Nanostructured Chitosan/Maghemite Composites Thin Film for Potential Optical Detection of Mercury Ion by Surface Plasmon Resonance Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagent and Materials
2.2. Preparation of Chemical
2.3. Preparation of Thin Film
2.4. Instrumental
3. Results and Discussion
3.1. FTIR Analysis
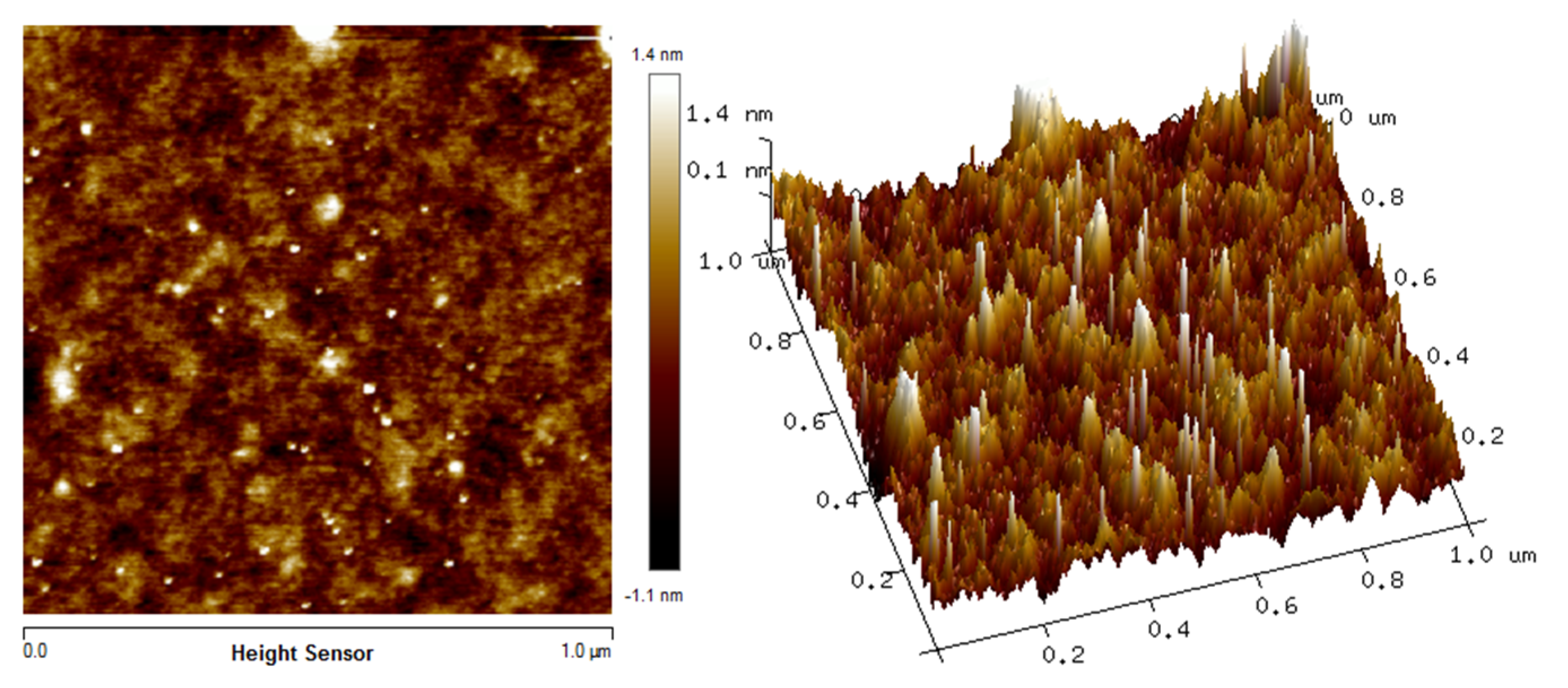
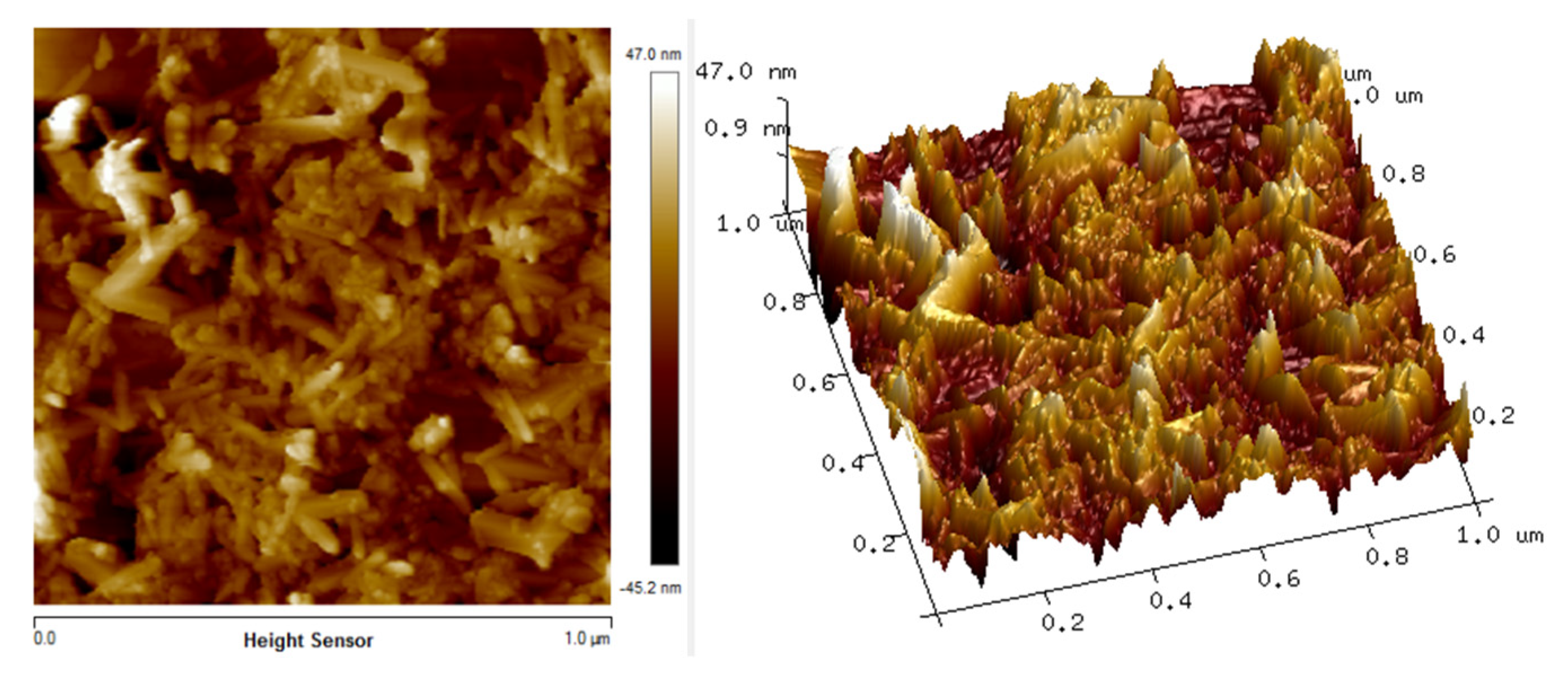
3.2. Surface Morphology
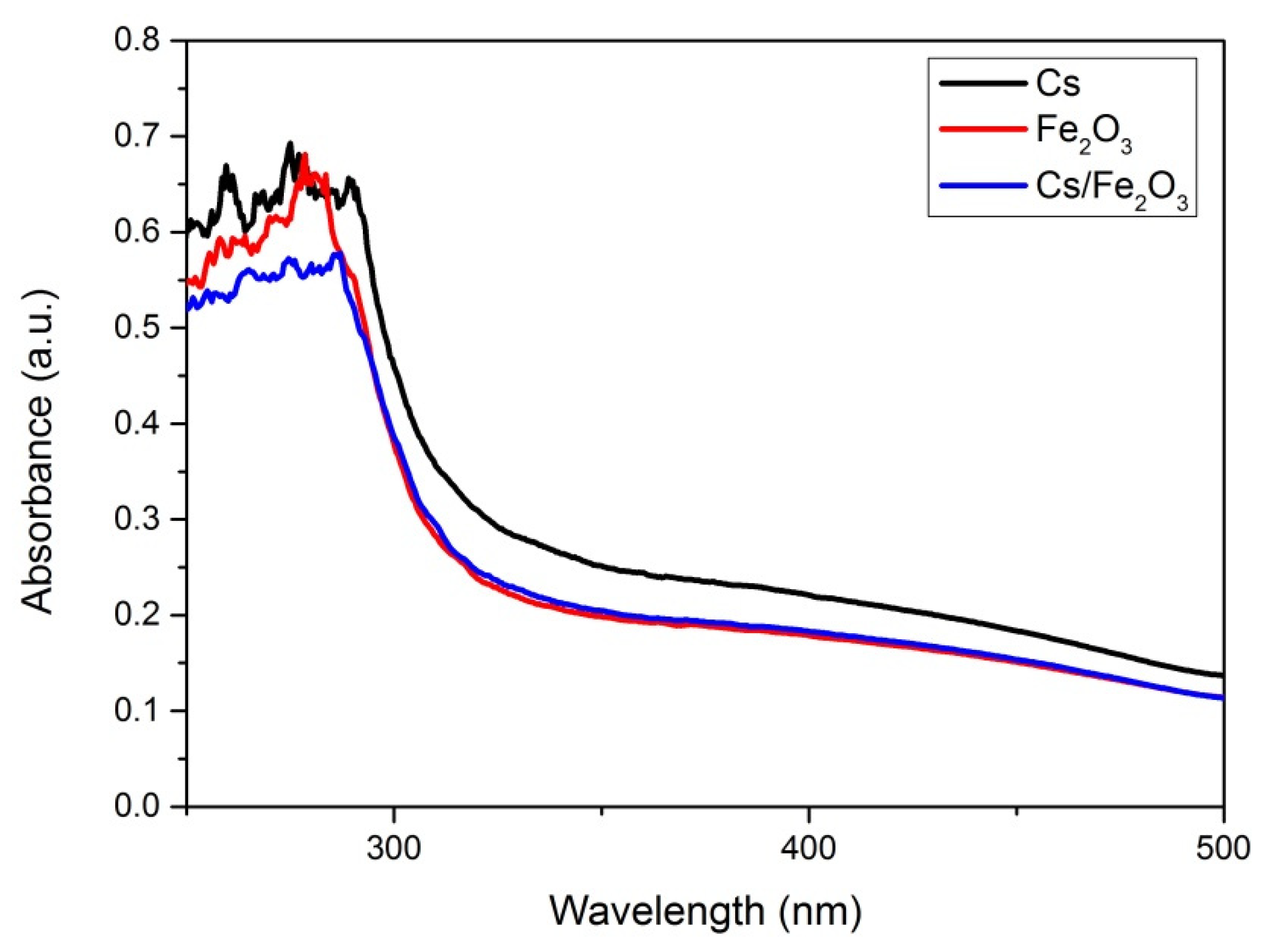
3.3. Optical Studies
3.4. Optical-Based Sensing of Hg2+
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iwan, A.; Sek, D. Processible polyazomethines and polyketanils: From aerospace to light-emitting diodes and other advanced applications. Prog. Polym. Sci. 2008, 33, 289–345. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A.; Yusof, N.A. X-ray photoelectron spectroscopy and atomic force microscopy studies on crosslinked chitosan thin film. Int. J. Phys. Sci. 2011, 6, 2744–2749. [Google Scholar]
- Martino, A.D.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef]
- Prashanth, K.V.H.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential an-overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem Lett. 2017, 16, 101–112. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. Chem. Bio. Eng. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, W.; Liu, P.; Cheng, Q.; Tahirou, T.; Gu, W.; Li, B. Chitosan modification and pharmaceutical/biomedical applications. Mar. Drugs 2010, 8, 1962–1987. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A.; Ishak, N.S.; Omar, N.A.S.; Zainudin, A.A. Preparation, characterization and optical properties of ionophore doped chitosan biopolymer thin film and its potential application for sensing metal ion. Optik 2015, 126, 4688–4692. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization and potential application of chitosan, chitosan derivatives and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Deel. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Omar, N.A.; Zainudin, A.A.; Daniyal, W.M.E.M.M. Optical and structural characterization of immobilized 4-(2-pyridylazo) resorcinol in chitosan-graphene oxide composite thin film and its potential for Co2+ sensing using surface plasmon resonance technique. Results Phys. 2018, 11, 118–122. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Al-Rekabi, S.H.; Mahdi, M.A.; Omar, N.A.S. Incorporation of surface plasmon resonance with novel valinomycin doped chitosan-graphene oxide thin film for sensing potassium ion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 191, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, A.; Salahuddin, N.; Matsuda, A.; Kawamura, G.; Elfiky, M. Fabrication of biosensor based on chitosan-ZnO/Polypyrrole nanocomposite modified carbon paste electrode for electroanalytical application. Mater. Sci. Eng. C 2017, 80, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Saleviter, S.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Abdullah, J.; Sadrolhosseini, A.R.; Omar, N.A.S. Design and analysis of surface plasmon resonance optical sensor for determining cobalt ion based on chitosan-graphene oxide decorated quantum dots-modified gold active layer. Opt. Express 2019, 27, 32294–32307. [Google Scholar] [CrossRef]
- Mariani, F.Q.; Borth, K.W.; Müller, M.; Dalpasquale, M.; Anaissi, F.J. Sustainable innovative method to synthesize different shades of iron oxide pigments. Dyes Pigments 2017, 137, 403–409. [Google Scholar] [CrossRef]
- Machala, L.; Zboril, R.; Gedanken, A. Amorphous Iron(III) Oxide- A review. Phys. Chem. B 2007, 111, 4003–4018. [Google Scholar] [CrossRef]
- Machala, L.; Tucek, J.; Zboril, R. Polymorphous transformations of nanometric iron(III) oxide: A review. Chem. Mater. 2011, 23, 3255–3272. [Google Scholar] [CrossRef]
- Zboril, R.; Mashlan, M.; Petridis, D. Iron(III) oxides from thermal processes- synthesis, structural, and magnetic properties, mossbauer spectroscopy characterization and applications. Chem. Mater. 2002, 14, 969–982. [Google Scholar] [CrossRef]
- Al-Rekabi, S.H.; Kamil, Y.M.; Bakar, M.H.A.; Fen, Y.W.; Lim, H.N.; Kanagesan, S.; Mahdi, M.A. Hydrous ferric oxide-magnetite-reduced graphene oxide nanocomposite for optical detection of arsenic using surface plasmon resonance. Opt. Laser Technol. 2019, 111, 417–423. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Dias, H.V.; Kharissova, O.V. Mini-Review: Ferrite nanoparticles in the catalysis. Arab. J. Chem. 2019, 12, 1234–1246. [Google Scholar] [CrossRef]
- Usman, U.A.; Yusoff, I.; Raoov, M.; Hodgkinson, J. The economic potential of the African iron-ore tailings: Synthesis of magnetite for the removal of trace metals in groundwater—A review. Environ. Earth Sci. 2019, 615, 1–22. [Google Scholar] [CrossRef]
- Lapo, B.; Demey, H.; Zapata, J.; Romero, C.; Sastre, A.M. Sorption of Hg(II) and Pb(II) ions on chitosan-Iron(III) from aqueous solutions: Single and binary systems. Polymers 2018, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G. Mercury exposure and heart diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef]
- Li, P.; Feng, X.B.; Qiu, G.L.; Shang, L.H.; Li, Z.G. Mercury pollution in Asia: A review of the contaminated sites. J. Hazard. Mater. 2009, 168, 591–601. [Google Scholar] [CrossRef]
- Reilly, S.B.; Lettmeier, B.; Matteucci, R.; Beinhoff, C.; Siebert, U.; Drasch, V. Mercury as a serious health hazard for children in gold mining areas. Environ. Res. 2008, 107, 89–97. [Google Scholar]
- Miretzky, P.; Cirelli, A.F. Hg(II) removal from water by chitosan and chitosan derivatives: A review. J. Hazard. Mater. 2009, 167, 10–23. [Google Scholar] [CrossRef]
- Roshidi, M.D.A.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Daniyal, W.M.E.M.M. Optical studies of graphene oxide/poly(amidoamine) dendrimer composite thin film and its potential for sensing Hg2+ using surface plasmon resonance spectroscopy. Sens. Mater. 2018, 31, 1147–1156. [Google Scholar] [CrossRef]
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Zainudin, A.A. Optical and surface plasmon resonance sensing properties for chitosan/carboxyl-functionalized graphene quantum dots thin film. Optik 2019, 178, 802–812. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Moksin, M.M.; Talib, Z.A.; Yusof, N.A. Surface plasmon resonance optical sensor for mercury ion detection by crosslinked chitosan thin film. J. Optoelectron. Adv. Mater. 2011, 13, 279–285. [Google Scholar]
- Chen, Z.; Han, K.; Zhang, Y. Reflective fiber surface plasmon resonance sensor for high-sensitive mercury ion detection. Appl. Sci. 2019, 9, 1480. [Google Scholar] [CrossRef]
- Uglov, A.N.; Bessmertnykh-Lemeune, A.; Guilard, R.; Averin, A.D.; Beletskaya, I.P. Optical methods for the detection of heavy metal ions. Russ. Chem. Rev. 2014, 83, 196–224. [Google Scholar] [CrossRef]
- Tang, W.; Li, J.; Guo, Q.; Nie, G. An ultrasensitive electrochemiluminescence assay for Hg2+ through graphene quantum dots and poly(5-formylindole) nanocomposite. Sens. Actuators B Chem. 2019, 282, 824–830. [Google Scholar] [CrossRef]
- Wang, B.B.; Jin, J.C.; Xu, Z.Q.; Jiang, Z.W.; Li, X.; Jiang, F.L.; Liu, Y. Single-step synthesis of highly photoluminescent carbon dots for rapid detection of Hg2+ with excellent sensitivity. J. Colloid Interface Sci. 2019, 551, 101–110. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Wang, B.; Luo, L.; Xu, Z.; Tian, X. A novel surface plasmon resonance sensor based on a functionalized graphene oxide/molecularimprinted polymer composite for chiral recognition of L-tryptophan. RSC Adv. 2018, 8, 32538–32544. [Google Scholar] [CrossRef]
- Kurihara, K.; Nakamura, K.; Suzuki, K. Asymmetric SPR sensor response curve-fitting equation for the accurate determination of SPR resonance angle. Sens. Actuators B Chem. 2002, 86, 49–57. [Google Scholar] [CrossRef]
- Xue, T.; Qi, K.; Hu, C. Novel SPR sensing platform based on superstructure MoS2 nanosheets for ultrasensitive detection of mercury ion. Sens. Actuators B Chem. 2019, 284, 589–594. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Detection of mercury and copper ions using surface plasmon resonance optical sensor. Sens. Mater. 2011, 23, 325–334. [Google Scholar]
- Ramdzan, N.S.M.; Fen, Y.W.; Anas, N.A.A.; Omar, N.A.S.; Saleviter, S. Development of biopolymer and conducting polymer-based optical sensors for heavy metal ion detection. Molecules 2020, 25, 2548. [Google Scholar] [CrossRef]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Lokman, N.F.; Azeman, N.H.; Suja, F.; Arsad, N.; Bakar, A.A. Sensitivity enhancement of Pb(II) ion detection in rivers using SPR-based Ag metallic layer coated with chitosan–graphene oxide nanocomposite. Sensors 2019, 19, 5159. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.S.; Fen, Y.W. Recent development of SPR spectroscopy as potential method for diagnosis of dengue virus E-protein. Sens. Rev. 2018, 38, 106–116. [Google Scholar] [CrossRef]
- Fujii, E.; Koike, T.; Nakamura, K.; Sasaki, S.; Kurihara, K.; Citterio, D.; Suzuki, K. Application of an absorption-based surface plasmon resonance principle to the development of SPR ammonium ion and enzyme sensors. Anal. Chem. 2002, 74, 6106–6110. [Google Scholar] [CrossRef]
- Eddin, F.B.K.; Fen, Y.W. Recent Advances in electrochemical and optical sensing of dopamine. Sensors 2020, 20, 1039. [Google Scholar]
- Fen, Y.W.; Yunus, W.M.M. Surface plasmon resonance spectroscopy as an alternative for sensing heavy metal ions: A review. Sens. Rev. 2013, 33, 305–314. [Google Scholar]
- Victoria, S. Application of surface plasmon resonance (SPR) for the detection of single viruses and single biological nano-objects. J. Bacteriol. Parasitol. 2012, 3, 1–3. [Google Scholar] [CrossRef]
- Jia, Y.; Peng, Y.; Bai, J.; Zhang, X.; Cui, Y.; Ning, B.; Cui, J.; Gao, Z. Magnetic nanoparticle enhanced surface plasmon resonance sensor for estradiol analysis. Sens. Actuators B Chem. 2017, 254, 629–635. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Saleviter, S.; Fen, Y.W. Development of surface plasmon resonance spectroscopy for metal ion detection. Sens. Mater. 2018, 30, 2023–2038. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Saleviter, S.; Daniyal, W.M.E.M.M.; Anas, N.A.A.; Ramdzan, N.S.M.; Roshidi, M.D.A. Development of a graphene-based surface plasmon resonance optical sensor chip for potential biomedical application. Materials 2019, 12, 1928. [Google Scholar] [CrossRef]
- Zhou, C.; Zou, H.; Li, M.; Sun, C.; Ren, D.; Li, Y. Fiber optic surface plasmon resonance sensor for detection of E. coli O157:H7 based on antimicrobial peptides and AgNPs-rGo. Biosens. Bioelectron. 2018, 117, 347–353. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M.; Saleviter, S. Development of graphene quantum dots-based optical sensor for toxic metal ion detection. Sensors 2019, 19, 3850. [Google Scholar] [CrossRef] [PubMed]
- Abdi, M.M.; Abdullah, L.C.; Sadrolhosseini, A.R.; Yunus, W.M.M.; Moksin, M.F.; Tahir, P.M. Surfa ce Plasmon Resonance Sensing Detection of Mercury and Lead Ions Based on Conducting Polymer Composite. PLoS ONE 2011, 6, 24578. [Google Scholar] [CrossRef] [PubMed]
- Kamaruddin, N.H.; Bakar, A.A.A.; Mobarak, N.N.; Zan, M.S.D.; Arsad, N. Binding affinity of a highly sensitive Au/Ag/Au/chitosan-graphene oxide sensor based on direct detection of Pb2+ and Hg2+ ions. Sensors 2017, 17, 2277. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Naseri, M.; Rasyid, S.A. Polypyrrole-chitosan/nickel-ferrite nanoparticle composite layer for detecting heavy metal ions using surface plasmon resonance technique. Opt. Laser Technol. 2017, 93, 216–223. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Surface plasmon resonance optical sensor for detection of essential heavy metal ions with potential for toxicity: Copper, zinc and manganese ions. Sens. Lett. 2011, 9, 1704–1711. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Exploration of surface plasmon resonance for sensing copper ion based on nanocrystalline cellulose-modified thin film. Opt. Express 2018, 26, 34880–34893. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Omar, N.A.S.; Zainudin, A.A.; Yusof, N.A. Development of optical sensor for determination of Co(II) based on surface plasmon resonance phenomenon. Sens. Lett. 2017, 15, 862–867. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Surface plasmon resonance optical sensor for detection of Pb2+ based on immobilized p-tert-butylcalix[4]arene-tetrakis in chitosan thin film as an active layer. Sens. Actuators B Chem. 2012, 171–172, 287–293. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Characterization of the optical properties of heavy metal ions using surface plasmon resonance technique. Opt. Photonics. 2011, 1, 116–123. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A.; Yusof, N.A. Development of surface plasmon resonance sensor for determining zinc ion using novel active nanolayers as probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 134, 48–52. [Google Scholar] [CrossRef]
- Roshidi, D.A.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Omar, N.A.S.; Zulholinda, M. Structural and optical properties of chitosan–poly(amidoamine) dendrimer composite thin film for potential sensing Pb2+ using an optical spectroscopy. Optik 2019, 185, 351–358. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optical properties of chitosan/hydroxyl-functionalized graphene quantum dots thin film for potential optical detection of ferric(III) ion. Opt. Laser Technol. 2019, 120, 105724. [Google Scholar] [CrossRef]
- Rajput, S.; Singh, L.P.; Pittman, C.U., Jr.; Mohan, D. Lead (Pb2+) and copper (Cu2+) remediation from water using superparamagnetic maghemite (γ-Fe2O3) nanoparticles synthesized by Flame Spray Pyrolysis (FSP). J. Colloid Interface Sci. 2016, 492, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Nasser, A.M.B. Synthesis and characterization of maghemite iron oxide (γ-Fe2O3) nanofibers: Novel semiconductor with magnetic feature. J. Mater. Sci. 2016, 47, 6237–6245. [Google Scholar]
- Coates, J. Encyclopedia of Analytical Chemistry; John Wiley & Sons Ltd.: Chichester, NH, USA, 2006; pp. 10815–10837. [Google Scholar]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S. Structural, optical and sensing properties of ionophore doped graphene based bionanocomposite thin film. Optik 2017, 144, 308–315. [Google Scholar] [CrossRef]
- Vujtek, M.; Zboril, R.; Kubinek, R.; Mashlan, M. Ultrafine particles of iron(III) oxides by view of AFM–Novel route for study of polymorphism in nano-world. Nanomaterials 2003, 3, 1–8. [Google Scholar]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Saleviter, S.; Omar, N.A.S. Preparation and characterization of hexadecyltrimethyl-ammonium bromide modified nanocrystalline cellulose/graphene oxide composite thin film and its potential in sensing copper ion using surface plasmon resonance technique. Optik 2018, 173, 71–77. [Google Scholar] [CrossRef]
- Rattana, T.; Chaiyakun, S.; Witit-anun, N.; Nuntawong, N.; Chindaudom, P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar] [CrossRef]
- Kumar, S.; Koh, J. Physiochemical and optical properties of chitosan based graphene oxide bionanocomposite. Int. J. Biol. Macromol. 2014, 70, 559–564. [Google Scholar] [CrossRef]
- Abdulla, H.; Abbo, A. Optical and electrical properties of thin films of polyaniline and polypyrrole. Int. J. Electrochem. Sci. 2012, 7, 10666–10678. [Google Scholar]
- Dhlamini, M.S.; Noto, L.L.; Mothudi, B.M.; Chithambo, M.; Mathevula, L.E. Structural and optical properties of sol-gel derived α-Fe2O3 nanoparticles. J. Lumin. 2017, 192, 879–887. [Google Scholar]
- Al-Kuhaili, M.F.; Saleem, M.; Durrani, S.M.A. Optical properties of iron oxide (α-Fe2O3) thin films deposited by the reactive evaporation of iron. J. Alloys Compd. 2012, 521, 178–182. [Google Scholar] [CrossRef]
- He, Y.P.; Miao, Y.M.; Li, C.R.; Wang, S.Q.; Cao, L.; Xie, S.S.; Yang, G.Z.; Zou, B.S.; Burda, C. Size and structure effect on optical transitions of iron oxide nanocrystals. Rev. B Condens. Matter. 2005, 71, 1–9. [Google Scholar] [CrossRef]
- Deotale, A.J.; Nandedkar, R.V. Correlation between particle size, strain and band gap of iron oxide nanoparticles. Mater. Today 2016, 3, 2069–2076. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A. Analysis of Pb(II) ion sensing by crosslinked chitosan thin film using surface plasmon resonance spectroscopy. Optik 2013, 124, 126–133. [Google Scholar] [CrossRef]
- Lokman, N.F.; Bakar, A.A.A.; Suja, F.; Abdullah, H.; Rahman, W.B.W.A.; Yaacob, M.H. Highly sensitive SPR response of Au/chitosan/graphene oxide nanostructured thin films toward Pb(II) ions. Sens. Actuators B Chem. 2014, 195, 459–466. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Utilization of chitosan-based sensor thin films for the detection of lead ion by surface plasmon resonance optical sensor. IEEE Sens. J. 2013, 13, 1413–1418. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Optical properties of crosslinked chitosan thin film as copper ion detection using surface plasmon resonance technique. Opt. Appl. 2011, 41, 999–1013. [Google Scholar]







| Ref. | Sensing Layer | LOD |
|---|---|---|
| [38] | MMW chitosan (glutaraldehyde-crosslinked) | 2.49 µM |
| [52] | Polypyrrole-chitosan conducting polymer composite | 2.50 µM |
| [53] | Chitosan/graphene oxide | 0.50 µM |
| [54] | Polypyrrole-chitosan/nickel-ferrite nanoparticles | 1.94 µM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Daniyal, W.M.E.M.M.; Hashim, H.S.; Nasrullah, M. Nanostructured Chitosan/Maghemite Composites Thin Film for Potential Optical Detection of Mercury Ion by Surface Plasmon Resonance Investigation. Polymers 2020, 12, 1497. https://doi.org/10.3390/polym12071497
Fauzi NIM, Fen YW, Omar NAS, Saleviter S, Daniyal WMEMM, Hashim HS, Nasrullah M. Nanostructured Chitosan/Maghemite Composites Thin Film for Potential Optical Detection of Mercury Ion by Surface Plasmon Resonance Investigation. Polymers. 2020; 12(7):1497. https://doi.org/10.3390/polym12071497
Chicago/Turabian StyleFauzi, Nurul Illya Muhamad, Yap Wing Fen, Nur Alia Sheh Omar, Silvan Saleviter, Wan Mohd Ebtisyam Mustaqim Mohd Daniyal, Hazwani Suhaila Hashim, and Mohd Nasrullah. 2020. "Nanostructured Chitosan/Maghemite Composites Thin Film for Potential Optical Detection of Mercury Ion by Surface Plasmon Resonance Investigation" Polymers 12, no. 7: 1497. https://doi.org/10.3390/polym12071497
APA StyleFauzi, N. I. M., Fen, Y. W., Omar, N. A. S., Saleviter, S., Daniyal, W. M. E. M. M., Hashim, H. S., & Nasrullah, M. (2020). Nanostructured Chitosan/Maghemite Composites Thin Film for Potential Optical Detection of Mercury Ion by Surface Plasmon Resonance Investigation. Polymers, 12(7), 1497. https://doi.org/10.3390/polym12071497






