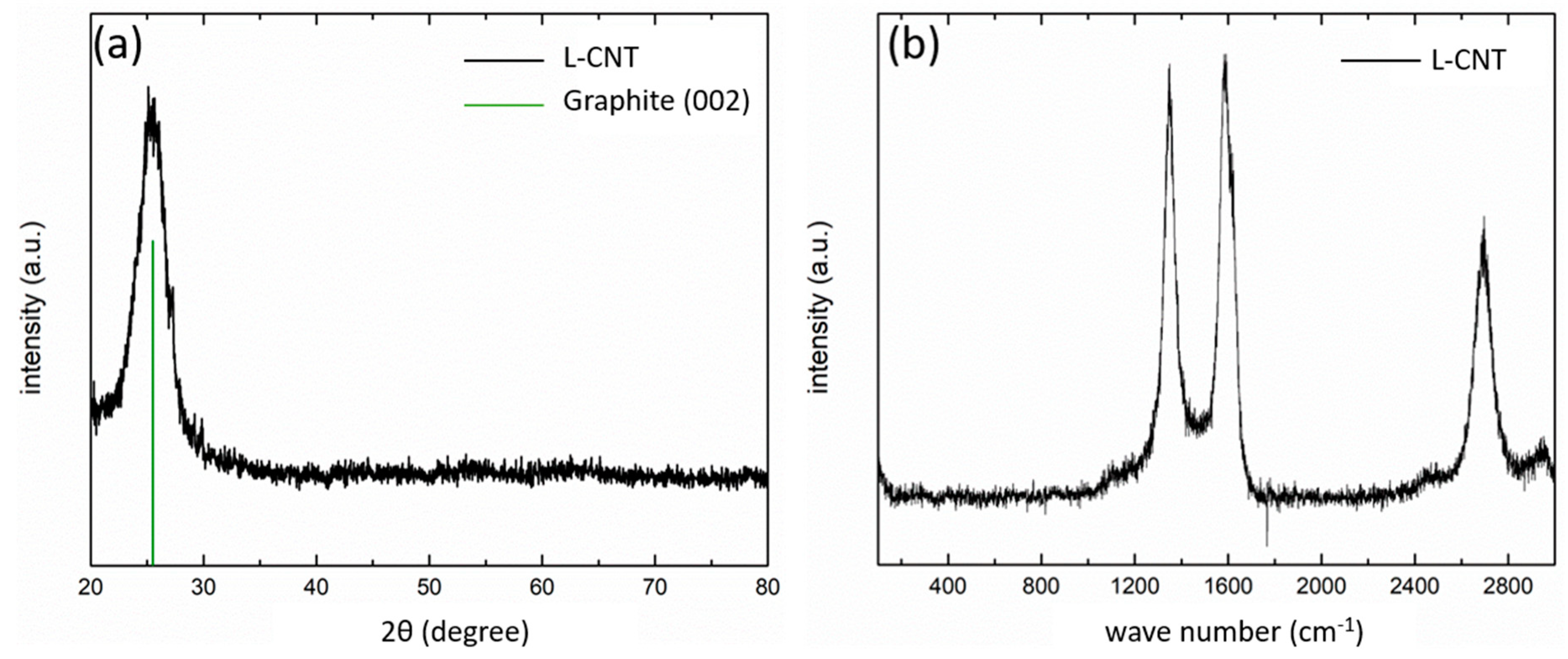
3.1. Morphology and Characteristics of L-CNT
3.2. Electrochemical Properties of L-CNT-Containing LNCM Electrodes and Cells
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.; Wang, Z.; Chen, L.; Huang, X. Research on advanced materials for Li-ion batteries. Adv. Mater. 2009, 21, 4593–4607. [Google Scholar] [CrossRef]
- Wallace, C.G.; Chen, J.; Mozer, A.J.; Forsyth, M.; MacFarlane, D.R.; Wang, C. Nanoelectrodes: Energy conversion and storage. Mater. Today 2009, 12, 20–27. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Rozier, P.; Tarascon, J.-M. Review—Li-Rich Layered Oxide Cathodes for Next-Generation Li-Ion Batteries: Chances and Challenges. J. Electrochem. Soc. 2015, 162, 2490–2499. [Google Scholar] [CrossRef]
- Myung, S.-T.; Amine, K.; Sun, Y.-K. Surface modification of cathode materials from nano- to microscale for rechargeable lithium-ion batteries. J. Mater. Chem. 2010, 20, 7074–7095. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, Y.; Amine, K.; Sun, Y.-K. Role of surface coating on cathode materials for lithium-ion batteries. J. Mater. Chem. 2010, 20, 7606–7612. [Google Scholar] [CrossRef]
- Rosina, K.J.; Jiang, M.; Zeng, D.; Salager, E.; Best, A.S.; Grey, C.P. Structure of aluminum fluoride coated Li[Li1/9Ni1/3Mn5/9]O2 cathodes for secondary lithium-ion batteries. J. Mater. Chem. 2012, 22, 20602–22610. [Google Scholar] [CrossRef]
- Mun, J.; Park, J.-H.; Choi, W.; Benayad, A.; Park, J.-H.; Lee, J.-M.; Doo, S.-G.; Oh, S.M. New dry carbon nanotube coating of over-lithiated layered oxide cathode for lithium ion batteries. J. Mater. Chem. 2014, 2, 19670–19677. [Google Scholar] [CrossRef] [Green Version]
- Anupriya, K.H.; Ranjusha, R.; Nair, S.V.; Balakrishnan, A.; Subramanian, K.R.V. Defining role of the surface and bulk contributions in camphoric carbon grafted lithium nickel manganese oxide powders fo lithium ion batteries. Ceram. Int. 2015, 41, 3269–3276. [Google Scholar] [CrossRef]
- Niketic, S.; Macneil, M.C.D.; Abu-Lebdeh, Y. Improving the performance of high voltage LiMn1.5Ni0.5O4 cathode material by carbon coating. J. Power Sources 2014, 271, 285–290. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef]
- Jiang, C.; Hosono, E.; Zhou, H. Nanomaterials for lithium ion batteries. Nano Today 2006, 1, 28–33. [Google Scholar] [CrossRef]
- Whittingham, M.S. Materials challenges facing electrical energy storage. MRS Bull. 2008, 33, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhang, Q.; Yu, Z.; Qu, M. The effect of different kinds of nano-carbon conductive additives in lithium ion batteries on the resistance and electrochemical behavior of the LiCoO2 composite cathodes. Solid State Ionics 2008, 179, 263–268. [Google Scholar]
- Panitz, J.-C.; Novak, P.; Haas, O. Raman microscopy applied to rechargeable lithium-ion cells—Steps towards in situ raman imaging with increased optical efficiency. Appl. Spectrosc. 2001, 55, 1131–1137. [Google Scholar] [CrossRef]
- Wilhelm, H.; Lelaurain, M.; McRae, E.; Humbert, B. Raman spectroscopic studies on well-defined carbonaceous materials of strong two-dimensional character. J. Appl. Phys. 1998, 84, 6552–6558. [Google Scholar] [CrossRef]
- Sheem, K.; Lee, Y.H.; Lim, H.S. High-density positive electrodes containing carbon nanotubes for use in Li-ion cells. J. Power Sources 2006, 158, 1425–1430. [Google Scholar] [CrossRef]
- Rubino, R.S.; Gan, H.; Takeuchi, E.S. A Study of Capacity Fade in Cylindrical and Prismatic Lithium-Ion Batteries. J. Electrochem. Soc. 2001, 148, A1029–A1033. [Google Scholar] [CrossRef]
- Shim, E.G.; Nam, T.H.; Kim, J.G.; Kim, H.S.; Moon, S.I. Effect of the concentration of diphenyloctyl phosphate as a flame-retarding additive on the electrochemical performance of lithium-ion batteries. Electrochim. Acta 2009, 54, 2276–2283. [Google Scholar] [CrossRef]
- Li, Y.H.; Lee, M.L.; Wang, F.M.; Yang, C.R.; Chua, P.J.; Pan, J.P. Electrochemical characterization of a branched oligomer as a high-temperature and long-cycle-life additive for lithium-ion batteries. Electrochim. Acta 2012, 85, 72–77. [Google Scholar] [CrossRef]
- Holzapfel, M.; Martinent, A.; Alloin, F.; Le Gorrec, B.; Yazami, R.; Montella, C. First lithiation and charge/discharge cycles of graphite materials, investigated by electrochemical impedance spectroscopy. J. Electroanal. Chem. 2003, 546, 41–50. [Google Scholar] [CrossRef]
- Piao, T.; Park, S.M.; Doh, C.H.; Moon, S.I. Intercalation of Lithium Ions into Graphite Electrodes Studied by AC Impedance Measurements. J. Electrochem. Soc. 1999, 146, 2794–2798. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.O.; Park, S.M. Intercalation Mechanism of Lithium Ions into Graphite Layers Studied by Nuclear Magnetic Resonance and Impedance Experiments. J. Electrochem. Soc. 2001, 148, 194–199. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.S.; Xu, K.; Jow, T.R. Charge and discharge characteristics of a commercial LiCoO2-based 18650 Li-ion battery. J. Power Sources 2006, 160, 1403–1409. [Google Scholar] [CrossRef]
- Ong, T.S.; Yang, H. Lithium intercalation into mechanically milled natural graphite: Electrochemical and kinetic characterization. J. Electrochem. Soc. 2002, 149, 1–8. [Google Scholar] [CrossRef]
- Zhuang, Q.C.; Wei, T.; Du, L.L.; Cui, Y.L.; Fang, L.; Sun, S.G. An Electrochemical Impedance Spectroscopic Study of the Electronic and Ionic Transport Properties of Spinel LiMn2O4. J. Phys. Chem. C 2011, 114, 8614–8621. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Shi, P. Electrochemical impedance study of lithium intercalation into MCMB electrode in a gel electrolyte. Electrochim. Acta 2004, 49, 1475–1482. [Google Scholar] [CrossRef]
- Tsai, H.L.; Hsieh, C.T.; Li, J.; Gandomi, Y. Enabling high rate charge and discharge capability, low internal resistance, and excellent cyceability for Li-ion batteries utilizing graphene additives. Electrochim. Acta 2018, 273, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.T.; Pai, C.T.; Chen, Y.F.; Yu, P.Y.; Juang, R.S. Electrochemical performance of lithium iron phosphate cathodes at various temperatures. Electrochim. Acta 2014, 115, 96–102. [Google Scholar] [CrossRef]
- Xiong, Z.; Yun, Y.S.; Jin, H.J. Applications of carbon nanotubes for lithium ion battery anodes. Material 2013, 6, 1138–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwon, H.; Hong, J.; Kim, H.; Seo, D.H.; Jeon, S.; Kang, K. Recent progress on flexible lithium rechargeable batteries. Energy Environ. Sci. 2014, 7, 538–551. [Google Scholar] [CrossRef]



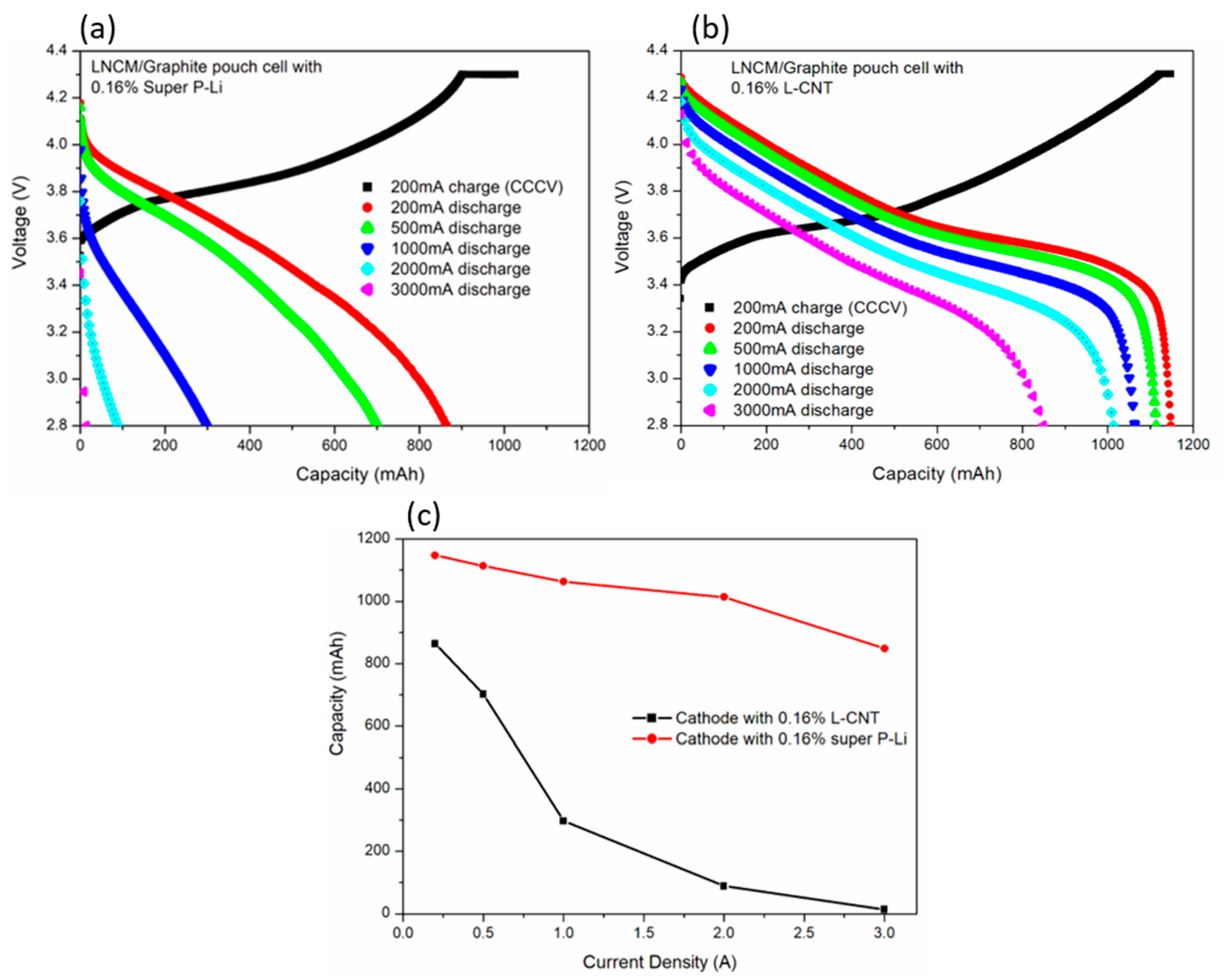



| Cell No. | 200 mA Discharge | 500 mA Discharge | 1000 mA Discharge | 2000 mA Discharge | 3000 mA Discharge | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Capacity (mAh, mAh/g) | Energy density (Wh/kg) (Wh/L) | Capacity (mAh, mAh/g) | Energy density (Wh/kg) (Wh/L) | Capacity (mAh, mAh/g) | Energy density (Wh/kg) (Wh/L) | Capacity (mAh, mAh/g) | Energy density (Wh/kg) (Wh/L) | Capacity (mAh, mAh/g) | Energy density (Wh/kg) (Wh/L) | |
| SP 016 | 864.1, 129.4 | 164.2 405.7 | 702.5, 105.2 | 132.0 326.2 | 297.2, 44.50 | 51.51 127.2 | 88.8, 13.30 | 14.41 35.59 | 13.9, 2.081 | 2.19 5.41 |
| LCNT 016 | 1147.7, 170.0 | 223.6 549.3 | 1113.3, 164.96 | 218.5 536.9 | 1063.3, 157.46 | 205.3 504.5 | 1013.5, 150.08 | 191.2 469.1 | 848.5, 125.65 | 157.0 385.7 |
| Cell (Electrode) | Re (Ω) | Rsei (Ω) | Rct (Ω) | Rcell (Ω) | DLi (cm2s−1) |
|---|---|---|---|---|---|
| LCNT-LNCM/Graphite | 0.035 | 0.015 | 0.061 | 0.11 | 6.25 × 10−7 |
| Super P-LNCM/Graphite | 0.067 | 0.083 | 0.42 | 0.57 | 2.86 × 10−9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, S.-H.; Chen, Y.-R.; Tsou, Y.-L.; Chang, T.-L.; Lai, H.-Z.; Lee, C.-Y. Applications of Long-Length Carbon Nano-Tube (L-CNT) as Conductive Materials in High Energy Density Pouch Type Lithium Ion Batteries. Polymers 2020, 12, 1471. https://doi.org/10.3390/polym12071471
Tsai S-H, Chen Y-R, Tsou Y-L, Chang T-L, Lai H-Z, Lee C-Y. Applications of Long-Length Carbon Nano-Tube (L-CNT) as Conductive Materials in High Energy Density Pouch Type Lithium Ion Batteries. Polymers. 2020; 12(7):1471. https://doi.org/10.3390/polym12071471
Chicago/Turabian StyleTsai, Shan-Ho, Ying-Ru Chen, Yi-Lin Tsou, Tseng-Lung Chang, Hong-Zheng Lai, and Chi-Young Lee. 2020. "Applications of Long-Length Carbon Nano-Tube (L-CNT) as Conductive Materials in High Energy Density Pouch Type Lithium Ion Batteries" Polymers 12, no. 7: 1471. https://doi.org/10.3390/polym12071471
APA StyleTsai, S.-H., Chen, Y.-R., Tsou, Y.-L., Chang, T.-L., Lai, H.-Z., & Lee, C.-Y. (2020). Applications of Long-Length Carbon Nano-Tube (L-CNT) as Conductive Materials in High Energy Density Pouch Type Lithium Ion Batteries. Polymers, 12(7), 1471. https://doi.org/10.3390/polym12071471





