Design and Preparation of New Multifunctional Hydrogels Based on Chitosan/Acrylic Polymers for Drug Delivery and Wound Dressing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Hydrogel Preparation
2.1.1. Chitosan Chemical Modification
2.1.2. Hydrogels Preparation
2.1.3. l-Arginine Coupling with the Hydrogels Network
2.2. Hydrogels Characterization
2.2.1. Structural and Morphological Analysis
2.2.2. Fluid Retention Study
2.2.3. Drug Release Study
2.2.4. In Vitro Degradation Study
2.2.5. In Vitro Cytocompatibility Study
2.2.6. Hemostatic Properties
3. Results and Discussions
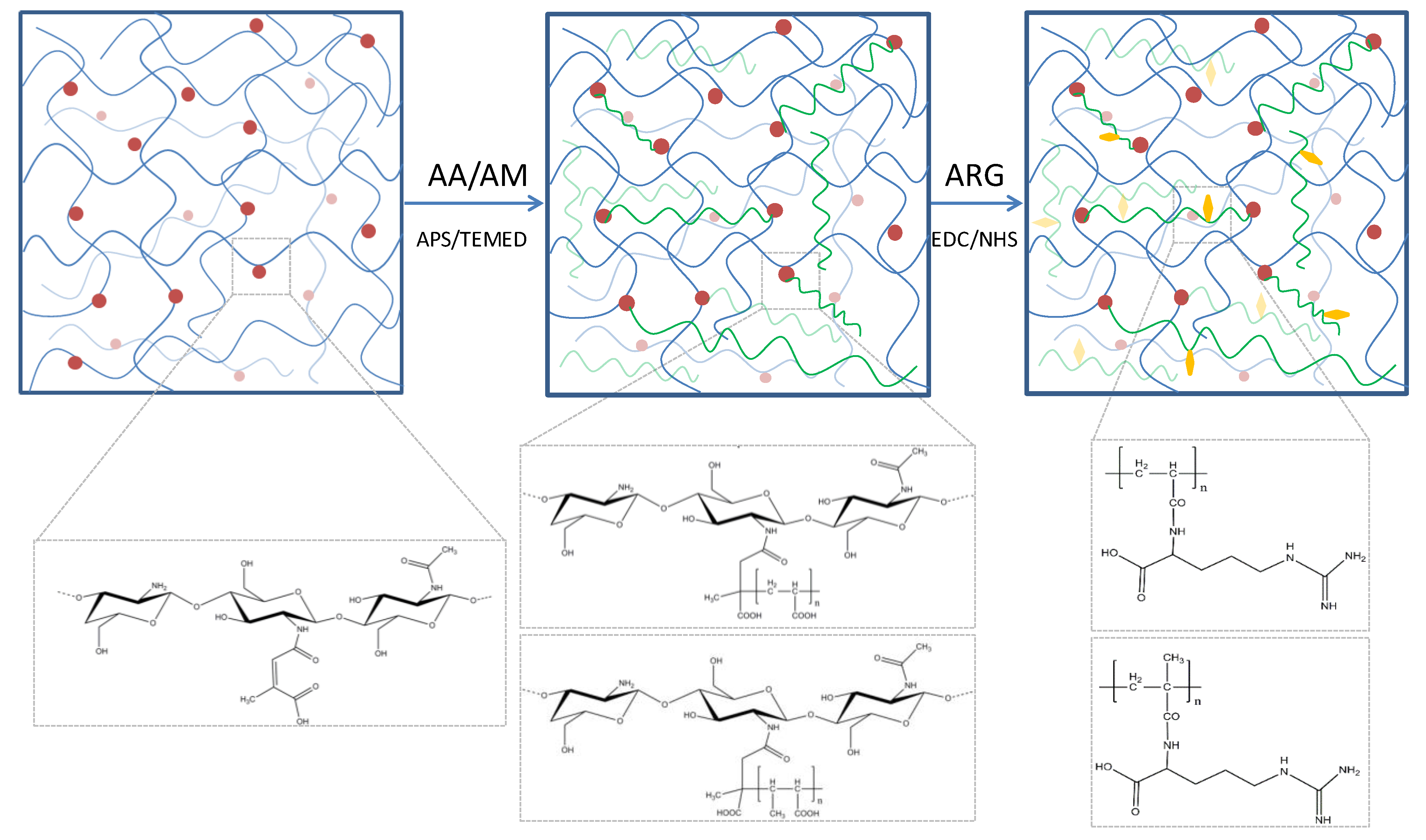
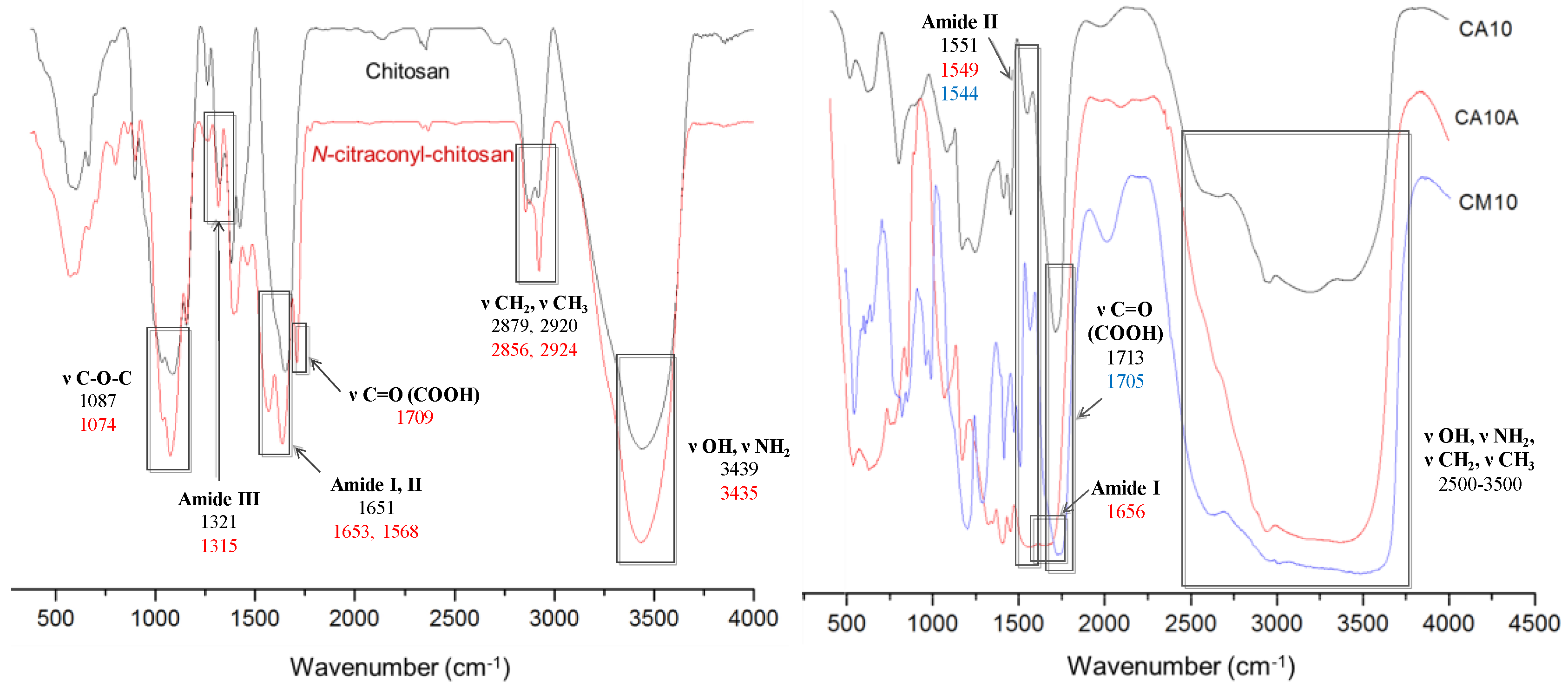
3.1. Hydrogels Structure
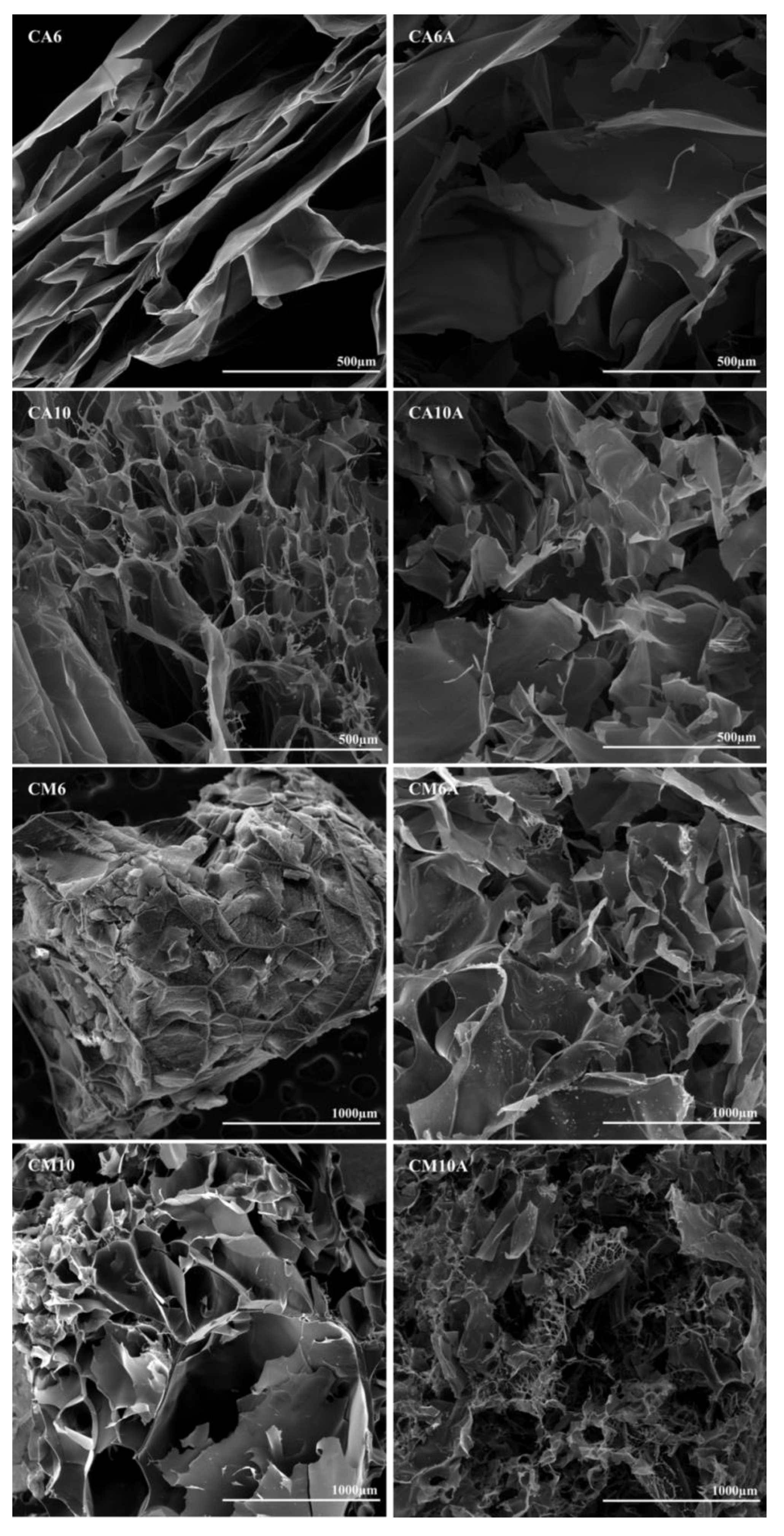
3.2. Hydrogels Morphology
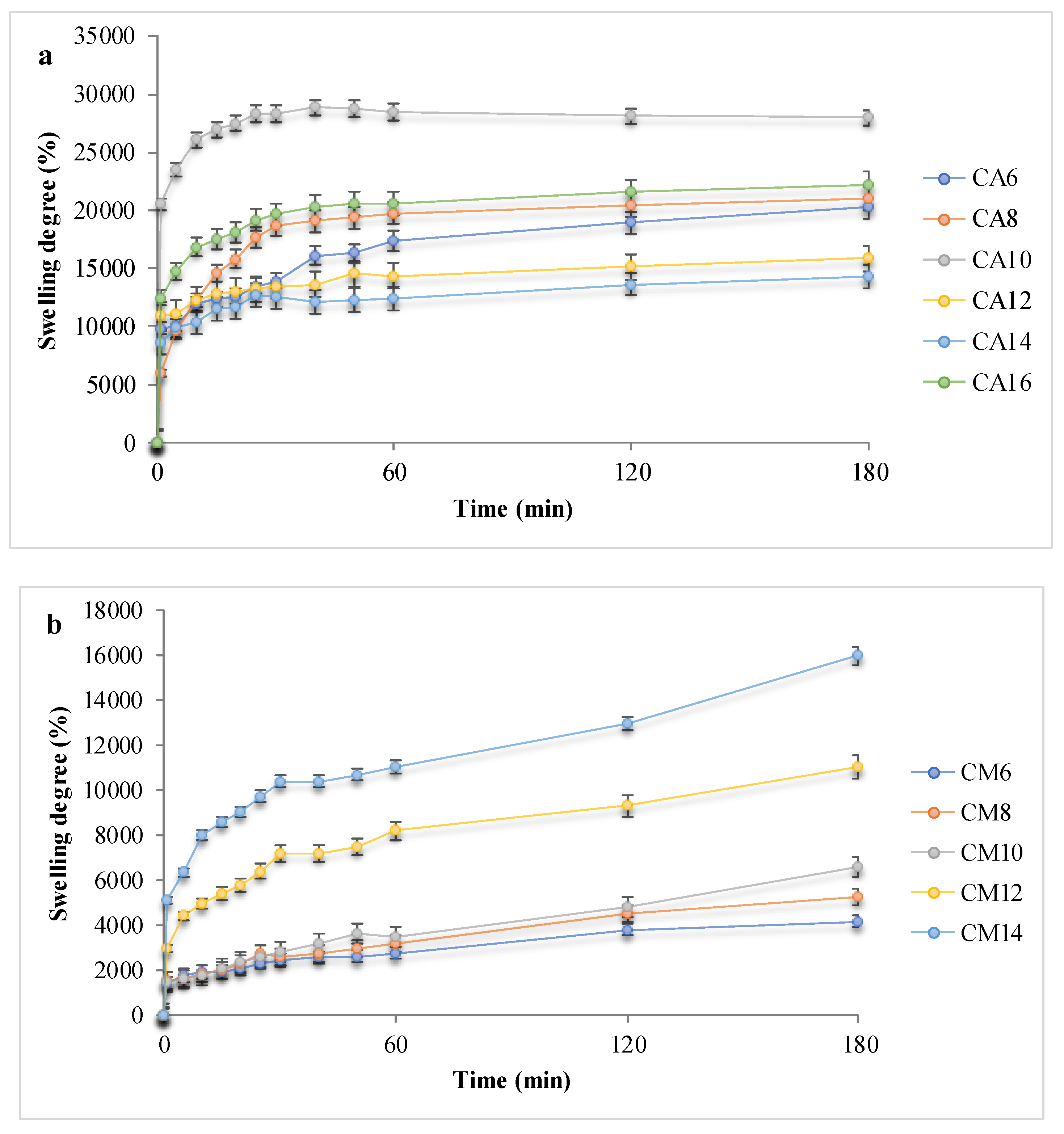
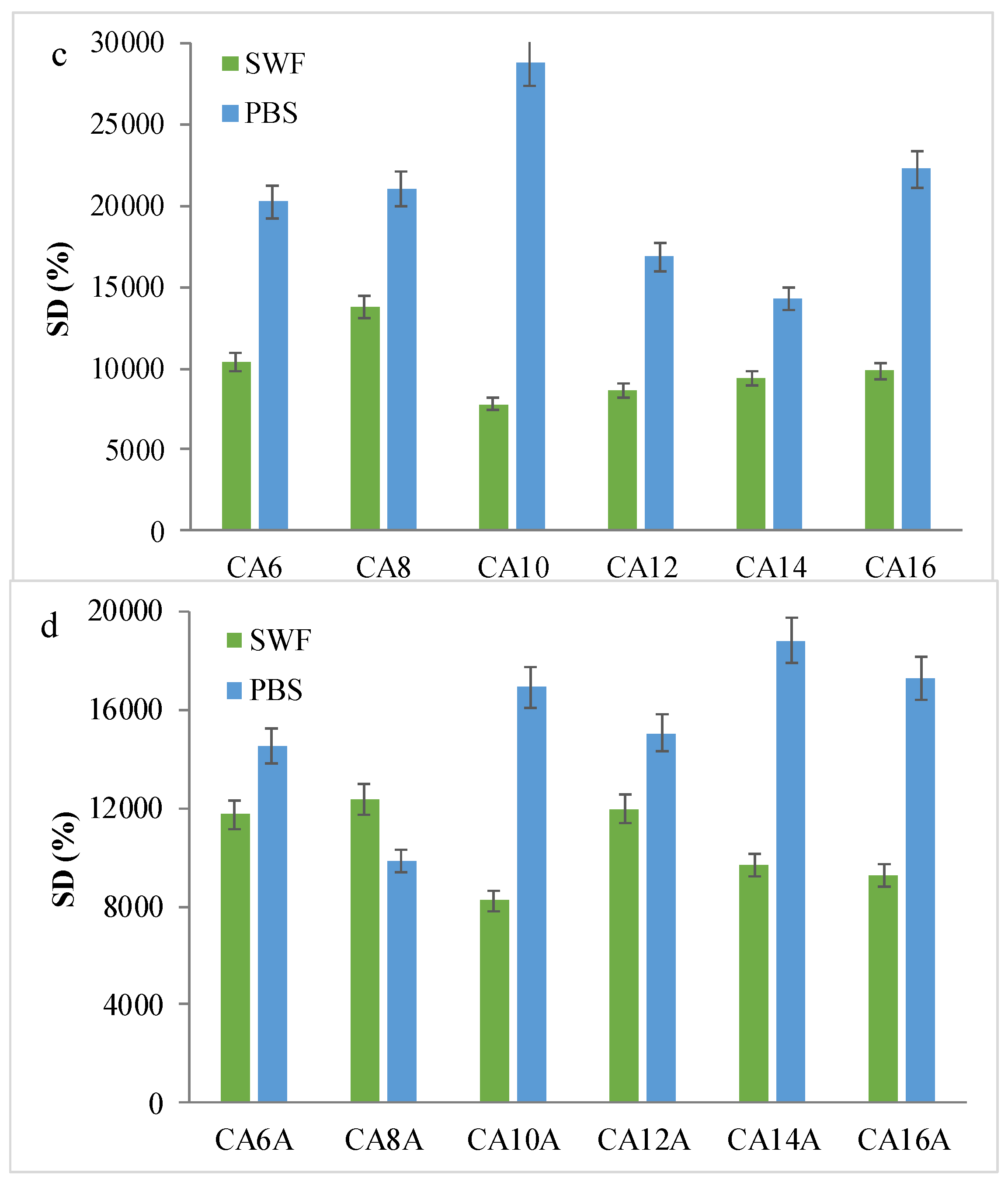
3.3. Swelling Behavior in Simulated Physiological Conditions
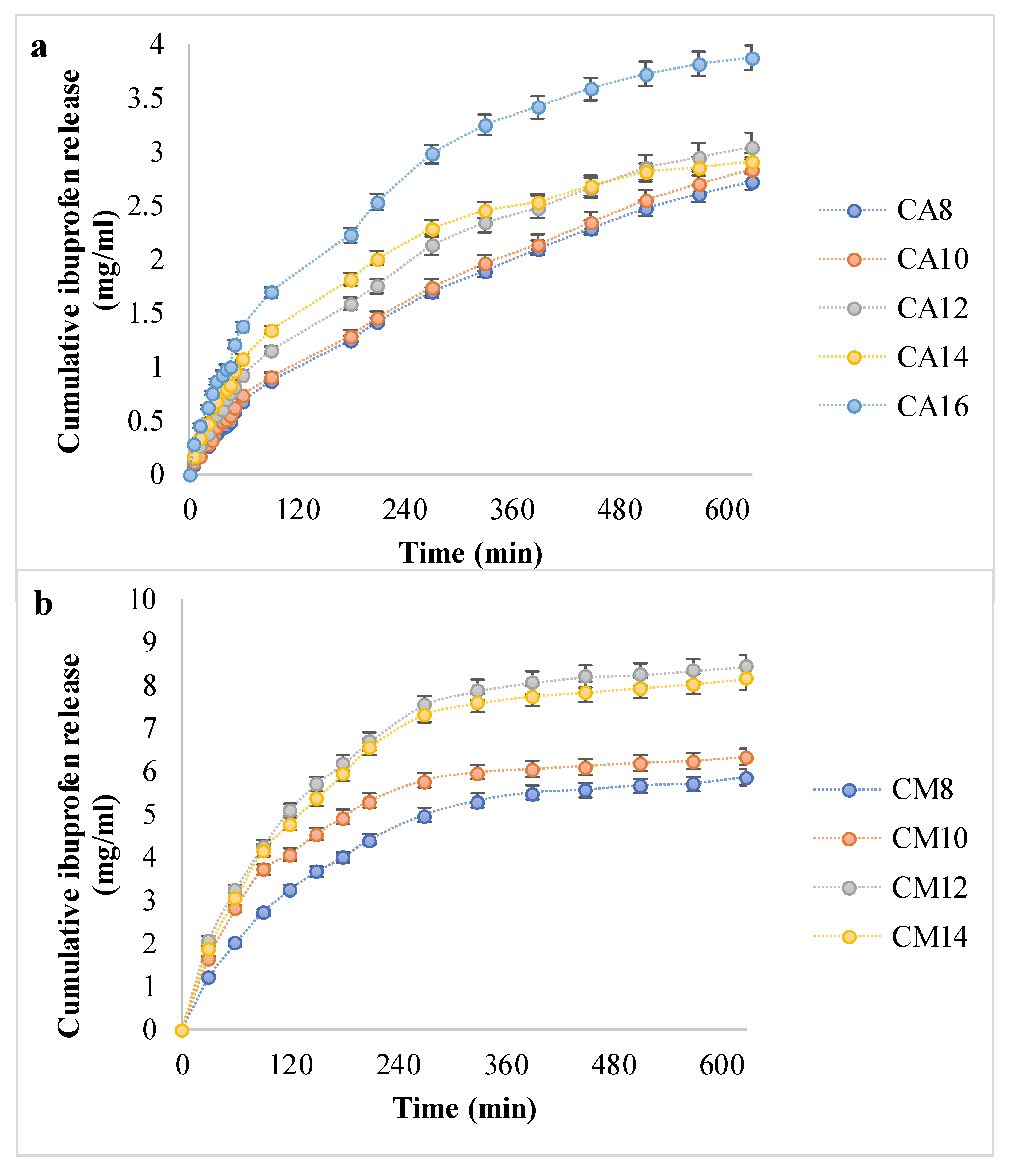
3.4. Ibuprofen Release Profiles and Kinetics
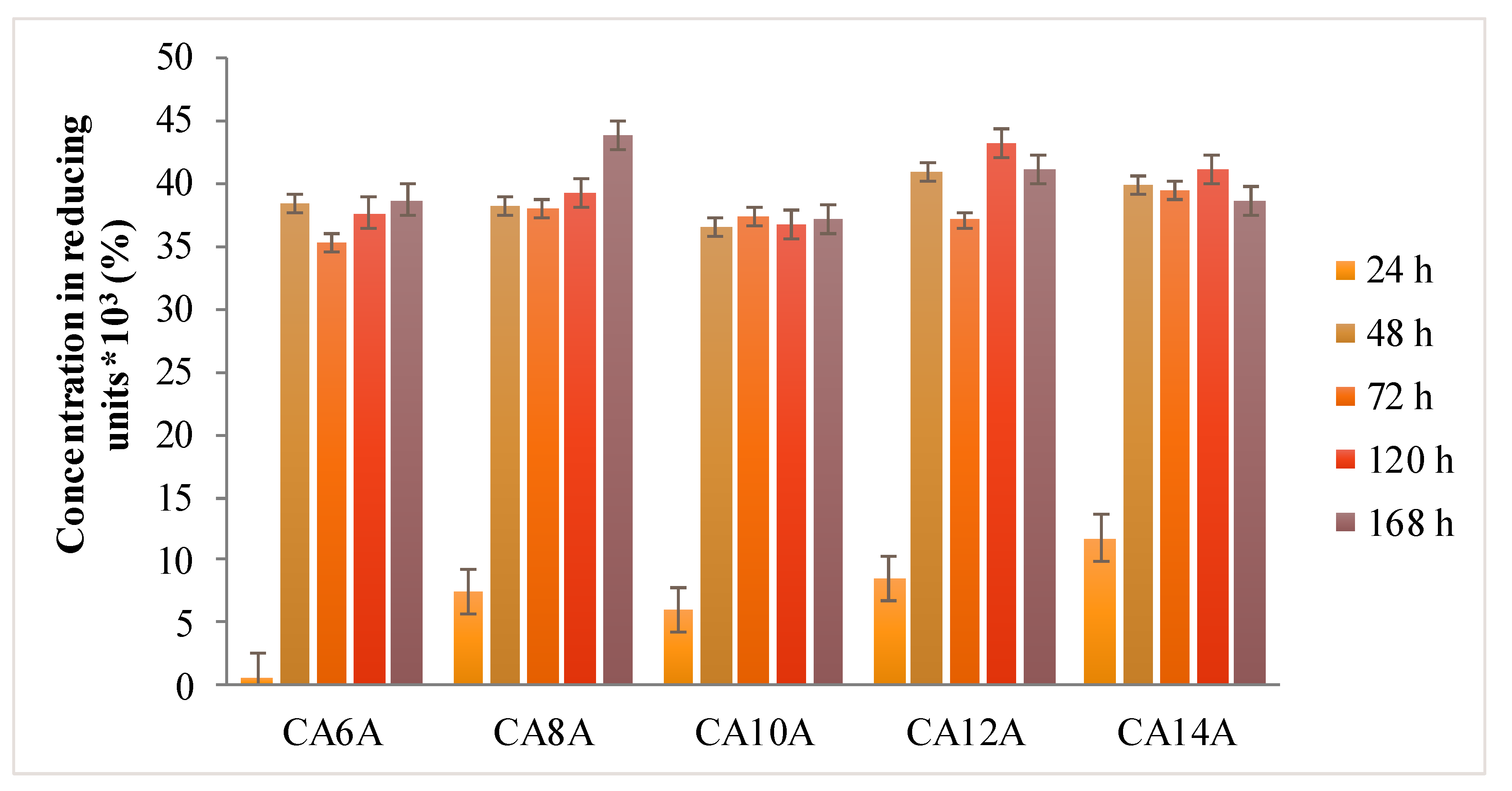
3.5. The In Vitro Hydrogels Degradation
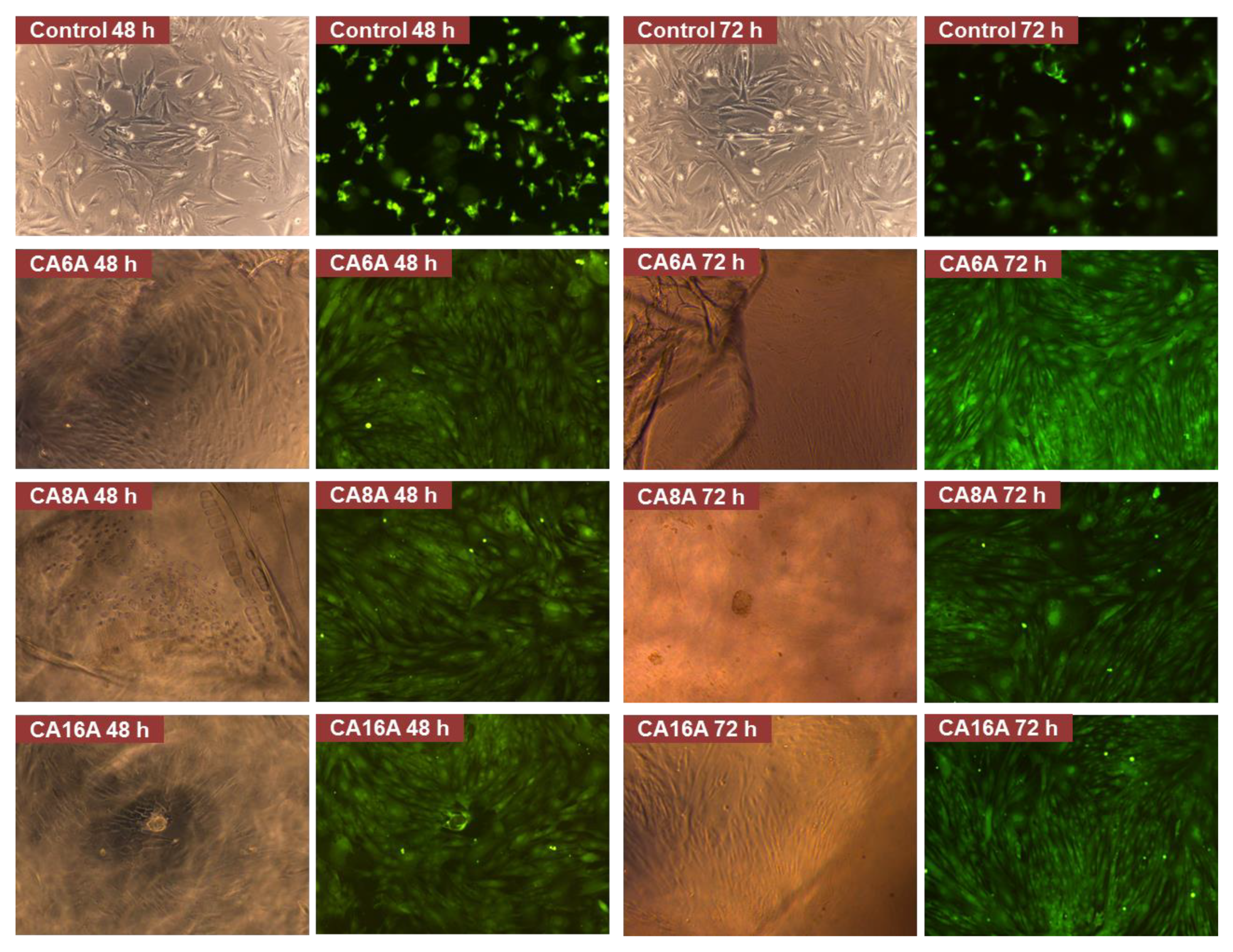
3.6. Hydrogels Cytocompatibility
3.7. Hemostatic Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ullah, F.; Othman, M.B.; Javed, F.; Ahmad, Z.; Akil, H. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The Production and Application of Hydrogels for Wound Management: A Review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A Review on Polymeric Hydrogel Membranes for Wound Dressing Applications: PVA-Based Hydrogel Dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Baron, R.I.; Culica, M.E.; Biliuta, G.; Bercea, M.; Gherman, S.; Zavastin, D.; Ochiuz, L.; Avadanei, M.; Coseri, S. Physical Hydrogels of Oxidized Polysaccharides and Poly(Vinyl Alcohol) For Wound Dressing Applications. Materials 2019, 12, 1569. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Matthews, K.H. Drug delivery dressings. In Advanced Wound Repair Therapies; Farrar, D., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 361–394. [Google Scholar]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016, 10, 27–37. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-Based Biomaterials for Tissue Engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan Based Hydrogels and Their Applications for Drug Delivery in Wound Dressings: A Review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Ferfera-Harrar, H.; Aiouaz, N.; Dairi, N. Synthesis and Properties of Chitosan Graft-Polyacrylamide Gelatin Superabsorbent Composites for Wastewater. Int. J. Chem. Mol. Eng. 2015, 9, 849–856. [Google Scholar]
- Sakthivel, M.; Franklin, D.S.; Guhanathan, S. PH-Sensitive Itaconic Acid Based Polymeric Hydrogels for Dye Removal Applications. Ecotoxicol. Environ. Saf. 2016, 134, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, L.; Ren, X.; Song, H.; Wang, A. Removal of Methyl Violet from Aqueous Solutions Using Poly (Acrylic Acid-Co-Acrylamide)/Attapulgite Composite. J. Environ. Sci. 2010, 22, 7–14. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.Y.; Kim, S.S.; Lee, Y.M.; Lee, K.H.; Kim, S.J. Synthesis and characteristics of interpenetrating polymer network hydrogel composed of chitosan and poly(acrylic acid). J. Appl. Polym. Sci. 1999, 73, 113–120. [Google Scholar] [CrossRef]
- Lee, J.S.; Kumar, R.N.; Rozman, H.D.; Azemi, B.M.N. Pasting, Swelling and Solubility Properties of UV Initiated Starch-Graft-Poly(AA). Food Chem. 2005, 91, 203–211. [Google Scholar] [CrossRef]
- Athawale, V.; Lele, V. Recent Trends in Hydrogels Based on Starch-Graft-Acrylic Acid: A Review. Starch/Stärke 2001, 53, 7–13. [Google Scholar] [CrossRef]
- Prabaharam, M. Graft Copolymerization of Acrylic Monomers on Chitosan and Its Derivatives: Recent Developments and Applications. In Chitin and Chitosan Derivatives: Advances in Drug Discovery and Developments; Se-Kwon, K., Ed.; CRC Press: Boca Raton, FL, USA, 2014; p. 167. [Google Scholar]
- Dos Santos, K.S.C.R.; Coelho, J.F.J.; Ferreira, P.; Pinto, I.; Lorenzetti, S.G.; Ferreira, E.I.; Higa, O.Z.; Gil, M.H. Synthesis and Characterization of Membranes Obtained by Graft Copolymerization of 2-Hydroxyethyl Methacrylate and Acrylic Acid onto Chitosan. Int. J. Pharm. 2006, 310, 37–45. [Google Scholar] [CrossRef]
- Duceac, I.A.; Lobiuc, A.; Coseri, S.; Verestiuc, L. Tunable Hydrogels Based on Chitosan, Collagen and Poly(Acrylic Acid) for Regenerative Medicine. In Proceedings of the EHB 2019: IEEE International Conference on e-Health and Bioengineering, Iasi, Romania, 21–23 November 2019; Volume 2, pp. 3–6. [Google Scholar]
- Dwivedi, R.; Singh, A.K.; Dhillon, A. pH-Responsive Drug Release from Dependal-M Loaded Polyacrylamide Hydrogels. J. Sci. Adv. Mater. 2017, 2, 45–50. [Google Scholar] [CrossRef]
- Chuah, C.; Wang, J.; Tavakoli, J.; Tang, Y. Novel Bacterial Cellulose-Poly(Acrylic Acid) Hybrid Hydrogels with Controllable Antimicrobial Ability as Dressings for Chronic Wounds. Polymers 2018, 10, 1323. [Google Scholar] [CrossRef]
- Hoffman, W.S. A rapid photoelectric method for the determination of glucose in blood and urine. J. Biol. Chem. 1937, 120, 51. [Google Scholar]
- Singh, B.; Sharma, A.; Sharma, A.; Dhiman, A. Design of Antibiotic Drug Loaded Carbopol- Hydrogel for Wound Dressing Applications. Am. J. Drug Deliv. Ther. 2017, 4, 1–9. [Google Scholar]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A Simple Equation for Description of Solute Release II. Fickian and Anomalous Release from Swellable Devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Blanco, A.; González, G.; Casanova, E.; Pirela, M.E.; Briceño, A. Mathematical Modeling of Hydrogels Swelling Based on the Finite Element Method. Appl. Math. 2013, 4, 161–170. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani-Farahani, S.; Vasheghani-Farahani, E. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. (Eng. Ed.) 2010, 19, 375–398. [Google Scholar]
- Mignon, A.; De Belie, N.; Dubruel, P.; Van Vlierberghe, S. Superabsorbent Polymers: A Review on the Characteristics and Applications of Synthetic, Polysaccharide-Based, Semi-Synthetic and ‘Smart’ Derivatives. Eur. Polym. J. 2019, 117, 165–178. [Google Scholar] [CrossRef]
- Cheng, W.M.; Hu, X.M.; Wang, D.M.; Liu, G.H. Preparation and Characteristics of Corn Straw-co-AMPS-co-AA Superabsorbent Hydrogel. Polymers 2015, 7, 2431–2445. [Google Scholar] [CrossRef]
- Khan, H.; Chaudhary, J.P.; Meena, R. Anionic Carboxymethylagarose-Based PH-Responsive Smart Superabsorbent Hydrogels for Controlled Release of Anticancer Drug. Int. J. Biol. Macromol. 2019, 124, 1220–1229. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.H.A.; Pereira, A.G.B.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogel composite made of cellulose nanofibrils and chitosan-graft-poly(acrylic acid). Carbohydr. Polym. 2012, 87, 2038–2045. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent Hydrogels Based on Polysaccharides for Application in Agriculture as Soil Conditioner and Nutrient Carrier: A Review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Kollár, J.; Mrlik, M.; Moravcikova, D.; Ivan, B.; Mosnacek, J. Effect of monomer content and external stimuli on properties of renewable Tulipalin A-based superabsorbent hydrogels. Eur. Polym. J. 2019, 115, 99–106. [Google Scholar] [CrossRef]
- Rao, P.N.P.; Knaus, E.E. Evolution of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): Cyclooxygenase (COX) Inhibition and Beyond. J. Pharm. Pharm. Sci. 1997, 11, 81–110. [Google Scholar] [CrossRef]
- Rodríguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Interactions of Ibuprofen with Cationic Polysaccharides in Aqueous Dispersions and Hydrogels: Rheological and Diffusional Implications. Eur. J. Pharm. Sci. 2003, 20, 429–438. [Google Scholar] [CrossRef]
- Si, H.; Xing, T.; Ding, Y.; Zhang, H.; Yin, R.; Zhang, W. 3D Bioprinting of the Sustained Drug Release Wound Dressing with Double-Crosslinked Hyaluronic-Acid-Based Hydrogels. Polymers 2019, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Tzereme, A.; Christodoulou, E.; Kyzas, G.Z.; Kostoglou, M.; Bikiaris, D.N.; Lambropoulou, D.A. Chitosan Grafted Adsorbents for Diclofenac Pharmaceutical Compound Removal from Single-Component Aqueous Solutions and Mixtures. Polymers 2019, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Sattari, S.; Tehrani, A.D.; Adeli, M. pH-Responsive Hybrid Hydrogels as Antibacterial and Drug Delivery Systems. Polymers 2018, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Pistone, A.; Iannazzo, D.; Celesti, C.; Scolaro, C.; Giofré, S.V.; Romeo, R.; Visco, A. Chitosan/PAMAM/Hydroxyapatite Engineered Drug Release Hydrogels with Tunable Rheological Properties. Polymers 2020, 12, 754. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of Sustained- Action Medication. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Mankotia, P.; Choudhary, S.; Sharma, K.; Kumar, V.; Kaur Bhatia, J.; Parmar, A.; Sharma, S.; Sharma, V. Neem Gum Based PH Responsive Hydrogel Matrix: A New Pharmaceutical Excipient for the Sustained Release of Anticancer Drug. Int. J. Biol. Macromol. 2019, 142, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Frohm, M.; Gunne, H.; Bergman, A.C.; Agerberth, B.; Bergman, T.; Boman, A.; Lidén, S.; Jörnvall, H.; Boman, H.G. Biochemical and Antibacterial Analysis of Human Wound and Blister Fluid. Eur. J. Biochem. 1996, 237, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Iacob, A.T.; Drăgan, M.; Ghețu, N.; Pieptu, D.; Vasile, C.; Buron, F.; Routier, S.; Giusca, S.E.; Caruntu, I.D.; Profire, L. Preparation, Characterization and Wound Healing Effects of New Membranes Based on Chitosan, Hyaluronic Acid and Arginine Derivatives. Polymers 2018, 10, 607. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, F.; Hua, Y.; Zhang, X.; Ni, C.; Pan, D.; Zhang, Y.; Jiang, D.; Yang, L.; Lin, Q.; et al. A strongly adhesive hemostatic hydrogel for the repair of arterial and heart bleeds. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, J.I.; Kulkarni, R.; Kim, M.A.; Hwang, J.S.; Na, M.K.; Bae, J.S. Antithrombotic and antiplatelet activities of small-molecule alkaloids from Scolopendra subspinipes mutilans. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Behrens, A.M.; Sikorski, M.J.; Kofinas, P. Hemostatic strategies for traumatic and surgical bleeding. J. Biomed. Mater. Res. Part A 2014, 102, 4182–4194. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.Y.; Lu, S.T.; Li, P.W.; Li, S.D. Chitosan-based composite materials for prospective hemostatic applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef]
- Yang, J.; Tian, F.; Wang, Z.; Wang, Q.; Zeng, Y.-J.; Chen, S.-Q. Effect of chitosan molecular weight and deacetylation degree on hemostasis. J. Biomed. Mater. Res. B. Appl. Biomater. 2008, 84, 131–137. [Google Scholar] [CrossRef]
- Kang, P.L.; Chang, S.J.; Manousakas, I.; Lee, C.W.; Yao, C.H.; Lin, F.H.; Kuo, S.M. Development and assessment of hemostasis chitosan dressings. Carbohydr. Polym. 2011, 85, 565–570. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Witte, M.B.; Barbul, A. Arginine physiology and its implication for wound healing. Wound Repair Regen. 2003, 11, 419–423. [Google Scholar] [CrossRef] [PubMed]


| Code * | Cross-Linker | Molar Ratio (%) AA/AM: APS/TEMED | Cross-Linking Reaction Yield (%) | N-Citraconyl-Chitosan (%) | Synthetic Polymer (%) |
|---|---|---|---|---|---|
| CA6 | PAA | 0.6 | 79.13 | 25.89 | 74.11 |
| CA8 | 0.8 | 76.26 | 23.09 | 76.91 | |
| CA10 | 1 | 59.12 | 29.74 | 70.26 | |
| CA12 | 1.2 | 63.96 | 25.95 | 74.05 | |
| CA14 | 1.4 | 60.23 | 20.30 | 79.70 | |
| CA16 | 1.6 | 58.5 | 34.42 | 65.58 | |
| CM6 | PAM | 0.6 | 59.21 | 17.99 | 82.01 |
| CM8 | 0.8 | 51.44 | 20.88 | 79.12 | |
| CM10 | 1 | 48.03 | 22.16 | 77.84 | |
| CM12 | 1.2 | 44.17 | 26.88 | 73.12 | |
| CM14 | 1.4 | 41.36 | 33.56 | 66.44 |
| Hydrogel | Monomer Type | APS (%) | Presence of Arginine | Pore Dimension (μm) | ||
|---|---|---|---|---|---|---|
| Min | Average | Max | ||||
| CA6 | AA | 0.6% | ✗ | 55.2 | 148.0 | 448.4 |
| CA6A | 0.6% | ✓ | 145.3 | 318.6 | 526.0 | |
| CA10 | 1% | ✗ | 91.4 | 185.9 | 387.5 | |
| CA10A | 1% | ✓ | 150.4 | 240.1 | 372.6 | |
| CM6 | AM | 0.6% | ✗ | 149.7 | 383.9 | 907.8 |
| CM6A | 0.6% | ✓ | 287.6 | 539.8 | 899.4 | |
| CM10 | 1% | ✗ | 251.0 | 548.5 | 1885.0 | |
| CM10A | 1% | ✓ | 13.6 | 41.8 | 73.1 | |
| Hydrogel | k | n | Hydrogel | k | n |
|---|---|---|---|---|---|
| CA6 | 0.028 | 0.790096 | CM6 | 0.0338 | 0.720515 |
| CA8 | 0.0397 | 0.759804 | CM8 | 0.0409 | 0.665587 |
| CA10 | 0.0093 | 0.938351 | CM10 | 0.0478 | 0.614805 |
| CA12 | 0.0132 | 0.893475 | CM12 | 0.0375 | 0.715687 |
| CA14 | 0.0153 | 0.879649 | CM14 | 0.0339 | 0.732957 |
| CA16 | 0.0187 | 0.868381 |
| Correlation Coefficient (r2) | Release Rate Constant, k | Release Exponent, n | ||||
|---|---|---|---|---|---|---|
| Zero Order | First Order | Higuchi | Korsmeyer–Peppas | |||
| CA16 | 0.9545 | 0.8342 | 0.9838 | 0.9936 | 0.0158 | 0.6234 |
| CM12 | 0.9236 | 0.858 | 0.9954 | 0.9947 | 0.0025 | 0.5907 |
| Parameter | Blood | CA6A | CA10A | CM6A | CM10A |
|---|---|---|---|---|---|
| PT (s) | 13.1 ± 1.2 | 10. 2 ± 0.5 | 10. 2 ± 0.5 | 11.3 ± 0.4 | 11.0 ± 0.5 |
| INR | 1.07 ± 0.12 | 0.70 ± 0.13 | 0.70 ± 0.16 | 0.79 ± 0.18 | 0.76 ± 0.21 |
| Fibrinogen (mg/dl) | 390 ± 15 | 460 ± 21 | 421 ± 12 | 447 ± 21 | 410 ± 33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duceac, I.A.; Verestiuc, L.; Dimitriu, C.D.; Maier, V.; Coseri, S. Design and Preparation of New Multifunctional Hydrogels Based on Chitosan/Acrylic Polymers for Drug Delivery and Wound Dressing Applications. Polymers 2020, 12, 1473. https://doi.org/10.3390/polym12071473
Duceac IA, Verestiuc L, Dimitriu CD, Maier V, Coseri S. Design and Preparation of New Multifunctional Hydrogels Based on Chitosan/Acrylic Polymers for Drug Delivery and Wound Dressing Applications. Polymers. 2020; 12(7):1473. https://doi.org/10.3390/polym12071473
Chicago/Turabian StyleDuceac, Ioana A., Liliana Verestiuc, Cristina D. Dimitriu, Vasilica Maier, and Sergiu Coseri. 2020. "Design and Preparation of New Multifunctional Hydrogels Based on Chitosan/Acrylic Polymers for Drug Delivery and Wound Dressing Applications" Polymers 12, no. 7: 1473. https://doi.org/10.3390/polym12071473
APA StyleDuceac, I. A., Verestiuc, L., Dimitriu, C. D., Maier, V., & Coseri, S. (2020). Design and Preparation of New Multifunctional Hydrogels Based on Chitosan/Acrylic Polymers for Drug Delivery and Wound Dressing Applications. Polymers, 12(7), 1473. https://doi.org/10.3390/polym12071473








