New Variants of Nitroxide Mediated Polymerization
Abstract
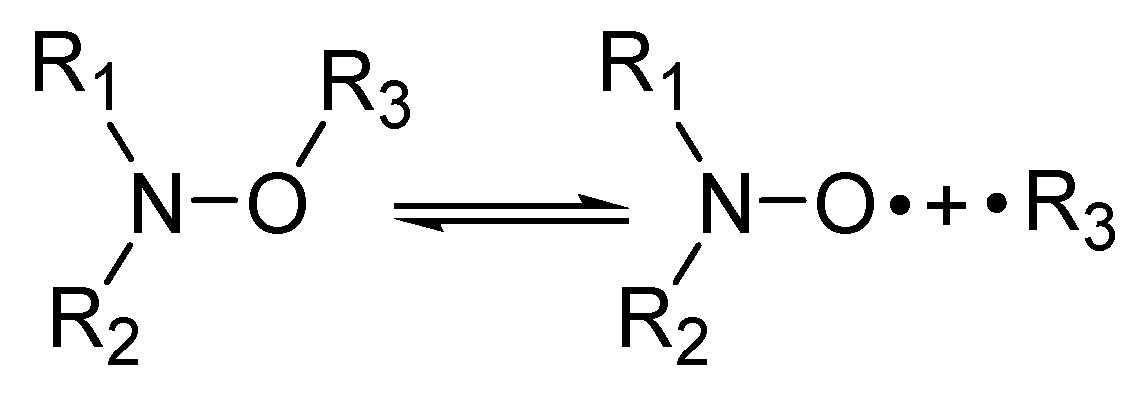
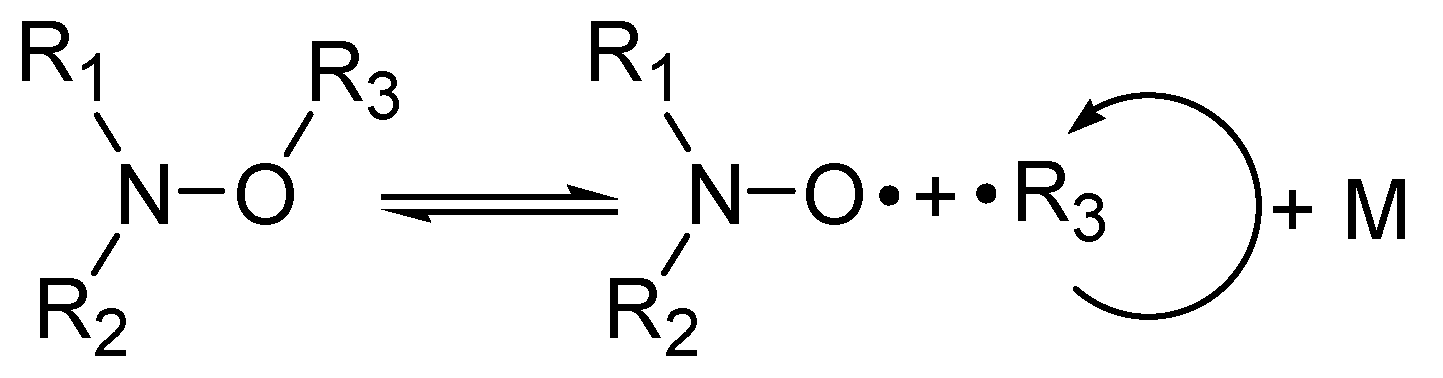
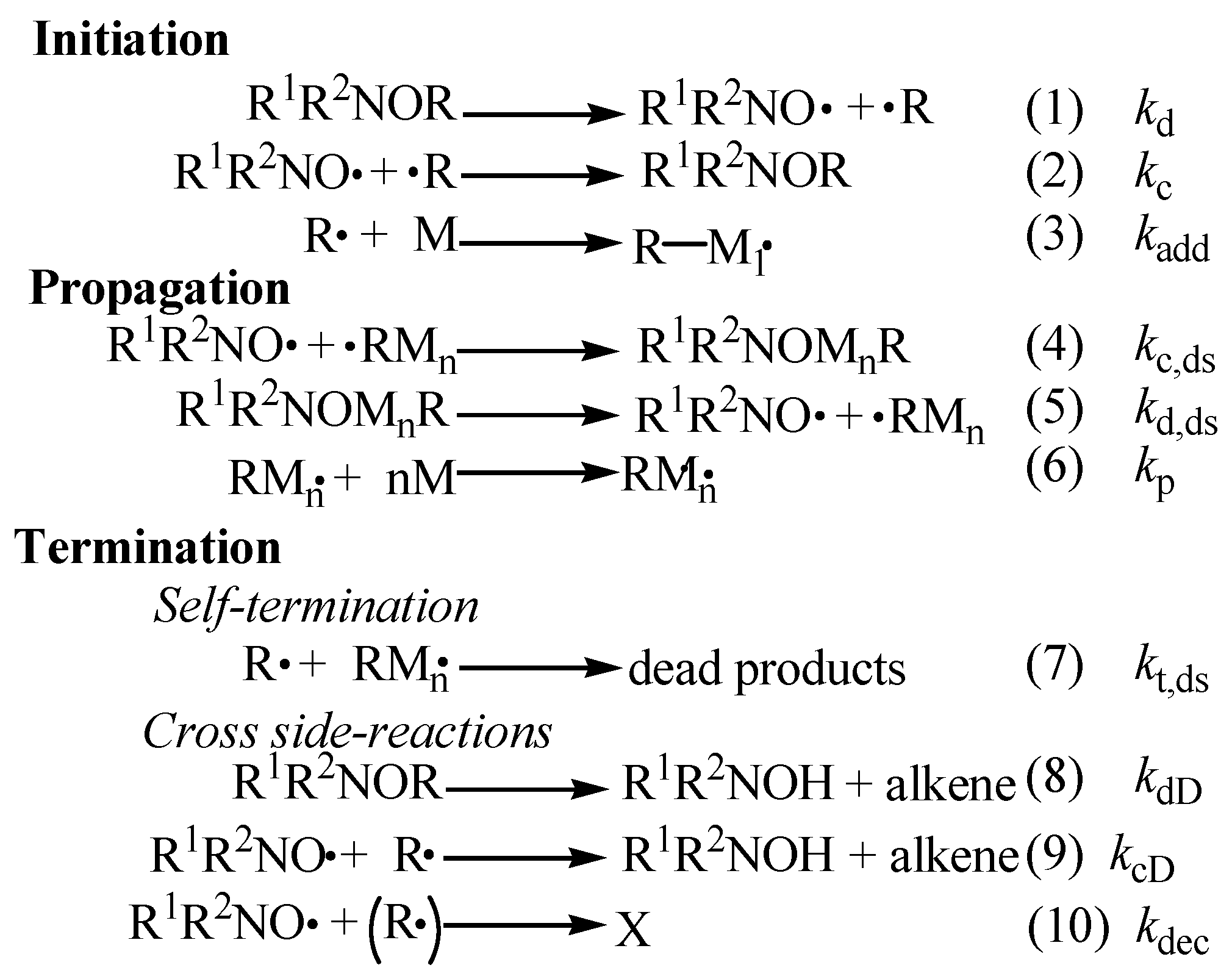
1. Introduction
2. Generality in NMP
3. Enhanced Spin Capture Polymerization ESCP
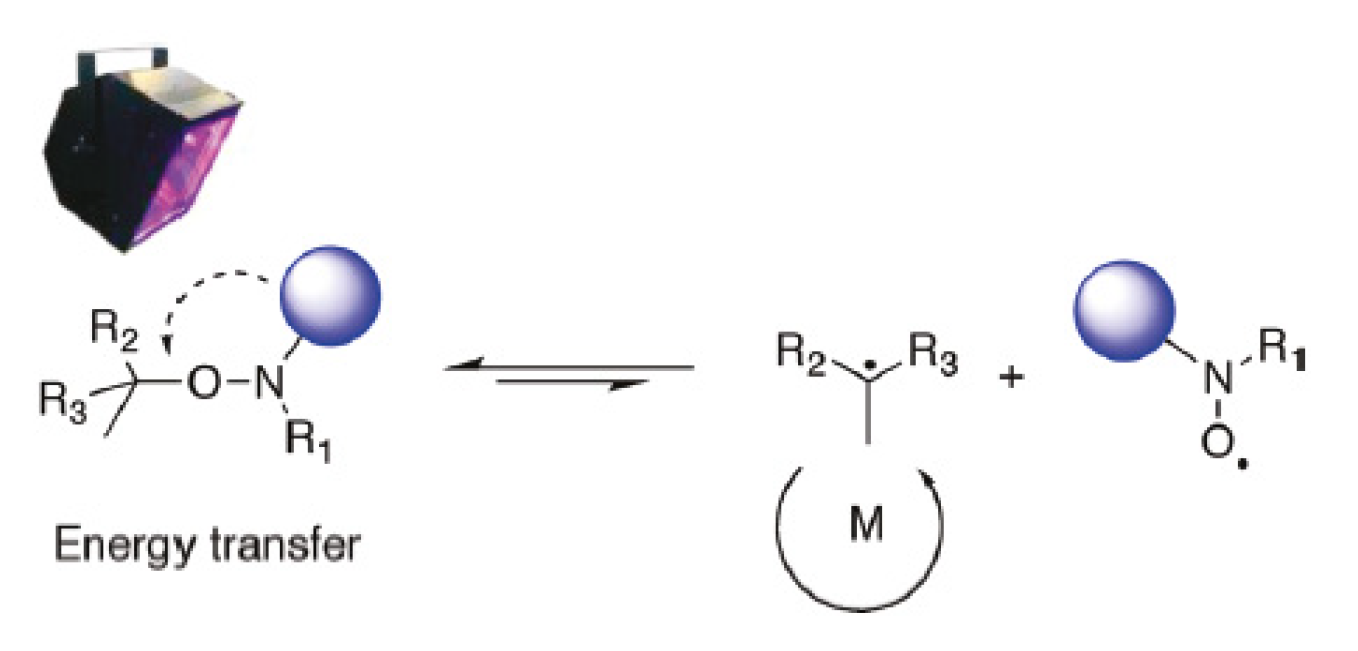
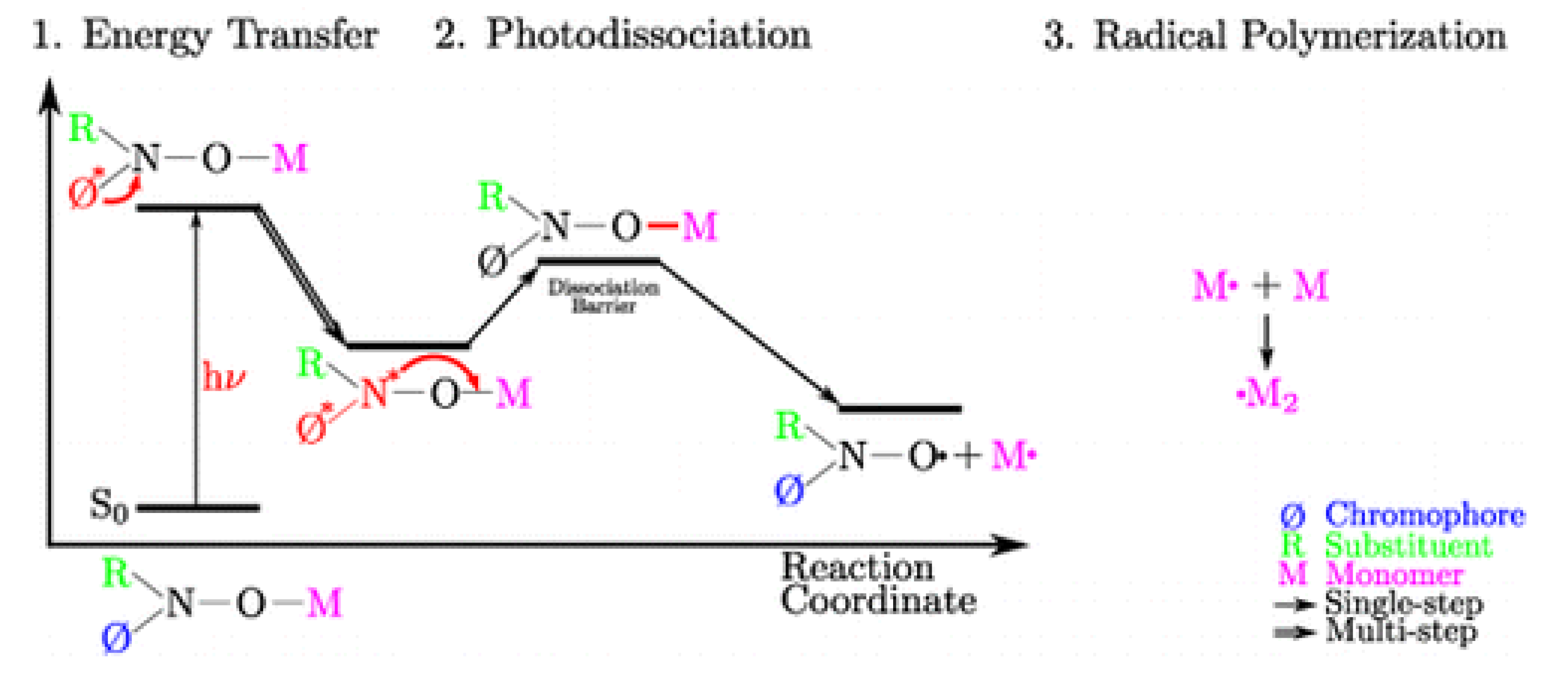
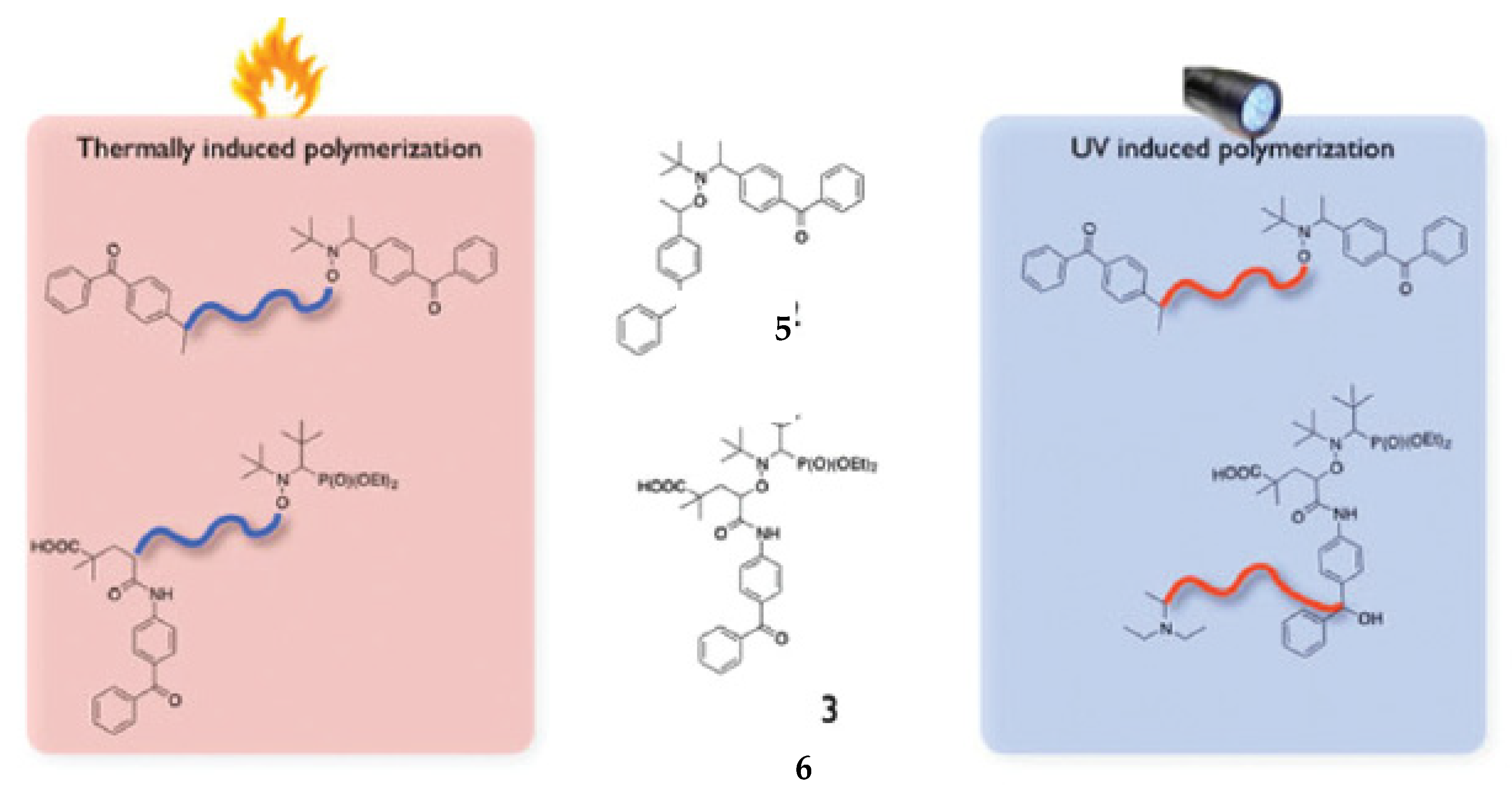
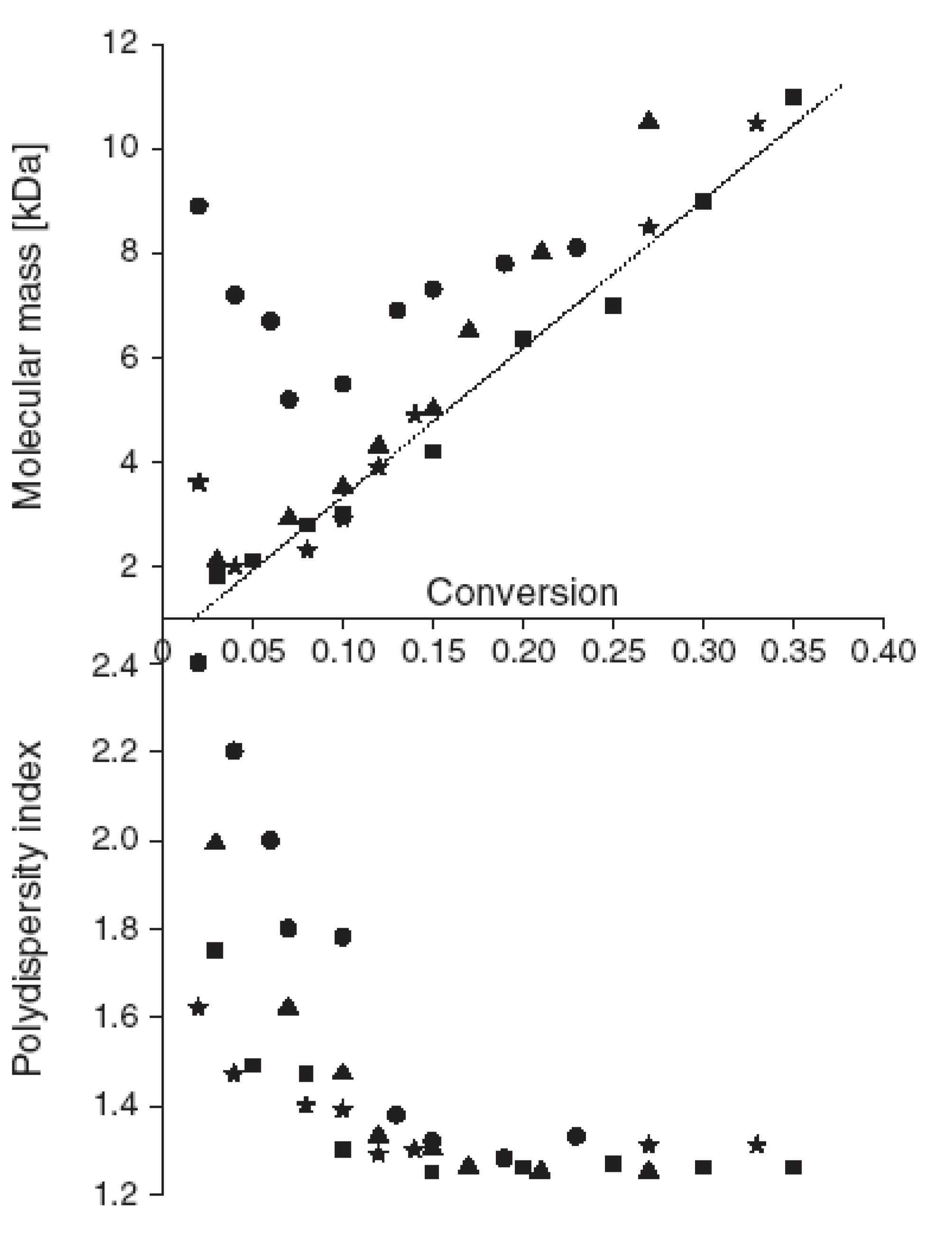
4. Nitroxide Mediated PhotoPolymerization NMP2
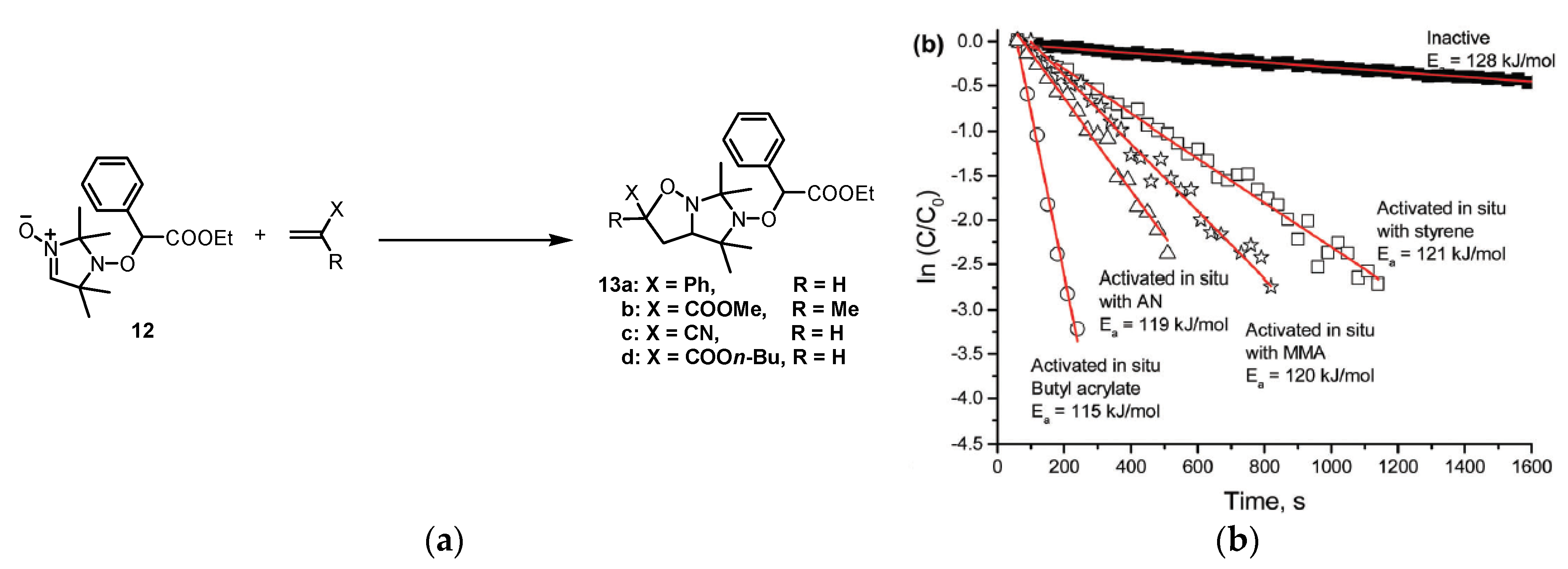
5. Chemically Initiated Nitroxide Mediated Polymerization CI-NMP
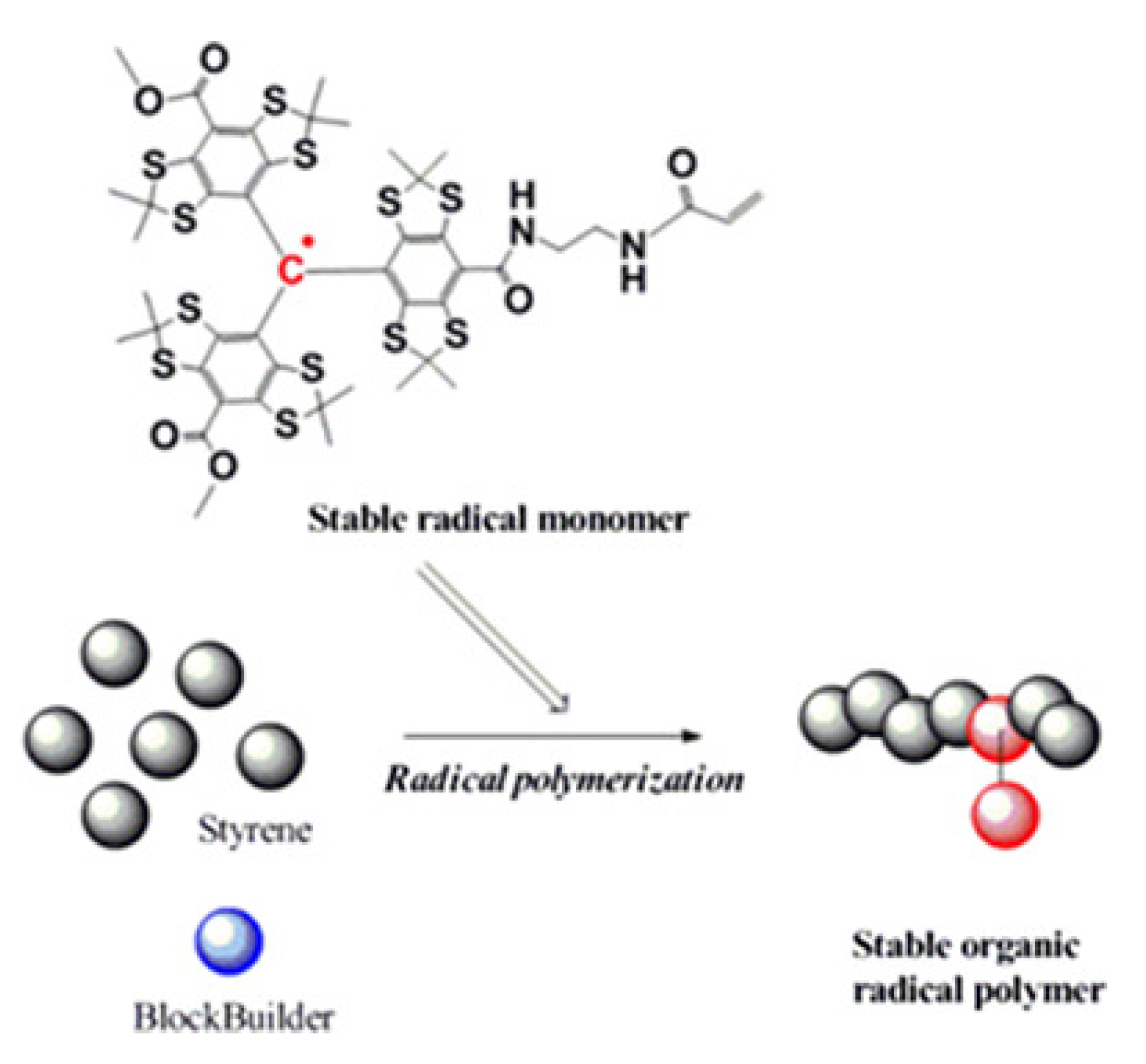
6. Spin Labeled Nitroxide Mediated Polymerization SL-NMP
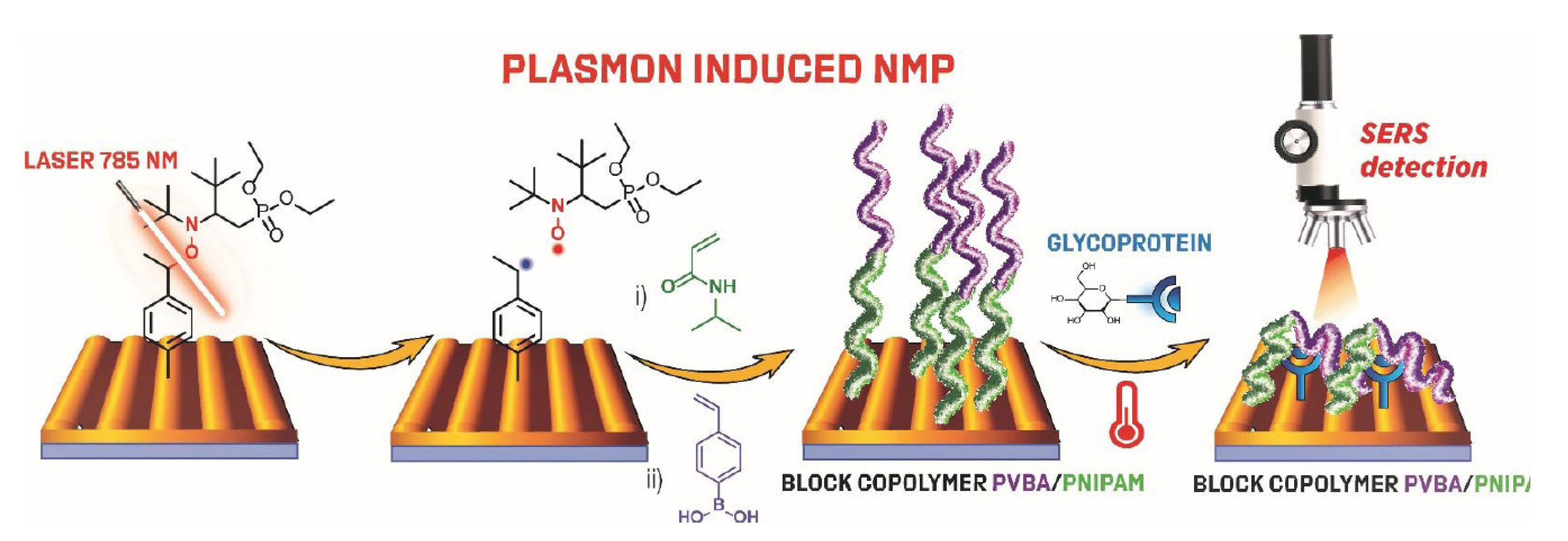
7. Plasmon Initiated Nitroxide Mediated Polymerization PI-NMP
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jones, L.W.; Major, R.T. Substituted O-alkyl hydroxylamines chemically related to medicinally valuable amines. J. Am. Chem. Soc. 1927, 49, 1527–1540. [Google Scholar] [CrossRef]
- Kovtun, G.A.; Aleksandrov, A.L.; Golubev, V.A. Interaction of Peroxide Radicals with Esters of Hydroxylamines. Russ. Chem. Bull. 1974, 23, 2115–2121, Izv. Akad. Nauk SSSR, Ser. Khim.1974, 2197–2203. [Google Scholar] [CrossRef]
- Solomon, D.H.; Rizzardo, E.; Cacioli, P. Polymerization Process and Polymers Produced Thereby. U.S. Patent 4,581,429, 4 August 1986. [Google Scholar]
- Bertin, D.; Gigmes, D.; Marque, S.R.A.; Tordo, P. Kinetic Subtleties of Nitroxide Mediated Polymerization. Chem. Soc. Rev. 2011, 40, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Matyjaszewski, K. Controlled Radical Polymerization. Current Opin. Solid State Materials Sci. 1996, 1996. 1, 769–776. [Google Scholar] [CrossRef]
- Bagryanskaya, E.G.; Marque, S.R.A. Kinetic Aspects of Nitroxide-Mediated Polymerization. In RSC Polymer Chemistry Series, n°=19, Nitroxide Mediated Polymerization: From Fundamentals to Applications in Materials Sciences; Gigmes, D., Ed.; Royal Society of Chemistry: London, UK, 2016; Chapter 2; pp. 45–113. [Google Scholar]
- Greszta, D.; Mardare, D.; Matyjaszewski, K. “Living” Radical Polymerization. 1. Possibilities and Limitations. Macromolecules 1994, 27, 638–644. [Google Scholar] [CrossRef]
- Kreutzer, J.; Yagci, Y. Metal Free Reversible-Deactivation Radical Polymerizations: Advances, Challenges, and Opportunities. Polymers 2018, 10, 35. [Google Scholar] [CrossRef]
- Grubbs, R.B. Nitroxide-Mediated Radical Polymerization: Limitations and Versatility. Polymer Rev. 2011, 51, 104–137. [Google Scholar] [CrossRef]
- Tebben, L.; Studer, A. Nitroxides: Applications in Synthesis and in Polymer Chemistry. Angew. Chem. Int. Ed. 2011, 50, 5034–5068. [Google Scholar] [CrossRef]
- Fischer, H. The Persistent Radical Effect: A Principle for Selective Radical Reactions and Living Radical Polymerizations. Chem. Rev. 2001, 101, 3581–3610. [Google Scholar] [CrossRef]
- Fischer, H.; Souaille, M. The Persistent Radical Effect in Living Radical Polymerization - Borderline Cases and Side-Reactions. Macromol. Symp. 2001, 174, 231–240. [Google Scholar] [CrossRef]
- Fortunatti, C.; Sarmoria, C.; Brandolin, A.; Asteasuain, M. Theoretical Analysis of Nitroxide-Mediated Copolymerization of Styrene and A-Methyl-Styrene Under Different Operating Policies and Reactor Designs. Macromol. React. Engineer. 2013, 8, 260–281. [Google Scholar] [CrossRef]
- Fukuda, T.; Goto, A.; Ohno, K. Mechanisms and Kinetics of Living Radical Polymerizations. Macromol. Rapid Commun. 2000, 21, 151–165. [Google Scholar] [CrossRef]
- Goto, A.; Fukuda, T. Kinetics of Living Radical Polymerization. Progr. Polym. Sci. 2004, 29, 329–385. [Google Scholar] [CrossRef]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/Living Radical Polymerization: Features, Developments, and Perspectives. Progr. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Gao, H.; Matyjaszewski, K. Synthesis of Functional Polymers with Controlled Architecture by CRP of Monomers in the Presence of Cross-Linkers: From Stars to Gels. Progr. Polym. Sci. 2009, 34, 317–350. [Google Scholar] [CrossRef]
- Grishin, D.F.; Grishin, I.D. Controlled Radical Polymerization: Prospects for Application for Industrial Synthesis of Polymers. Russ. J. Appl. Chem. 2012, 84, 2021–2028. [Google Scholar] [CrossRef]
- Kermagoret, A.; Gigmes, D. Combined Nitroxide Mediated Radical Polymerization Techniques for Block Copolymer Synthesis. Tetrahedron 2016, 72, 7672–7685. [Google Scholar] [CrossRef]
- Destarac, M. Industrial Development of Reversible-Deactivation Radical Polymerization: Is the Induction Period Over? Polym. Chem. 2018, 9, 4947–4967. [Google Scholar] [CrossRef]
- Destarac, M. Controlled Radical Polymerization: Industrial Stakes, Obstacles and Achievements. Macromol. React. Engineer. 2010, 4, 165–179. [Google Scholar] [CrossRef]
- Sciannamea, V.; Jérôme, R.; Detrembleur, C. In-Situ Nitroxide-Mediated Radical Polymerization (NMP) Processes: Their Understanding and Optimization. Chem. Rev. 2008, 108, 1104–1126. [Google Scholar] [CrossRef]
- Kolyakina, E.V.; Grishin, D.F. Nitroxide Radicals Formed in Situ as Polymer Chain Growth Regulators. Russ. Chem. Rev. 2009, 78, 535–568. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Ataman, N.C.; Mocny, P.; Wang, J.; Moraes, J.; Klok, H.A. Surface-Initiated Controlled Radical Polymerization: State-of-the-Art, Opportunities, and Challenges in Surface and Interface Engineering with Polymer Brushes. Chem. Rev. 2017, 117, 1105–1318. [Google Scholar] [CrossRef] [PubMed]
- Charleux, B.; Nicolas, J. Water-Soluble SG1-Based Alkoxyamines: A Breakthrough in Controlled/Living Free-Radical Polymerization in Aqueous Dispersed Media. Polymer 2007, 48, 5813–5833. [Google Scholar] [CrossRef]
- Zetterlund, P.B.; Thickett, S.C.; Perrier, S.; Bourgeat-Lami, E.; Lansalot, M. Controlled/Living Radical Polymerization in Dispersed Systems: An Update. Chem. Rev. 2015, 115, 9745–9800. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.F. Controlled/Living Radical Polymerization in Aqueous Dispersed Systems. Progr. Polym. Sci. 2008, 33, 365–398. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E. Alkoxyamine-Initiated Living Radical Polymerization: Factors Affecting Alkoxyamine Homolysis Rates. Macromolecules 1995, 28, 8722–8728. [Google Scholar] [CrossRef]
- Johnson, C.; Moad, G.; Solomon, D.H. The Application of Supercomputers in Modeling Chemical Reaction Kinetics: Kinetic Simulation of“Quasi-Living”Radical Polymerization. Aust. J. Chem. 1990, 43, 1215–1230. [Google Scholar] [CrossRef]
- Gigmes, D.; Marque, S.R.A. Nitroxide Mediated Polymerization and its Applications. In Encyclopedia of Radicals in Chemistry, Biology, and Materials; Chatgilialoglu, C., Studer, A., Eds.; Wiley: Chichester, UK, 2012; pp. 1813–1850. [Google Scholar]
- This term has been coined by Daikh, B.E.; Finke, R.G. The Persistent Radical Effect: A Prototype Example of Extreme, 105 to 1, Product Selectivity in a Free-Radical Reaction Involving Persistent. CoII[Macrocycle] and Alkyl Free Radicals. J. Am. Chem. Soc. 1992, 114, 2938–2943. [Google Scholar]
- Fischer, H. Unusual Selectivities of Radical Reactions by Internal Suppression of Fast Modes. J. Am. Chem. Soc. 1986, 108, 3925–3927. [Google Scholar] [CrossRef]
- Kothe, T.; Marque, S.; Martschke, R.; Popov, M.; Fischer, H. Radical Reaction Kinetics During Homolysis of N-alkoxyamines: Verification of the Persistent Radical Effect. J. Chem. Soc., Perkin Trans. 1998, 2, 1553–1559. [Google Scholar] [CrossRef]
- Yoshikawa, C.; Goto, A.; Fukuda, T. Quantitative Comparison of Theory and Experiment on Living Radical Polymerization Kinetics. 1. Nitroxide-Mediated Polymerization. Macromolecules 2002, 35, 5801–5807. [Google Scholar] [CrossRef]
- Tang, W.; Fukuda, T.; Matyjaszewski, K. Reevaluation of Persistent Radical Effect in NMP. Macromolecules 2006, 39, 4332–4337. [Google Scholar] [CrossRef]
- Ohno, K.; Tsujii, Y.; Miyamoto, T.; Fukuda, T.; Goto, M.; Kobayashi, K.; Akaike, T. Synthesis of a Well-Defined Glycopolymer by Nitroxide-Controlled Free Radical Polymerization. Macromolecules 1998, 31, 1064–1069. [Google Scholar] [CrossRef]
- Lutz, J.F.; Desmazes, P.L. The Persistent Radical Effect in Nitroxide Mediated Polymerization: Experimental Validity. Macromol. Rapid Commun. 2001, 22, 189–193. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-Mediated Polymerization. Progr. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Garcia-Valdez, O.; Champagne, P.; Cunningham, M.F. Graft Modification of Natural Polysaccharides via Reversible Deactivation Radical Polymerization. Progr. Polym. Sci. 2018, 76, 151–173. [Google Scholar] [CrossRef]
- Darabi, A.; Jessop, P.G.; Cunningham, M.F. CO2-Responsive Polymeric Materials: Synthesis, Self-Assembly, and Functional Applications. Chem. Soc. Rev. 2016, 45, 4391–4436. [Google Scholar] [CrossRef]
- Wong, E.H.H.; Junkers, T.; Barner-Kowollik, C. Enhanced Spin Capturing Polymerization: An Efficient and Versatile Protocol for Controlling Molecular Weight Distributions. J. Polym. Sci. A Polym. Chem. 2008, 46, 7273–7279. [Google Scholar] [CrossRef]
- Wong, E.H.H.; Stenzel, M.H.; Junkers, T.; Barner-Kowollik, C. The Kinetics of Enhanced Spin Capturing Polymerization: Influence of the Nitrone Structure. J. Polym. Sci. A Polym. Chem. 2009, 47, 1098–1107. [Google Scholar] [CrossRef]
- Wong, E.H.H.; Boyer, C.; Stenzel, M.H.; Barner-Kowollik, C.; Junkers, T. Spin Capturing with Nitrones: Radical Coupling Reactions with Concurrent Introduction of Mid-Chain Functionality. Chem. Commun. 2010, 46, 1959–1961. [Google Scholar] [CrossRef]
- Junkers, T.; Wong, E.H.H.; Stenzel, M.H.; Barner-Kowollik, C. Formation Efficiency of ABA Blockcopolymers via Enhanced Spin Capturing Polymerization (ESCP): Locating the Alkoxyamine Function. Macromolecules 2009, 42, 5027–5035. [Google Scholar] [CrossRef]
- Wong, E.H.H.; Junkers, T.; Barner-Kowollik, C. Nitrones in Synthetic Polymer Chemistry. Polym. Chem. 2011, 2, 1008–1010. [Google Scholar] [CrossRef]
- Dommanget, C.; Boisson, C.; Charleux, B.; D’Agosto, F.; Monteil, V.; Boisson, F.; Junkers, T.; Barner-Kowollik, C.; Guillaneuf, Y.; Gigmes, D. Enhanced Spin Capturing Polymerization of Ethylene. Macromolecules 2013, 46, 29–36. [Google Scholar] [CrossRef]
- Wong, E.H.H.; Stenzel, M.H.; Junkers, T.; Barner-Kowollik, C. Spin Capturing with “Clickable” Nitrones: Generation of Miktoarmed Star Polymers. Macromolecules 2010, 43, 3785–3793. [Google Scholar] [CrossRef]
- Nikitin, S.V.; Parkhomenko, D.A.; Edeleva, M.V.; Bagryanskaya, E.G. Enhanced spin capturing polymerization: Numerical investigation of mechanism. J. Polym. Sci.: Part. A: Polym. Chem. 2015, 53, 2546–2556. [Google Scholar] [CrossRef]
- Scaiano, J.C.; Connolly, T.J.; Mohtat, N.; Pliva, C.N. Exploratory Study of the Quenching of Photosensitizers by Initiators of Free Radical “Living” Polymerization. Can. J. Chem. 1997, 75, 92–97. [Google Scholar] [CrossRef]
- Yoshida, E. Photo-Living Radical Polymerization of Methyl Methacrylate by a Nitroxide Mediator. Colloid. Polym. Sci. 2008, 286, 1663–1666. [Google Scholar] [CrossRef]
- Yoshida, E. Nitroxide-Mediated Photo-Living Radical Polymerization of Methyl Methacrylate in Solution. Colloid. Polym. Sci. 2010, 288, 1639–1643. [Google Scholar] [CrossRef]
- Guillaneuf, Y.; Bertin, D.; Gigmes, D.; Versace, D.-L.; Lalevée, J.; Fouassier, J.P. Toward Nitroxide-Mediated Photopolymerization. Macromolecules 2010, 43, 2204–2212. [Google Scholar] [CrossRef]
- Guillaneuf, Y.; Versace, D.-L.; Bertin, D.; Lalevée, J.; Gigmes, D.; Fouassier, J.P. Importance of the Position of the Chromophore Group on the Dissociation Process of Light Sensitive Alkoxyamines. Macromol. Rapid Commun. 2010, 31, 1909–1913. [Google Scholar] [CrossRef] [PubMed]
- Huix-Rotllant, M.; Ferré, N. Theoretical Study of the Photochemical Initiation in Nitroxide-Mediated Photopolymerization. J. Phys. Chem. A 2014, 118, 4464–4470. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Tasdelen, M.A.; Laun, J.; Junkers, T.; Yagci, Y.; Matyjaszewski, K. Photomediated Controlled Radical Polymerization. Progr. Polym. Sci. 2016, 62, 73–125. [Google Scholar] [CrossRef]
- Morris, J.; Telitel, S.; Fairfull-Smith, K.E.; Bottle, S.E.; Lalevée, J.; Clément, J.-L.; Guillaneuf, Y.; Gigmes, D. Novel Polymer Synthesis Methodologies Using Combinations of Thermally- and Photochemically-Induced Nitroxide Mediated Polymerization. Polym. Chem. 2015, 6, 754–763. [Google Scholar] [CrossRef]
- Garra, P.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Photopolymerization Processes of Thick Films and in Shadow Areas: A Review for the Access to Composites. Polym. Chem. 2017, 8, 7088–7101. [Google Scholar] [CrossRef]
- Brémond, P.; Marque, S.R.A. First Proton Triggered C—ON Bond Homolysis in Alkoxyamines. Chem. Commun. 2011, 47, 4291–4293. [Google Scholar] [CrossRef] [PubMed]
- Edeleva, M.V.; Kirilyuk, I.A.; Zhurko, I.F.; Parkhomenko, D.A.; Tsentalovich, Y.P.; Bagryanskaya, E.G. pH-Sensitive C–ON Bond Homolysis of Alkoxyamines of Imidazoline Series with Multiple Ionizable Groups as an Approach for Control of Nitroxide Mediated Polymerization. J. Org. Chem. 2011, 76, 5558–5573. [Google Scholar] [CrossRef]
- Brémond, P.; Koïta, A.; Marque, S.R.A.; Pesce, V.; Roubaud, V.; Siri, D. Chemically Trigerred C—ON Bond Homolysis of Alkoxyamines. Quaternization of the Alkyl Fragments. Org. Lett. 2012, 14, 358–361. [Google Scholar] [CrossRef]
- Audran, G.; Bagryanskaya, E.; Bagryanskaya, I.; Brémond, P.; Edeleva, M.; Marque, S.R.A.; Parkhomenko, D.; Tretyakov, E.; Zhivetyeva, S. C—ON Bond Homolysis of Alkoxyamines Triggered by Paramagnetic Copper(II) Salts. Inorg. Chem. Frontier 2016, 3, 1464–1472. [Google Scholar] [CrossRef]
- Audran, G.; Bagryanskaya, E.; Bagryanskaya, I.; Edeleva, M.; Marque, S.R.A.; Parkhomenko, D.; Tretyakov, E.; Zhivetyeva, S. Zinc(II) Hexafluoroacetylacetonate Complexes of Alkoxyamines: NMR and Kinetic Investigations. First Step for a New Way to Prepare Hybrid Materials. ChemistrySelect 2017, 2, 3584–3593. [Google Scholar] [CrossRef]
- Edeleva, M.V.; Bagryanskaya, E.G.; Marque, S.R.A. Imidazoline and Imidazolidine Nitroxides as Controlling Agents in Nitroxide-Mediated Pseudo-living Radical Polymerization. Russ. Chem. Rev. 2018, 87, 328–349. [Google Scholar]
- Bagryanskaya, E.; Brémond, P.; Edeleva, M.; Marque, S.R.A.; Parkhomenko, D.; Roubaud, V.; Siri, D. Chemically Triggered C—ON Bond Homolysis in Alkoxyamines. Part 2: DFT Investigation and Application of the pH Effect on NMP. Macromol. Rapid Commun. 2012, 33, 152–157. [Google Scholar] [CrossRef]
- Audran, G.; Bagryanskaya, E.; Edeleva, M.; Marque, S.R.A.; Parkhomenko, D.; Tretyakov, E.; Zhivetyeva, S. Coordination-Initiated Nitroxide-Mediated Polymerization (CI-NMP). Aust. J. Chem. 2018, 71, 334–340. [Google Scholar] [CrossRef]
- Edeleva, M.; Morozov, D.; Parkhomenko, D.; Polienko, Y.; Iurchenkova, A.; Kirilyuk, I.; Bagryanskaya, E. Versatile approach to activation of alkoxyamine homolysis by 1, 3-dipolar cycloaddition for efficient and safe nitroxide mediated polymerization. Chemical Communications 2019, 55, 190–193. [Google Scholar] [CrossRef]
- Bagryanskaya, E.G.; Krumkacheva, O.; Fedin, M.V.; Marque, S.R.A. Development and Application of Spin Traps, Spin Probes, and Spin Labels. Methods Enzym. 2015, 563, 365–396. [Google Scholar]
- Gentilini, C.; Franchi, P.; Mileo, E.; Polizzi, S.; Lucarini, M.; Pasquato, L. Formation of Patches on 3D SAMs Driven by Thiols with Immiscible Chains Observed by ESR Spectroscopy. Angew. Chem. Int. Ed. 2009, 48, 3060–3064. [Google Scholar] [CrossRef]
- Ong, Q.; Luo, Z.; Stellacci, F. Characterization of Ligand Shell for Mixed-Ligand Coated Gold Nanoparticles. Acc. Chem. Res. 2017, 50, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Naveed, K.-U.-R.; Wang, L.; Yu, H.; Ullah, R.S.; Haroon, M.; Fahad, S.; Li, J.; Elshaarani, T.; Khan, R.U.; Nazir, A. Recent Progress in the Electron Paramagnetic Resonance Study of Polymers. Polym. Chem. 2018, 9, 3306–3335. [Google Scholar] [CrossRef]
- Ebdon, J.R.; Huckerby, T.N.; Hunt, B.J.; Rimmer, S. Radical Polymerizations of Methyl Methacrylate Initiated by Methyl 2-[(4-Diphenylmethylene)-2, 5-Cyclohexadienyl]-2-Methyl-Propanoate: A Model System for So-Called “quasi-living” polymerization of methyl methacrylate initiated by phenylazotriphenylmethane. Polymer 1998, 39, 4943–4948. [Google Scholar] [CrossRef]
- Otsu, T.; Yoshida, M.; Tazaki, T. A Model for Living Radical Polymerization. Makromol. Chem. Rapid Commun. 1982, 3, 133–140. [Google Scholar] [CrossRef]
- Acar, M.H.; Yagci, Y. Studies on the Block Copolymerization of Methacrylo-Nitrile and Hexafluorobutylmethacrlate Using Phenylazo-Triphenylmethane as Thermal Iniferter. J. Macromol. Sci.: Part. A – Chem. 1991, 28, 177–183. [Google Scholar] [CrossRef]
- Chernikova, E.V.; Garina, E.S.; Zeremskii, M.Y.; Olenin, A.V.; Lachinov, M.B.; Golubev, V.B. Quasiliving radical polymerization of methyl methacrylate in the presence of phenylazotriphenylmethane. Polym. Sci. Ser. A. 1995, 37, 988–993. [Google Scholar]
- Audran, G.; Bagryanskaya, E.; Bagryanskaya, I.; Brémond, P.; Edeleva, M.; Marque, S.R.A.; Parkhomenko, D.; Rogozhnikova, O.Y.; Tormyshev, V.M.; Tretyakov, E.V.; et al. Trityl-based Alkoxyamines as NMP Controlers and Spin-labels. Polym. Chem. 2016, 7, 6490–6499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Monteiro, M.J.; Jia, Z. Stable Organic Radical Polymers: Synthesis and Applications. Polym. Chem. 2016, 7, 5589–5614. [Google Scholar] [CrossRef]
- Hansen, K.-A.; Blinco, J.P. Nitroxide Radical Polymers – a Versatile Material Class for High-Tech Applications. Polym. Chem. 2018, 9, 1479–1516. [Google Scholar] [CrossRef]
- Edeleva, M.V.; Marque, S.R.A.; Rogozhnikova, O.Y.; Tormyshev, V.M.; Troitskaya, T.I.; Bagryanskaya, E.G. Radical Polymerization of Radical-labelled Monomers: The Triarylmethyl-based Radical Monomer as an Example. J. Polym. Sci.: Part. A: Polym. Chem. 2018, 56, 2656–2664. [Google Scholar] [CrossRef]
- See Accounts of Chemical Research special issue “Nanochemistry for Plasmonics and Plasmonics for Nanochemistry”.
- Nam, J.-M.; Liz-Marzán, L.; Halas, N. Chemical Nanoplasmonics: Emerging Interdisciplinary Research Field at Crossroads Between Nanoscale Chemistry and Plasmonics. Acc. Chem. Res. 2019, 52, 2995–2996. [Google Scholar] [CrossRef]
- Wang, P.; Nasir, M.E.; Krasavin, A.V.; Dickson, W.; Jiang, Y.; Zayats, A.V. Plasmonic Metamaterials for Nanochemistry and Sensing. Acc. Chem. Res. 2019, 52, 3018–3028. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Chen, X.-J.; Huang, Y.-F.; Wu, D.-Y.; Tian, Z.-Q. Plasmon-Mediated Chemical Reactions on Nanostructures Unveiled by Surface-Enhanced Raman Spectroscopy. Acc. Chem. Res. 2019, 52, 2784–2792. [Google Scholar] [CrossRef]
- Gargiulo, J.; Berté, R.; Li, Y.; Maier, S.A.; Cortés, E. From Optical to Chemical Hot Spots in Plasmonics. Acc. Chem. Res. 2019, 52, 2525–2535. [Google Scholar] [CrossRef]
- Murphy, C.J.; Chang, H.-H.; Falagan-Lotsch, P.; Gole, M.T.; Hofmann, D.M.; Hoang, K.N.L.; McClain, S.M.; Meyer, S.M.; Turner, J.G.; Unnikrishnan, M.; et al. Virus-Sized Gold Nanorods: Plasmonic Particles for Biology. Acc. Chem. Res. 2019, 52, 2124–2135. [Google Scholar] [CrossRef]
- Phan-Quang, G.C.; Han, X.; Koh, C.S.L.; Sim, H.Y.F.; Lay, C.L.; Leong, S.X.; Lee, Y.H.; Pazos-Perez, N.; Alvarez-Puebla, R.A.; Ling, X.Y. Three-Dimensional Surface-Enhanced Raman Scattering Platforms: Large-Scale Plasmonic Hotspots for New Applications in Sensing, Microreaction, and Data Storage. Acc. Chem. Res. 2019, 52, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yao, X.K.J. Recent development of plasmon-mediated photocatalysts and their potential in selectivity regulation. J. Mater. Chem. A 2018, 6, 1941–1946. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef] [PubMed]
- Kazuma, E.; Kim, Y. Mechanistic Studies of Plasmon Chemistry on Metal Catalysts. Angew. Chem. Int. Ed. 2019, 58, 4800–4808. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Chen, X.-J.; Yi, J.; Li, J.-F.; Wu, D.-Y.; Tian, Z.-Q. From plasmon-enhanced molecular spectroscopy to plasmon-mediated chemical reactions. Nat. Rev. Chem. 2018, 2, 216–230. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Chen, H.; Jiang, R.; Sun, L.-D.; Li, Q.; Wang, J.; Yu, J.C.; Yan, C.-H. Plasmonic Harvesting of Light Energy for Suzuki Coupling Reactions. J. Am. Chem. Soc. 2013, 135, 5588–5601. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Sarina, S.; Bo, A.; Jia, J.; Liu, H.; Arnold, D.P.; Huang, Y.; Wu, H.; Zhu, H. Visible Light-Driven Cross-Coupling Reactions at Lower Temperatures Using a Photocatalyst of Palladium and Gold Alloy Nanoparticles. ACS Catalysis 2014, 4, 1725–1734. [Google Scholar] [CrossRef]
- Guselnikova, O.; Olshtrem, A.; Kalachyova, Y.; Panov, I.; Postnikov, P.; Svorcik, V.; Lyutakov, O. Plasmon Catalysis on Bimetallic Surface—Selective Hydrogenation of Alkynes to Alkanes or Alkenes. The J. Phys. Chem. C 2018, 122, 26613–26622. [Google Scholar] [CrossRef]
- Landry, M.J.; Gellé, A.; Meng, B.Y.; Barrett, C.J.; Moores, A. Surface-Plasmon-Mediated Hydrogenation of Carbonyls Catalyzed by Silver Nanocubes under Visible Light. ACS Catalysis 2017, 7, 6128–6133. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Y.; Song, C.; Zheng, L.; Ma, N.; Liu, X.; Li, S.; Lin, L.; Li, M.; Xu, Y.; et al. Hybrid Au–Ag Nanostructures for Enhanced Plasmon-Driven Catalytic Selective Hydrogenation through Visible Light Irradiation and Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2018, 140, 864–867. [Google Scholar] [CrossRef]
- Chuang, C.-C.; Chu, H.-C.; Huang, S.-B.; Chang, W.-S.; Tuan, H.-Y. Laser-induced plasmonic heating in copper nanowire fabric as a photothermal catalytic reactor. Chem. Engineer. J. 2020, 379, 122285. [Google Scholar] [CrossRef]
- Guselnikova, O.; Postnikov, P.; Chehimi, M.M.; Kalachyovaa, Y.; Svorcik, V.; Lyutakov, O. Surface Plasmon-Polariton: A Novel Way To Initiate Azide–Alkyne Cycloaddition. Langmuir 2019, 35, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Connell, T.U.; Cadusch, J.J.; Roberts, A.; Chesman, A.S.R.; Gómez, D.E. Hot-Carrier Organic Synthesis via the Near-Perfect Absorption of Light. ACS Catalysis 2018, 8, 10331–10339. [Google Scholar] [CrossRef]
- Li, H.; Qin, F.; Yang, Z.; Cui, X.; Wang, J.; Zhangu, L. New Reaction Pathway Induced by Plasmon for Selective Benzyl Alcohol Oxidation on BiOCl Possessing Oxygen Vacancies. J. Am. Chem. Soc. 2017, 139, 3513–3521. [Google Scholar] [CrossRef]
- Ding, T.; Mertens, J.; Lombardi, A.; Scherman, O.A.; Baumberg, J.J. Light-Directed Tuning of Plasmon Resonances via Plasmon-Induced Polymerization Using Hot Electrons. ACS Photonics 2017, 4, 1453–1458. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Zhang, S.; Scherman, O.A.; Baumberg, J.J.; Ding, T.; Xu, H. Plasmon-directed polymerization: Regulating polymer growth with light. Nano Res. 2018, 11, 6384–6390. [Google Scholar] [CrossRef]
- Erzina, M.; Guselnikova, O.; Postnikov, P.; Elashnikov, R.; Kolska, Z.; Miliutina, E.; Svorcik, V.; Lyutakov, O. Plasmon-Polariton Induced, “From Surface” RAFT Polymerization, as a Way Toward Creation of Grafted Polymer Films with Thickness Precisely Controlled by Self-Limiting Mechanism. Adv. Mater. Interfaces 2018, 5, 1801042. [Google Scholar] [CrossRef]
- Guselnikova, O.; Marque, S.R.A.; Tretyakov, E.; Mares, D.; Jerabek, V.; Audran, G.; Joly, J.-P.; Trusova, M.; Švorčik, V.; Lyutakov, O.; et al. Unprecedented Plasmon-Induced Nitroxide-Mediated Polymerization (PI-NMP): A Method for Preparation of Functional Surfaces. J. Mat. Chem. A 2019, 7, 12414–12419. [Google Scholar] [CrossRef]

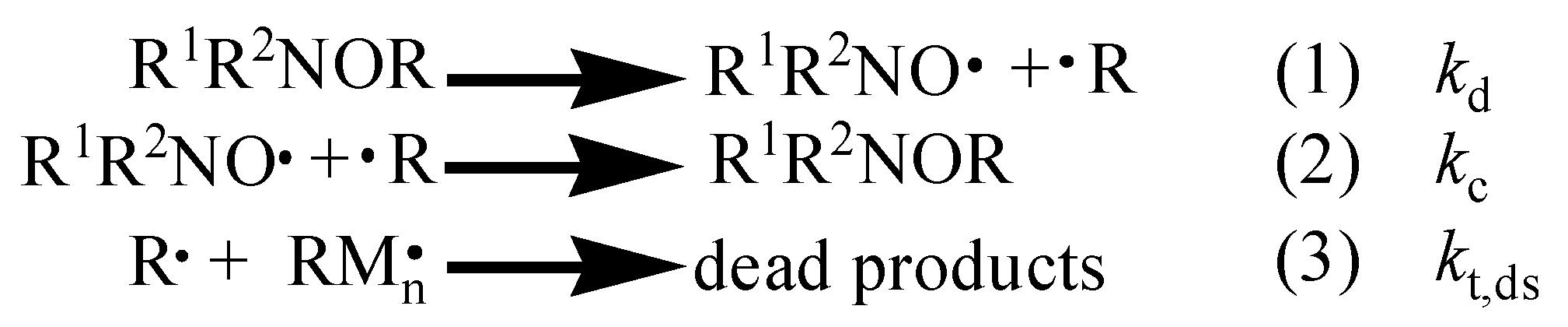





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Audran, G.; Bagryanskaya, E.G.; Marque, S.R.A.; Postnikov, P. New Variants of Nitroxide Mediated Polymerization. Polymers 2020, 12, 1481. https://doi.org/10.3390/polym12071481
Audran G, Bagryanskaya EG, Marque SRA, Postnikov P. New Variants of Nitroxide Mediated Polymerization. Polymers. 2020; 12(7):1481. https://doi.org/10.3390/polym12071481
Chicago/Turabian StyleAudran, Gérard, Elena G. Bagryanskaya, Sylvain R. A. Marque, and Pavel Postnikov. 2020. "New Variants of Nitroxide Mediated Polymerization" Polymers 12, no. 7: 1481. https://doi.org/10.3390/polym12071481
APA StyleAudran, G., Bagryanskaya, E. G., Marque, S. R. A., & Postnikov, P. (2020). New Variants of Nitroxide Mediated Polymerization. Polymers, 12(7), 1481. https://doi.org/10.3390/polym12071481







