Phenol-Furfural Resin/Montmorillonite Based High-Pressure Green Composite from Renewable Feedstock (Saccharum munja) with Improved Thermo-Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methodology
2.1. Materials
2.2. Extraction of Furfural from Saccharum Munja
2.3. Synthesis of Phenol-Furfural Resin
2.4. Exfoliation of Montmorillonite Clay
2.5. Synthesis of MMT/Phenol-Furfural Composite
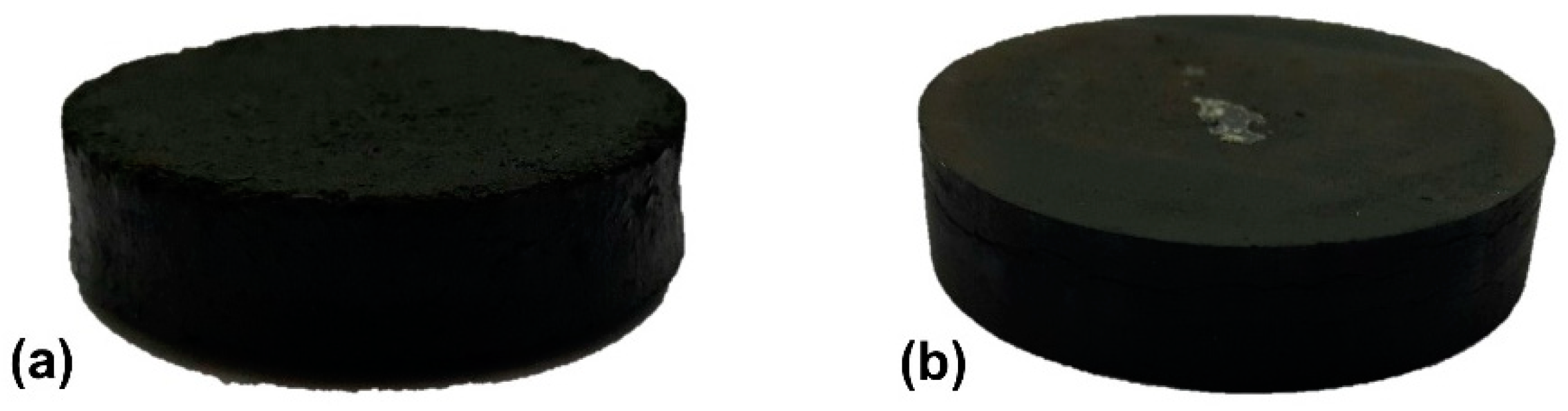
2.6. Manufacturing of High Pressure Composite
3. Results and Discussion
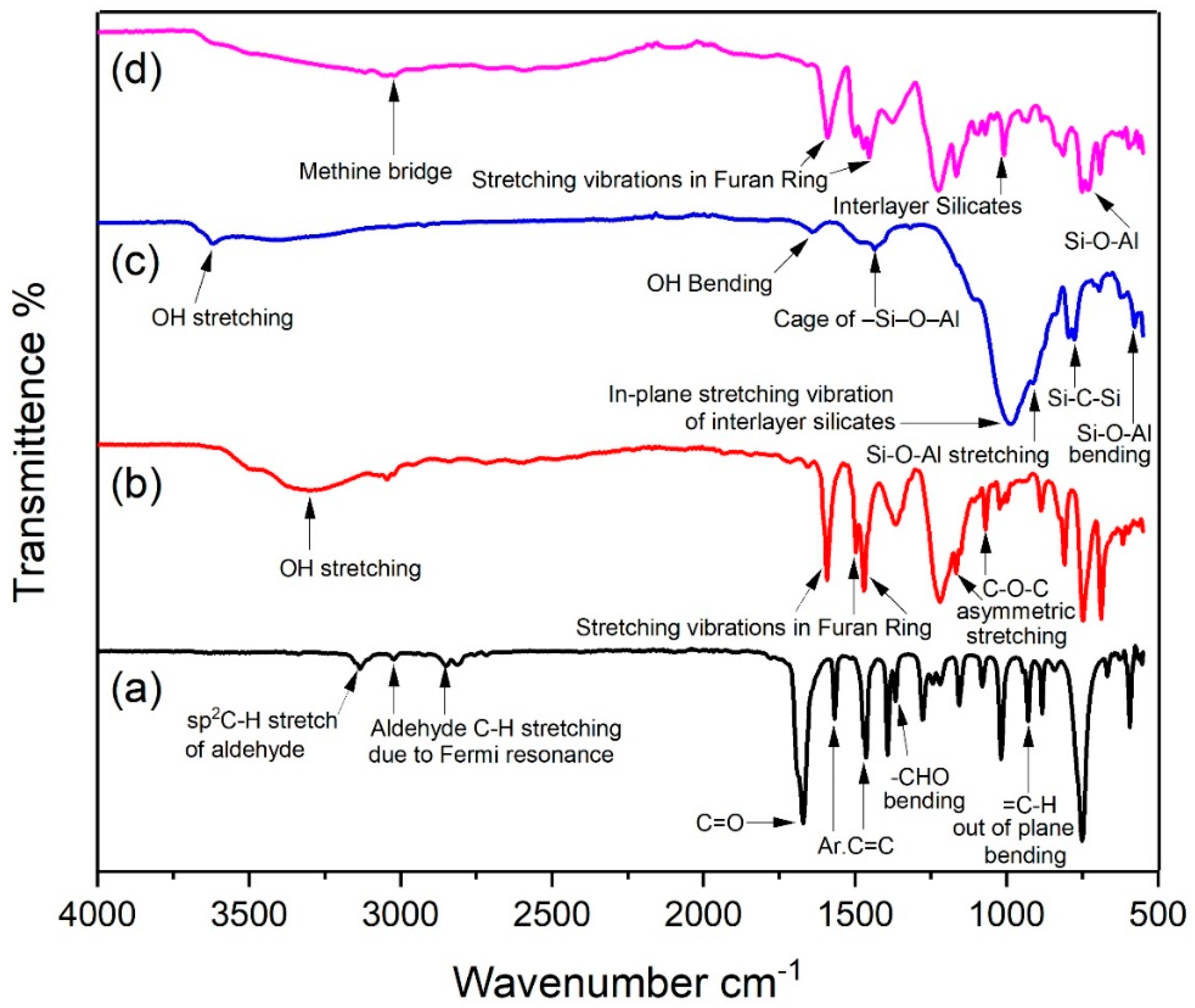
3.1. FTIR Results
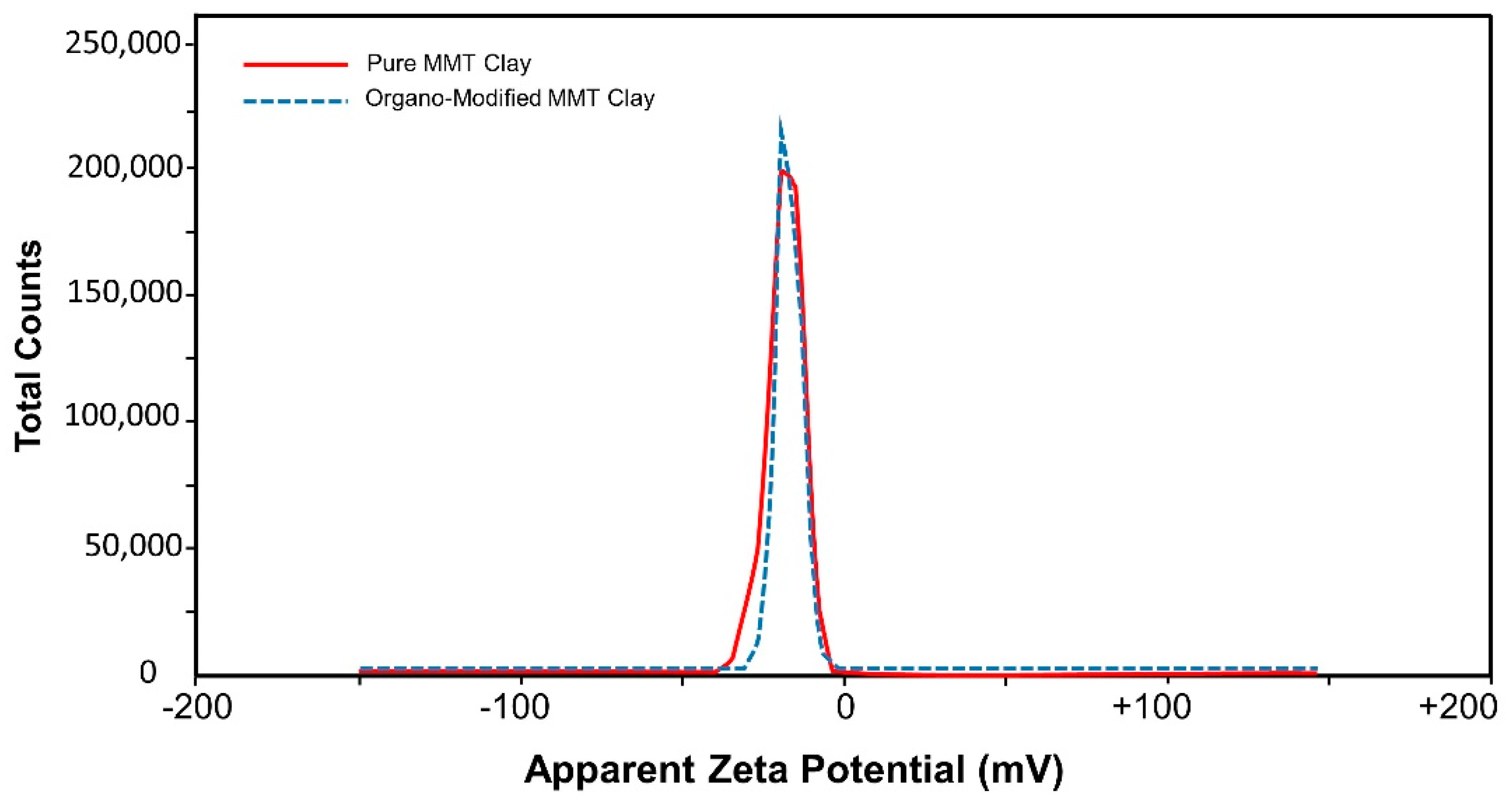
3.2. Particle Size Analysis
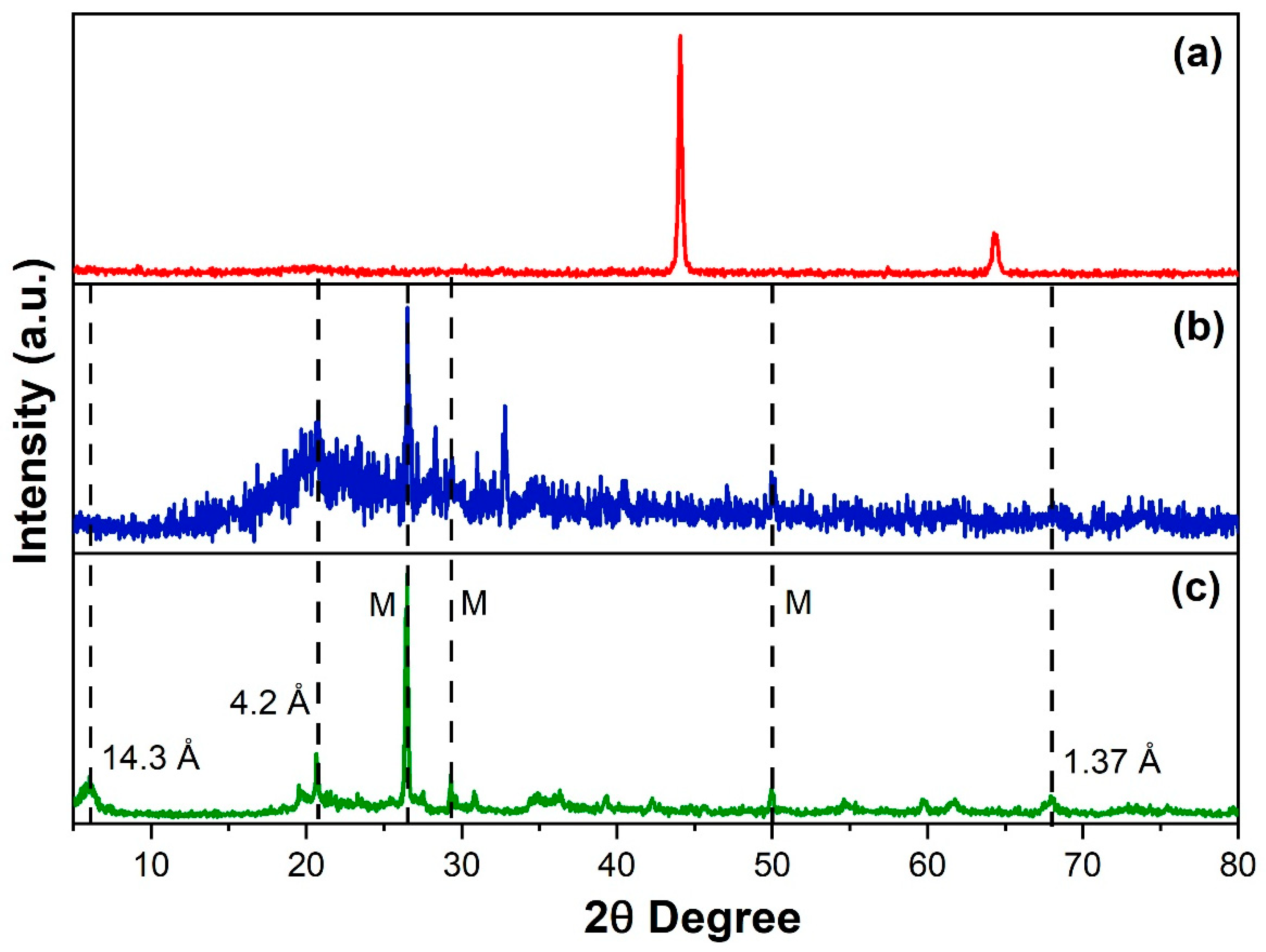
3.3. XRD Results
3.4. SEM Results
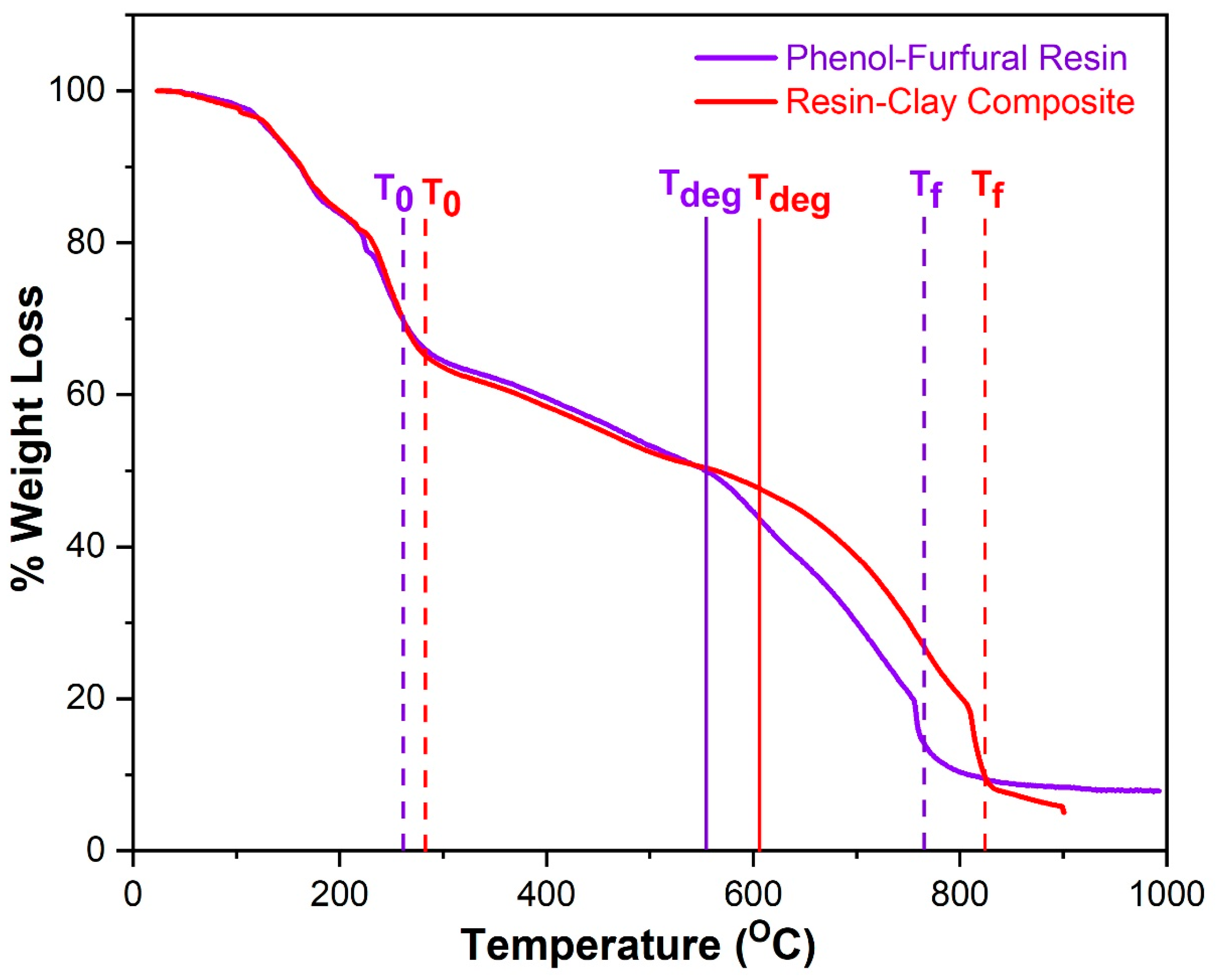
3.5. Thermogravimetric Analysis
3.6. Mechanical Testing
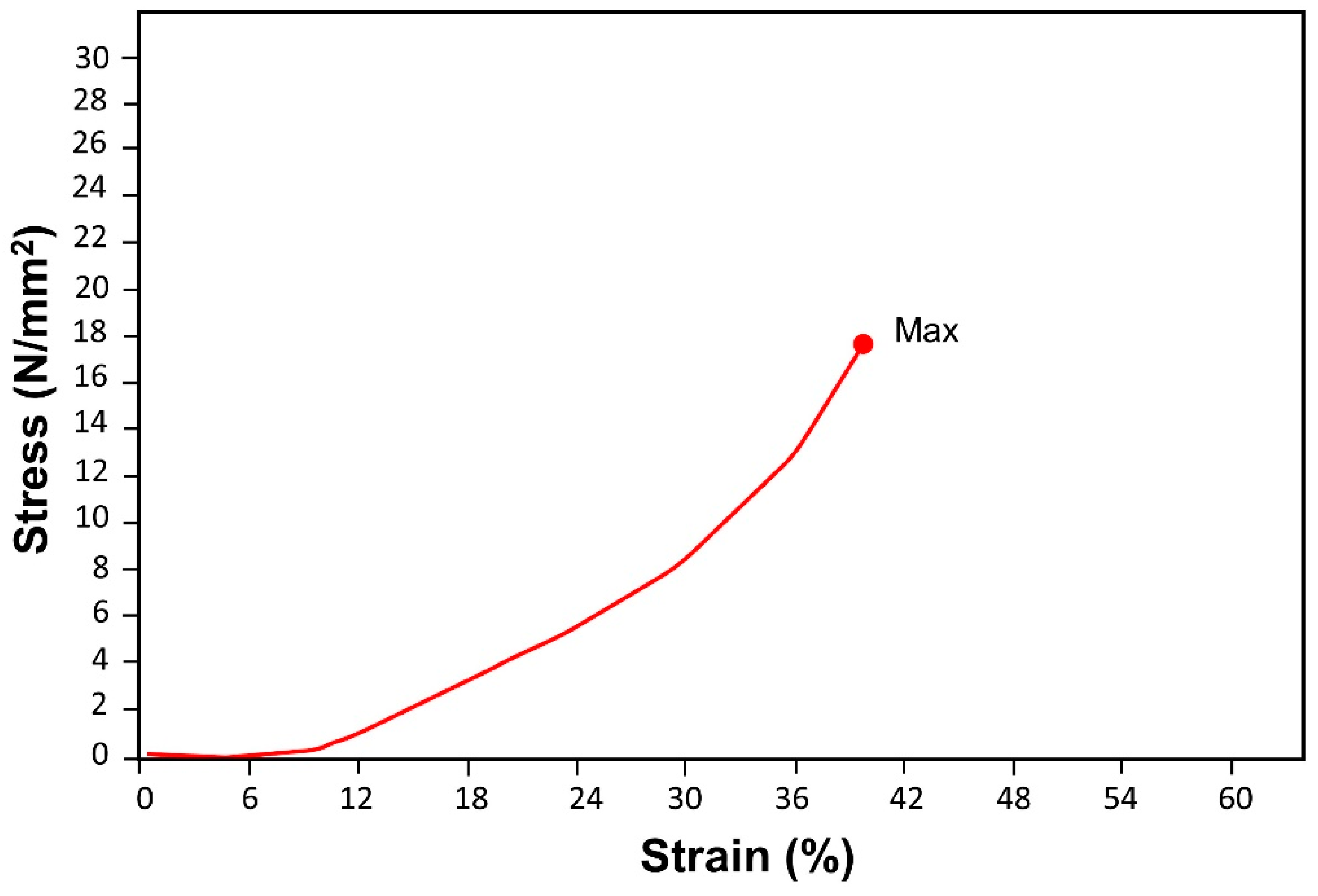
3.6.1. Compression Test
3.6.2. Rockwell Hardness Testing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, G.W.; Chheda, J.N.; Barrett, C.J.; Dumesic, J.A. Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates. Science 2005, 308, 1446–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tondi, G.; Schnabel, T. Bio-Based Polymers for Engineered Green Materials. Polymers 2020, 12, 775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Heo, Y.-J.; Park, M.; Park, S.-J. Recent advances in organic thermoelectric materials: Principle mechanisms and emerging carbon-based green energy materials. Polymers 2019, 11, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasudevan, P.; Gujral, G.; Madan, M. Saccharum munja Roxb., an underexploited weed. Biomass 1984, 4, 143–149. [Google Scholar] [CrossRef]
- Wu, Z.; Xi, X.; Lei, H.; Liang, J.; Liao, J.; Du, G. Study on Soy-Based Adhesives Enhanced by Phenol Formaldehyde Cross-Linker. Polymers 2019, 11, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhou, W.; Yang, L.; Chen, P.; Yan, C.; Cai, C.; Li, H.; Li, L.; Shi, Y. Glass fiber-reinforced phenol formaldehyde resin-based electrical insulating composites fabricated by selective laser sintering. Polymers 2019, 11, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurple, K.R. Foundry Resins. U.S. Patent 4,851,457, 25 July 1989. [Google Scholar]
- Cheng, S.; Yuan, Z.; Leitch, M.; Anderson, M.; Xu, C.C. Highly efficient de-polymerization of organosolv lignin using a catalytic hydrothermal process and production of phenolic resins/adhesives with the depolymerized lignin as a substitute for phenol at a high substitution ratio. Ind. Crop. Prod. 2013, 44, 315–322. [Google Scholar] [CrossRef]
- Wang, M.; Leitch, M.; Xu, C.C. Synthesis of phenol–formaldehyde resol resins using organosolv pine lignins. Eur. Polym. J. 2009, 45, 3380–3388. [Google Scholar] [CrossRef]
- Wang, M.; Xu, C.C.; Leitch, M. Liquefaction of cornstalk in hot-compressed phenol–water medium to phenolic feedstock for the synthesis of phenol–formaldehyde resin. Bioresour. Technol. 2009, 100, 2305–2307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chen, H.; Pizzi, A.; Li, Y.; Gao, Q.; Li, J. Characterization and application of urea-formaldehyde-furfural co-condensed resins as wood adhesives. BioResources 2014, 9, 6267–6276. [Google Scholar] [CrossRef] [Green Version]
- Lü, X.; Saka, S. New insights on monosaccharides’ isomerization, dehydration and fragmentation in hot-compressed water. J. Supercrit. Fluids 2012, 61, 146–156. [Google Scholar] [CrossRef]
- Garrett, E.R.; Dvorchik, B.H. Kinetics and mechanisms of the acid degradation of the aldopentoses to furfural. J. Pharm. Sci. 1969, 58, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Kenne, L.; Olsson, K.; Theander, O. The formation of 2-furaldehyde and formic acid from pentoses in slightly acidic deuterium oxide studied by 1H NMR spectroscopy. Carbohydr. Res. 1995, 276, 309–320. [Google Scholar] [CrossRef]
- Zhang, T.; Kumar, R.; Wyman, C.E. Enhanced yields of furfural and other products by simultaneous solvent extraction during thermochemical treatment of cellulosic biomass. RSC Adv. 2013, 3, 9809–9819. [Google Scholar] [CrossRef]
- Schuster, K.C.; Rohrer, C.; Eichinger, D.; Schmidtbauer, J.; Aldred, P.; Firgo, H. Environmentally friendly lyocell fibers. In Natural Fibers, Plastics and Composites; Springer: Boston, MA, USA, 2004; pp. 123–146. [Google Scholar]
- Lehnen, R.; Saake, B.; Nimz, H.H. Furfural and hydroxymethylfurfural as by-products of FORMACELL pulping. Holzforschung 2001, 55, 199–204. [Google Scholar] [CrossRef]
- Yi, J.; He, T.; Jiang, Z.; Li, J.; Hu, C. AlCl3 catalyzed conversion of hemicellulose in corn stover. Chin. J. Catal. 2013, 34, 2146–2152. [Google Scholar] [CrossRef]
- Carvalho, A.V.; da Lopes, A.M.; Bogel-Łukasik, R. Relevance of the acidic 1-butyl-3-methylimidazolium hydrogen sulphate ionic liquid in the selective catalysis of the biomass hemicellulose fraction. RSC Adv. 2015, 5, 47153–47164. [Google Scholar] [CrossRef] [Green Version]
- Brazdausks, P.; Vedernikovs, N.; Puke, M.; Kruma, I. Effect of the Acid Hydrolysis Temperature on the Conversion of Birch Wood Hemicelluloses into Furfural. in Key Engineering Materials. Key Eng. Mater. 2014, 604, 245–248. [Google Scholar] [CrossRef]
- Brazdausks, P.; Puke, M.; Vederņikovs, N.; Krūma, I. Influence of biomass pretreatment process time on furfural extraction from birch wood. Environ. Clim. Technol. 2013, 11, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.G.; Boyd, G.; Strickland, R. Adhesive properties of furfural-modified phenol-formaldehyde resins as oriented strandboard binders. Holzforschung 1994, 48, 262–267. [Google Scholar] [CrossRef]
- Brown, L.H. Resin forming reactions of furfural and phenol. Ind. Eng. Chem. 1952, 44, 2673–2675. [Google Scholar] [CrossRef]
- Pizzi, A.; Pasch, H.; Simon, C.; Rode, K. Structure of resorcinol, phenol, and furan resins by MALDI-TOF mass spectrometry and 13C NMR. J. Appl. Polym. Sci. 2004, 92, 2665–2674. [Google Scholar] [CrossRef]
- Pizzi, A.; Orovan, E.; Cameron, F. The development of weather-and boil-proof phenol-resorcinol-furfural cold-setting adhesives. Holz als Roh Werkst. 1984, 42, 467–472. [Google Scholar] [CrossRef]
- Oliveira, F.B.; Gardrat, C.; Enjalbal, C.; Frollini, E.; Castellan, A. Phenol–furfural resins to elaborate composites reinforced with sisal fibers—Molecular analysis of resin and properties of composites. J. Appl. Polym. Sci. 2008, 109, 2291–2303. [Google Scholar] [CrossRef]
- Brown, L.; Watson, D. Curing phenol-furfural resins. Ind. Eng. Chem. 1959, 51, 683–684. [Google Scholar] [CrossRef]
- Rivero, G.; Villanueva, S.; Manfredi, L.B. Furan resin as a replacement of phenolics: Influence of the clay addition on its thermal degradation and fire behaviour. Fire Mater. 2014, 38, 683–694. [Google Scholar] [CrossRef]
- Uddin, F. Montmorillonite: An introduction to properties and utilization. In Current Topics in the Utilization of Clay in Industrial and Medical Applications; IntechOpen: London, UK, 2018; p. 1. [Google Scholar]
- Uddin, F. Clays, nanoclays, and montmorillonite minerals. Metall. Mater. Trans. A 2008, 39, 2804–2814. [Google Scholar] [CrossRef]
- Byun, H.Y.; Choi, M.H.; Chung, I.J. Synthesis and characterization of resol type phenolic resin/layered silicate nanocomposites. Chem. Mater. 2001, 13, 4221–4226. [Google Scholar] [CrossRef]
- Kato, M.; Tsukigase, A.; Usuki, A.; Shimo, T.; Yazawa, H. Preparation and thermal properties of resole-type phenol resin–clay nanocomposites. J. Appl. Polym. Sci. 2006, 99, 3236–3240. [Google Scholar] [CrossRef]
- Choi, M.H.; Chung, I.J.; Lee, J.D. Morphology and curing behaviors of phenolic resin-layered silicate nanocomposites prepared by melt intercalation. Chem. Mater. 2000, 12, 2977–2983. [Google Scholar] [CrossRef]
- Pappas, J.; Patel, K.; Nauman, E. Structure and properties of phenolic resin/nanoclay composites synthesized by in situ polymerization. J. Appl. Polym. Sci. 2005, 95, 1169–1174. [Google Scholar] [CrossRef]
- Manfredi, L.B.; Puglia, D.; Kenny, J.M.; Vázquez, A. Structure-properties relationship in resol/montmorillonite nanocomposites. J. Appl. Polym. Sci. 2007, 104, 3082–3089. [Google Scholar] [CrossRef]
- Hu, X.; Sun, Z.; Zhu, X.; Sun, Z. Montmorillonite-synergized water-based intumescent flame retardant coating for plywood. Coatings 2020, 10, 109. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, J.; Fu, Y.; Chang, J. Synthesis and characterization of phenol–furfural resins using lignin modified by a low transition temperature mixture. RSC Adv. 2016, 6, 94588–94594. [Google Scholar] [CrossRef]
- Mohan Babu, K.; Mettilda, M. Studies on mechanical, thermal, and morphological properties of glass fibre reinforced polyoxymethylene nanocomposite. J. Appl. Chem. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Purbowatiningrum, R.S.; Hapsari, M.; Rafi’ah, F.H.; Haq, M.S. Synthesis of Furfural from Water Hyacinth (Eichornia croassipes). In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Purwokerto, Indonesia, 2017. [Google Scholar]
- Nsubuga, H.; Chanbasha, B.; Al-Muallem, H.A.S.; Kalanthoden, A.N. Isolation, characterization and evaluation of photochemical potential of rice husk-based furfural via continuous flow reactor. J. Environ. Chem. Eng. 2016, 4, 857–863. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, X.; Sun, Z. Efficient flame-retardant and smoke-suppression properties of MgAlCO3-LDHs on the intumescent fire retardant coating for steel structures. Prog. Org. Coat. 2019, 135, 291–298. [Google Scholar] [CrossRef]
- Akyüz, S.; Akyüz, T.; Yakar, A. FT-IR spectroscopic investigation of adsorption of 3-aminopyridine on sepiolite and montmorillonite from Anatolia. J. Mol. Struct. 2001, 565, 487–491. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, X.; Sun, Z. Effect of CaAlCO3-LDHs on fire resistant properties of intumescent fireproof coatings for steel structure. Appl. Surf. Sci. 2018, 457, 164–169. [Google Scholar] [CrossRef]
- Manfredi, L.B.; Puglia, D.; Tomasucci, A.; Kenny, J.M.; Vázquez, A. Influence of clay modification on the properties of resol nanocomposites. Macromol. Mater. Eng. 2008, 293, 878–886. [Google Scholar] [CrossRef]
- Zadaka, D.; Radian, A.; Mishael, Y.G. Applying zeta potential measurements to characterize the adsorption on montmorillonite of organic cations as monomers, micelles, or polymers. J. Colloid Interface Sci. 2010, 352, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Grim, R.E. Clay Mineralogy, 2nd ed.; McGraw-Hill: New York, NY, USA, 1968. [Google Scholar]
- Karthikeyan, G.; Pius, A.; Alagumuthu, G. Fluoride Adsorption Studies of Montmorillonite Clay. Indian J. Chem. Technol. 2005, 12, 263–272. [Google Scholar]
- Zulfiqar, S.; Sarwar, M.I.; Rasheed, N.; Yavuz, C.T. Influence of interlayer functionalization of kaolinite on property profile of copolymer nanocomposites. Appl. Clay Sci. 2015, 112, 25–31. [Google Scholar] [CrossRef]
- Puglia, D.; Kenny, J.M.; Manfredi, L.B.; Vázquez, A. Influence of the chemical composition on the thermal degradation and fire resistance of resol type phenolic resins. Mater. Eng. Modena 2001, 12, 55–72. [Google Scholar]
- ASTM D3500. Standard Test Methods for Structural Panels in Tension; ASTM International: West Conshohocken, PA, USA, 2014; Available online: www.astm.org (accessed on 10 May 2020).
- Kumar, M.S.S.; Raju, N.M.S.; Sampath, P.S.; Selvan, M.C.P. Influence of nanoclay on mechanical and thermal properties of glass fiber reinforced polymer nanocomposites. Polym. Compos. 2018, 39, 1861–1868. [Google Scholar] [CrossRef]
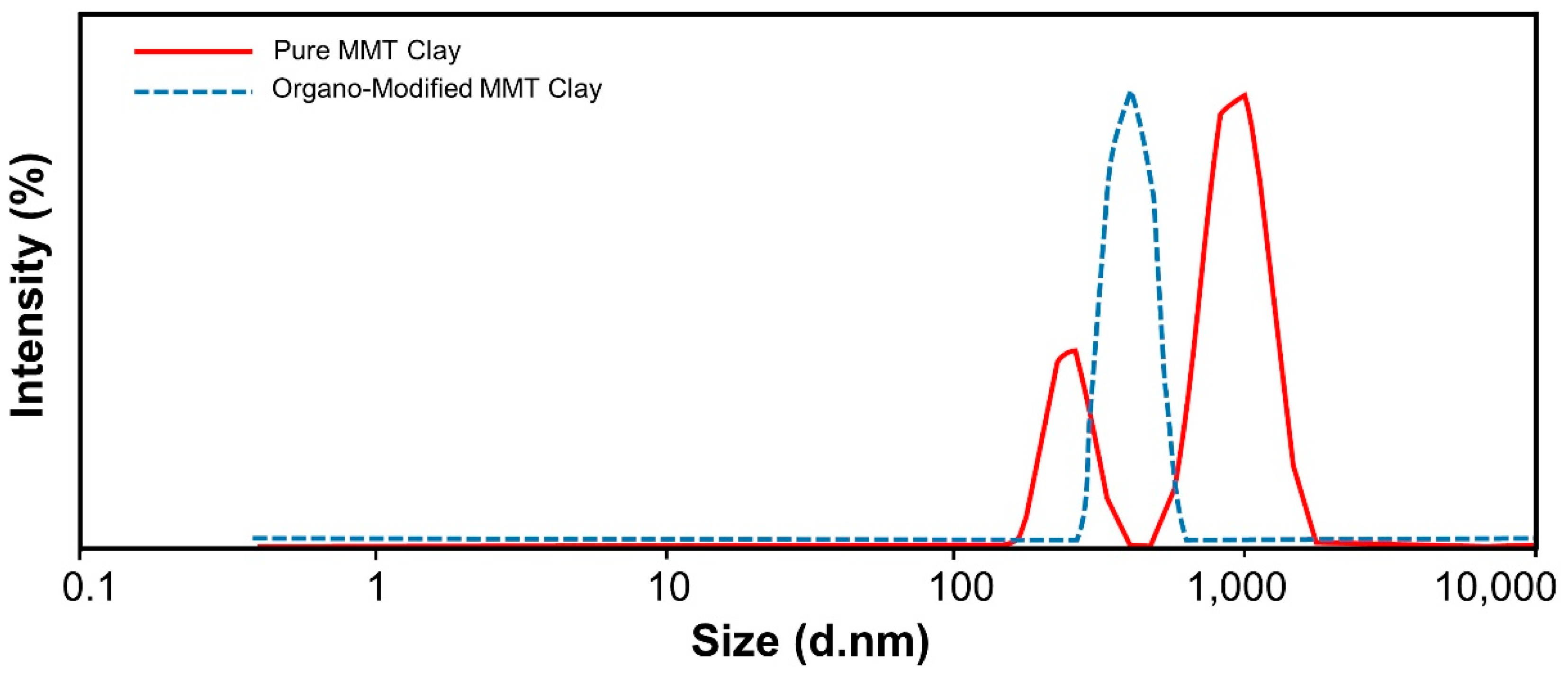



| Sample | Signal | Size (d.nm) | % Intensity | Std Deviation | Z-Average |
|---|---|---|---|---|---|
| Untreated Clay | Peak 1 | 932.2 | 74.8 | 226.3 | 856.8 d.nm |
| Peak 2 | 246.0 | 25.2 | 45.53 | ||
| Organo-Modified Clay | Peak 1 | 400.2 | 100 | 66.13 | 669.9 d.nm |
| Sample | Signal | Mean | Area | Std Deviation | Zeta Potential Avg. |
|---|---|---|---|---|---|
| Untreated Clay | Peak 1 | −19.2 mV | 100 % | 5.74 mV | −19.2 mV |
| Organo Modified Clay | Peak 1 | −17.6 mV | 100 % | 4.07 mV | −17.6 mV |
| Samples | * T0 | Tf | Tdeg | Increment |
|---|---|---|---|---|
| Pure munja resin | 266 °C | 765 °C | 550 °C | - |
| Munjaresin clay composite | 280 °C | 828 °C | 607 °C | 57 °C |
| Sample | Max_Force * (N) | Max_Stress (N/mm2) | Max_Disp. (mm) | Max_Strain (Areas %) |
|---|---|---|---|---|
| Saccharum munja plywood | 20,017.0 | 17.6450 | 8.32352 | 39.6358 |
| Material | RHB Values |
|---|---|
| High-Pressure Sample | 64.0 |
| Copper | 48.9 |
| Brass | 63.2 |
| Aluminium 6061 T6 | 63.5 |
| Cast Iron | 95.9 |
| Low Carbon Steel | 91.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asad, M.Z.; Mahmood, A.; Hussain Shah, S.T. Phenol-Furfural Resin/Montmorillonite Based High-Pressure Green Composite from Renewable Feedstock (Saccharum munja) with Improved Thermo-Mechanical Properties. Polymers 2020, 12, 1562. https://doi.org/10.3390/polym12071562
Asad MZ, Mahmood A, Hussain Shah ST. Phenol-Furfural Resin/Montmorillonite Based High-Pressure Green Composite from Renewable Feedstock (Saccharum munja) with Improved Thermo-Mechanical Properties. Polymers. 2020; 12(7):1562. https://doi.org/10.3390/polym12071562
Chicago/Turabian StyleAsad, Muhammad Zeeshan, Azhar Mahmood, and Syed Tasweer Hussain Shah. 2020. "Phenol-Furfural Resin/Montmorillonite Based High-Pressure Green Composite from Renewable Feedstock (Saccharum munja) with Improved Thermo-Mechanical Properties" Polymers 12, no. 7: 1562. https://doi.org/10.3390/polym12071562
APA StyleAsad, M. Z., Mahmood, A., & Hussain Shah, S. T. (2020). Phenol-Furfural Resin/Montmorillonite Based High-Pressure Green Composite from Renewable Feedstock (Saccharum munja) with Improved Thermo-Mechanical Properties. Polymers, 12(7), 1562. https://doi.org/10.3390/polym12071562







