Acrylic Bone Cement Incorporated with Low Chitosan Loadings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ABC
2.3. Characterization Physical, Chemical and Thermal of ABC
2.4. Determination of Handling Properties
2.5. Mechanical Properties
2.6. Assessment in a Simulated Biological Fluid (SBF)
2.6.1. Hydrolytic Degradation
2.6.2. Bioactivity in SBF
2.7. Statistical Analysis
3. Results
3.1. FTIR
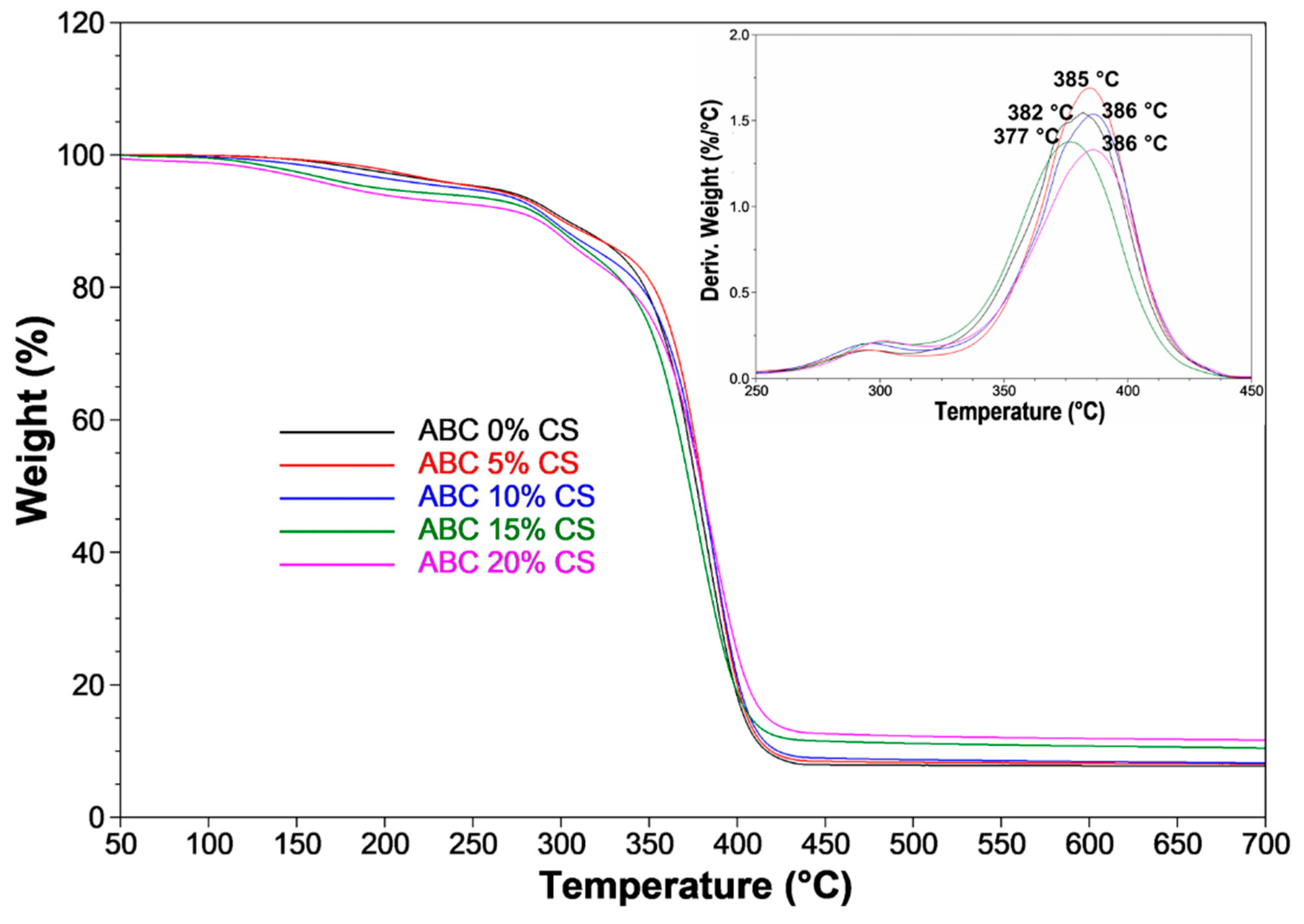
3.2. TGA
3.3. Handling Properties and Water Contact Angle
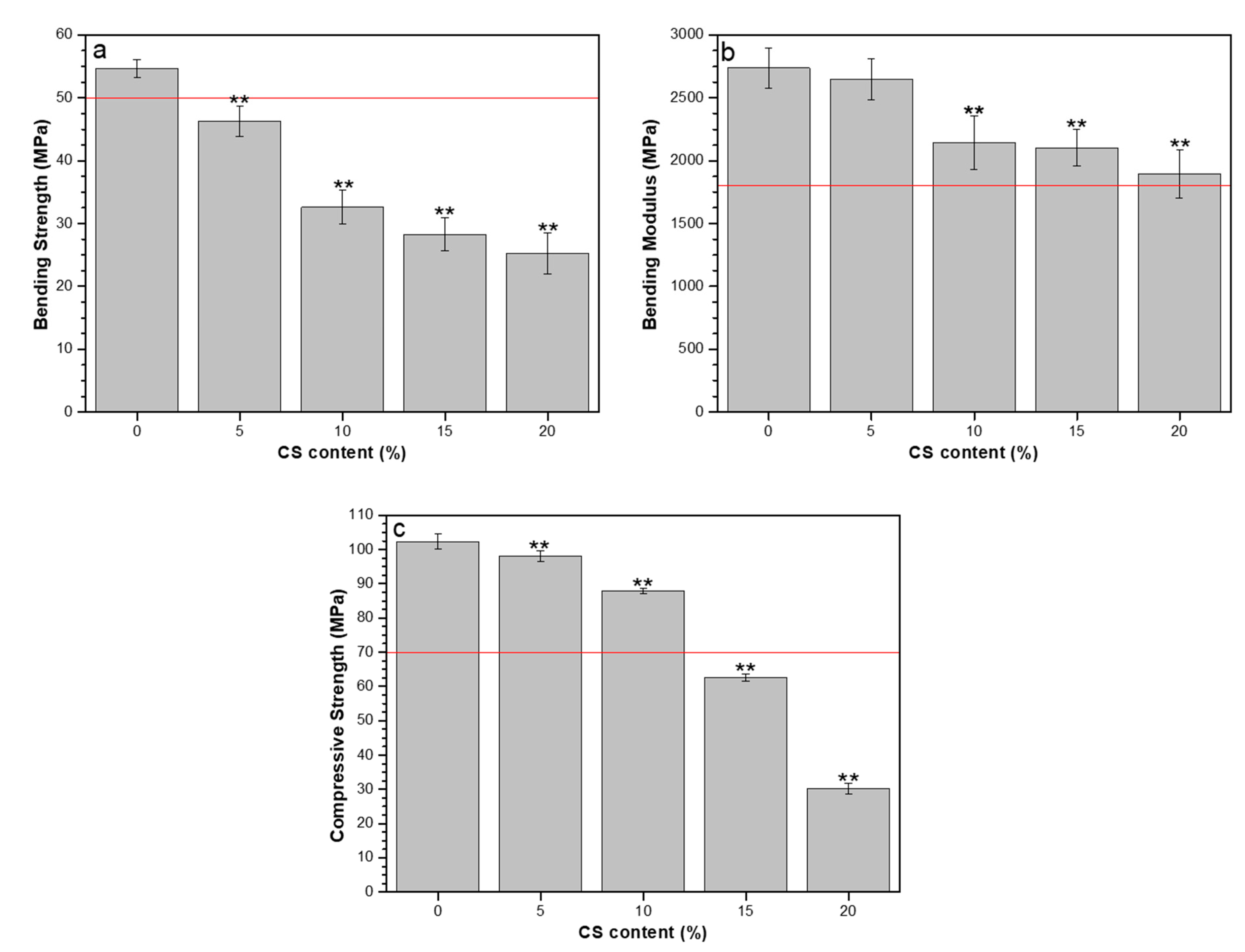
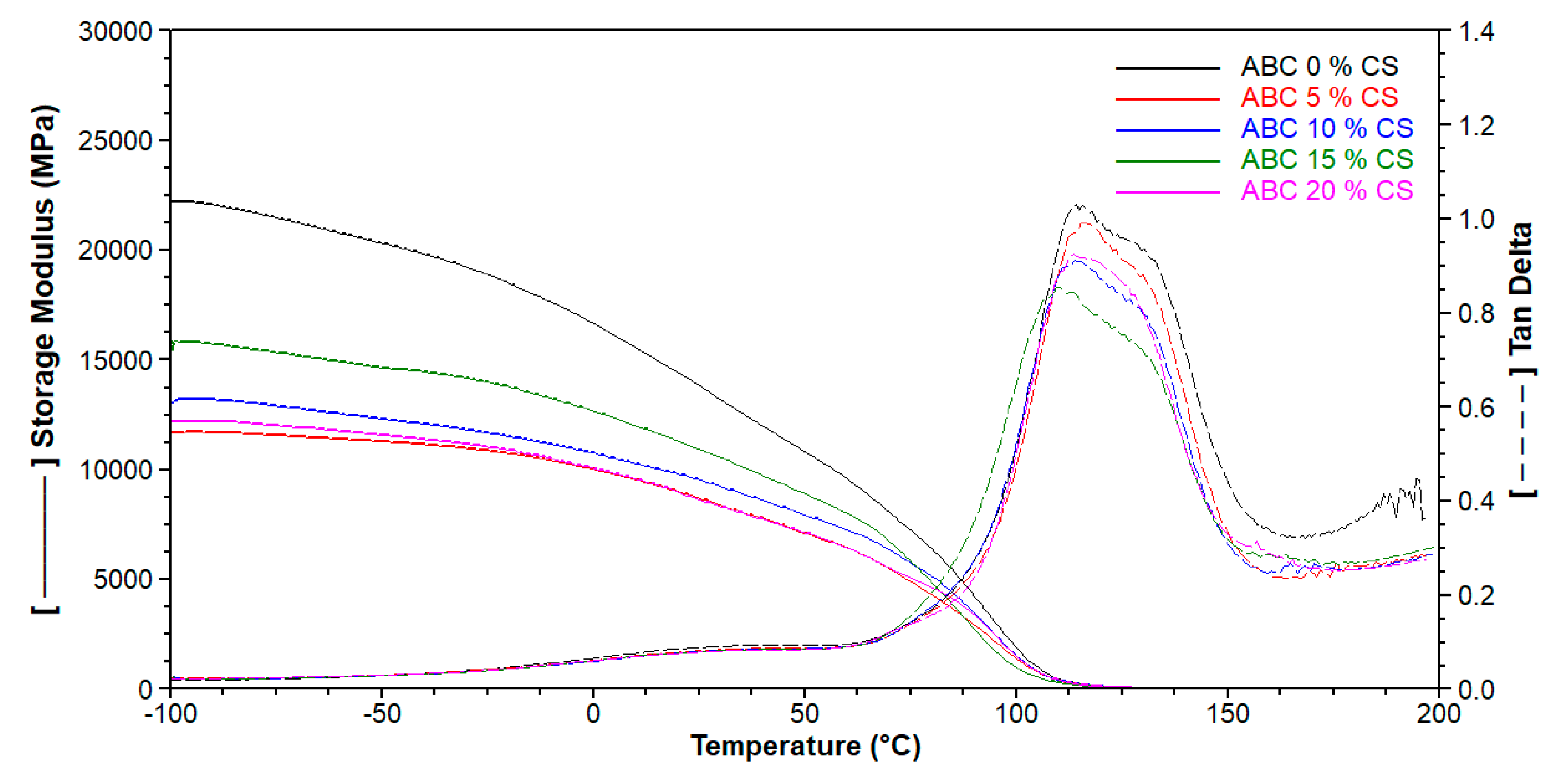
3.4. Mechanical Properties, Residual Monomer Content and DSC
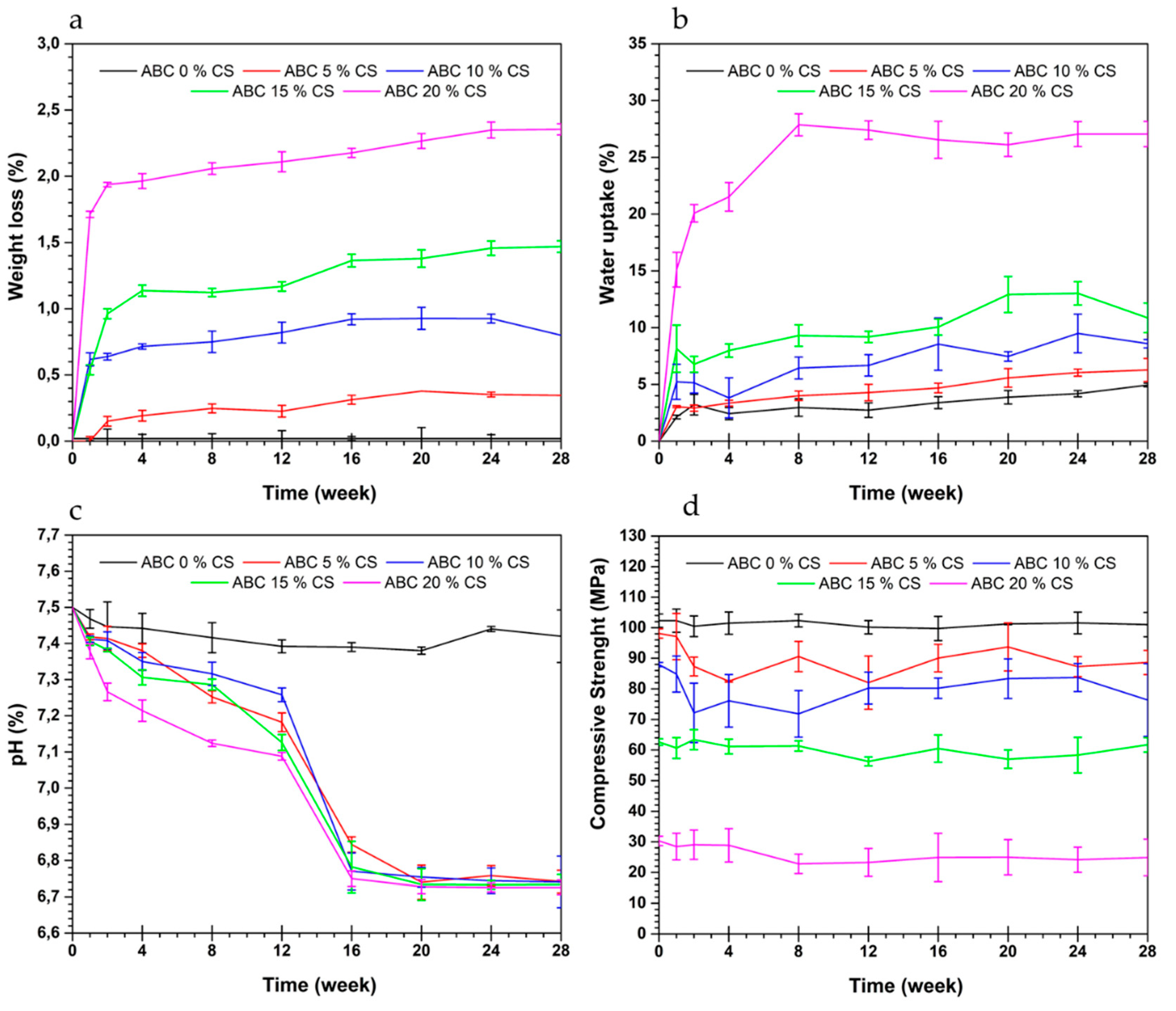
3.5. Hydrolytic Degradation
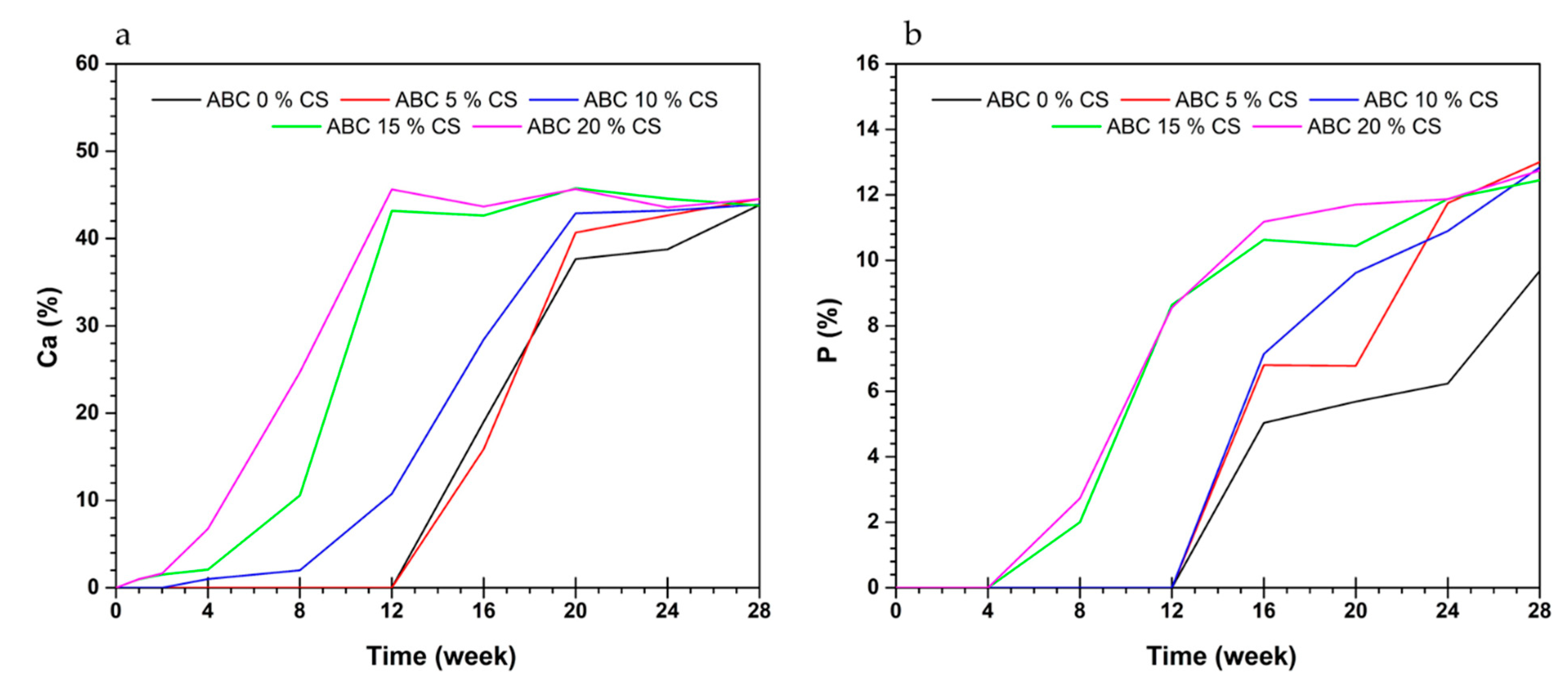
3.6. Bioactivity in SBF
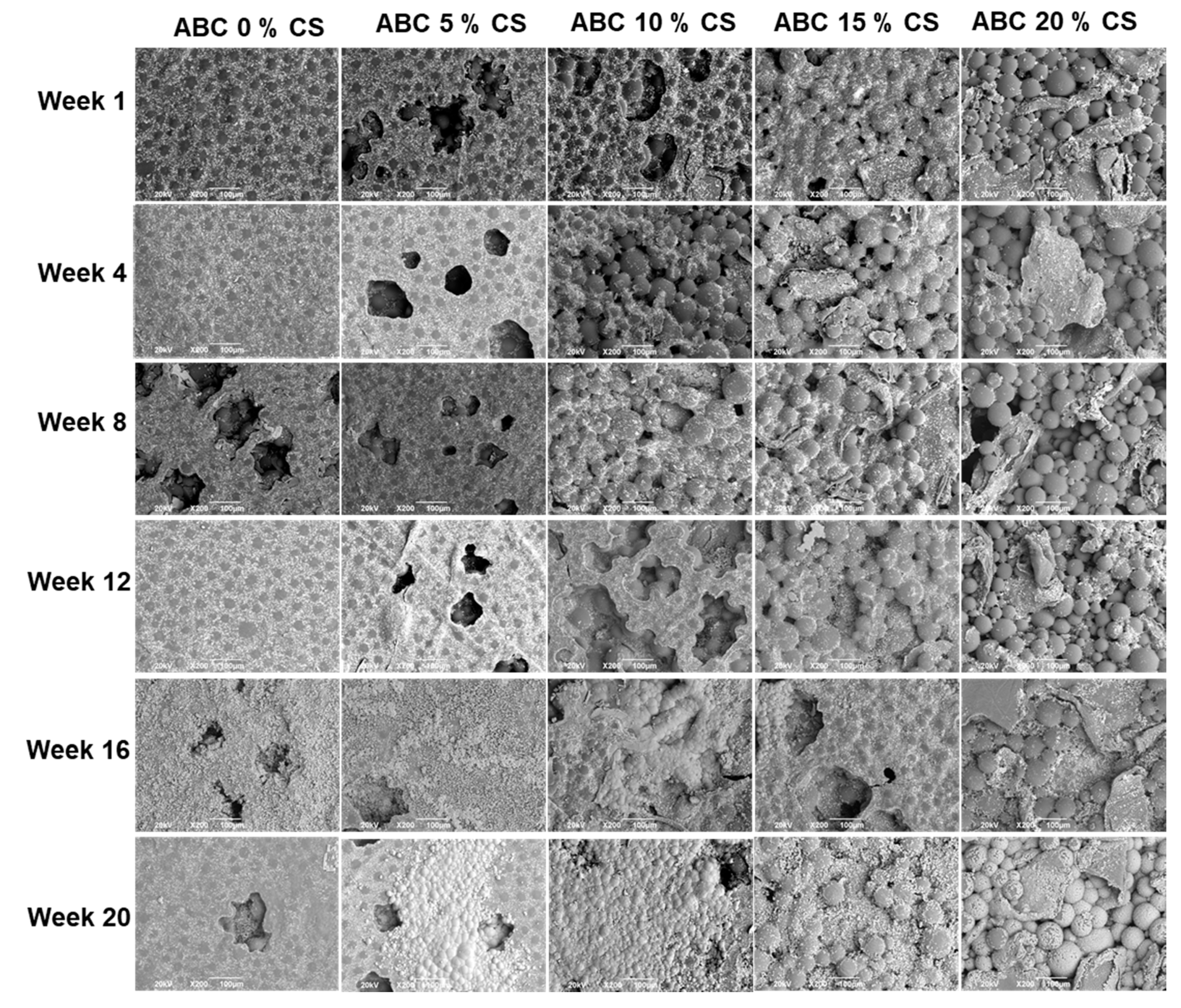
3.6.1. SEM
3.6.2. EDS
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Magnan, B.; Bondi, M.; Maluta, T.; Samaila, E.; Schirru, L.; Dall’Oca, C. Acrylic bone cement: Current concept review. Musculoskelet. Surg. 2013, 97, 93–100. [Google Scholar] [CrossRef]
- Khandaker, M.; Vaughan, M.B.; Morris, T.L.; White, J.J.; Meng, Z. Effect of additive particles on mechanical, thermal, and cell functioning properties of poly (methyl methacrylate) cement. Int. J. Nanomed. 2014, 2699–2712. [Google Scholar] [CrossRef] [PubMed]
- Soleymani Eil Bakhtiari, S.; Karbasi, S.; Hassanzadeh Tabrizi, S.A.; Ebrahimi-Kahrizsangi, R.; Salehi, H. Evaluation of the effects of chitosan/multiwalled carbon nanotubes composite on physical, mechanical and biological properties of polymethyl methacrylate-based bone cements. Mater. Technol. 2019, 35, 267–280. [Google Scholar] [CrossRef]
- Lewis, G. Alternative acrylic bone cement formulations for cemented arthroplasties: Present status. key issues, and future prospects. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 84, 301–319. [Google Scholar] [CrossRef]
- Wang, M.; Sa, Y.; Li, P.; Guo, Y.; Du, Y.; Deng, H.; Jiang, T.; Wang, Y. A versatile and injectable poly(methyl methacrylate) cement functionalized with quaternized chitosan-glycerophosphate/nanosized hydroxyapatite hydrogels. Mater. Sci. Eng. C 2018, 90, 264–272. [Google Scholar] [CrossRef]
- De Mori, A.; Di Gregorio, E.; Kao, A.P.; Tozzi, G.; Barbu, E.; Sanghani-Kerai, A.; Draheim, R.R.; Roldo, M. Antibacterial PMMA Composite Cements with Tunable Thermal and Mechanical Properties. ACS Omega 2019, 4, 19664–19675. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications—A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Barradas, A.M.C.; Yuan, H.; Van Blitterswijk, C.A.; Habibovic, P. Osteoinductive biomaterials: Current knowledge of properties, experimental models and biological mechanisms. Eur. Cells Mater. 2011, 21, 407–429. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Dunne, N.; Buchanan, F.; Hill, J.; Newe, C.; Tunney, M.; Brady, A.; Walker, G. In vitro testing of chitosan in gentamicin-loaded bone cement: No antimicrobial effect and reduced mechanical performance. Acta Orthop. 2008, 79, 851–860. [Google Scholar] [CrossRef]
- Lin, M.C.; Chen, C.C.; Wu, I.T.; Ding, S.J. Enhanced antibacterial activity of calcium silicate-based hybrid cements for bone repair. Mater. Sci. Eng. C 2020, 110, 110727. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Neoh, K.G.; Kang, E.T.; Wang, W. Antibacterial and mechanical properties of bone cement impregnated with chitosan nanoparticles. Biomaterials 2006, 27, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, M.; Bakhtiari, S.S.E.; Karbasi, S. Incorporation of chitosan/graphene oxide nanocomposite in to the PMMA bone cement: Physical, mechanical and biological evaluation. Int. J. Biol. Macromol. 2020, 149, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, M.; Yin, B.; Zou, J.; Zhang, W.; Wang, S.-Y. Effects of Incorporating Carboxymethyl Chitosan into PMMA Bone Cement Containing Methotrexate. PLoS ONE 2015, 10, 144407. [Google Scholar] [CrossRef] [PubMed]
- Endogan, T.; Kiziltay, A.; Kose, G.T.; Comunoglu, N.; Beyzadeoglu, T.; Hasirci, N. Acrylic Bone Cements: Effects of the Poly (methyl methacrylate) Powder Size and Chitosan Addition on Their Properties. J. Appl. Polym. Sci. Polym. Sci. 2014, 131, 39662. [Google Scholar] [CrossRef]
- Deb, S.; Koller, G. Chapter 8. Acrylic bone cement: Genesis and evolution. In Orthopaedic Bone Cements; Deb, S., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2008; pp. 167–182. ISBN 978-1-84569-517-0. [Google Scholar]
- Miyazaki, T.; Ohtsuki, C. Design of bioactive bone cement based on organic–inorganic hybrids. In Orthopaedic Bone Cements; Deb, S., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2008; p. 400. [Google Scholar]
- International Standard ISO 5833. Implants for Surgery-Acrylic Resin Cements. ISO: Geneva, Switzerland, 2002; pp. 1–22. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- ASTM F1635-16. Standard Test Method for In Vitro Degradation Testing of Hydrolytically Degradable Polymer Resins and Fabricated Forms for Surgical Implants. American Society of Testing Materials: West Conshohocken, PA, USA, 2016; pp. 1–5. [Google Scholar]
- Pahlevanzadeh, F.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Chen, X.B. Development of PMMA-Mon-CNT bone cement with superior mechanical properties and favorable biological properties for use in bone-defect treatment. Mater. Lett. 2019, 240, 9–12. [Google Scholar] [CrossRef]
- Vazquez-Lasa, B. Poly(methylmethacrylate) bone cement: Chemical composition and chemistry. In Orthopaedic Bone Cements; Deb, S., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2008; p. 400. [Google Scholar]
- Tan, H.; Guo, S.; Yang, S.; Xu, X.; Tang, T. Physical characterization and osteogenic activity of the quaternized chitosan-loaded PMMA bone cement. Acta Biomater. 2012, 8, 2166–2174. [Google Scholar] [CrossRef]
- Nazhat, S.N.; Cauich Rodriguez, J.V. Dynamic mechanical properties of bone cements. In Orthopaedic Bone Cements; Deb, S., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 296–310. [Google Scholar]
- Varlamov, V.P.; Il’ina, A.V.; Shagdarova, B.T.; Lunkov, A.P.; Mysyakina, I.S. Chitin/Chitosan and Its Derivatives: Fundamental Problems and Practical Approaches. Biochemistry 2020, 85, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Tiainen, H.; Wohlfahrt, J.C.; Verket, A.; Lyngstadaas, S.P.; Haugen, H.J. Bone formation in TiO 2 bone scaffolds in extraction sockets of minipigs. Acta Biomater. 2012, 8, 2384–2391. [Google Scholar] [CrossRef]
- He, Q.; Chen, H.; Huang, L.; Dong, J.; Guo, D.; Mao, M.; Kong, L.; Li, Y.; Wu, Z.; Lei, W. Porous Surface Modified Bioactive Bone Cement for Enhanced Bone Bonding. PLoS ONE 2012, 7, e42525. [Google Scholar] [CrossRef]
- May-Pat, A.; Herrera-Kao, W.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M.; Flores-Gallardo, S.G. Comparative study on the mechanical and fracture properties of acrylic bone cements prepared with monomers containing amine groups. J. Mech. Behav. Biomed. Mater. 2012, 6, 95–105. [Google Scholar] [CrossRef]
- Valencia Zapata, M.E.; Mina Hernandez, J.H.; Grande Tovar, C.D.; Valencia Llano, C.H.; Diaz Escobar, J.A.; Vázquez-Lasa, B.; San Román, J.; Rojo, L.; Rojo, L. Novel Bioactive and Antibacterial Acrylic Bone Cement Nanocomposites Modified with Graphene Oxide and Chitosan. Int. J. Mol. Sci. 2019, 20, 2938. [Google Scholar] [CrossRef] [PubMed]
- Kühn, K.-D. Bone Cements; Springer: Berlin, Germany, 2000; ISBN 9783642641152. [Google Scholar]
- Lewis, G. Properties of nanofiller-loaded poly (methyl methacrylate) bone cement composites for orthopedic applications: A review. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2017, 105, 1260–1284. [Google Scholar] [CrossRef] [PubMed]
- Depan, D.; Shah, J.S.; Misra, R.D.K. Degradation mechanism and increased stability of chitosan-based hybrid scaffolds cross-linked with nanostructured carbon: Process-structure-functional property relationship. Polym. Degrad. Stab. 2013, 98, 2331–2339. [Google Scholar] [CrossRef]
- Ruiz, S.; Tamayo, J.A.; Ospina, J.D.; Navia Porras, D.P.; Valencia Zapata, M.E.; Mina Hernandez, J.H.; Valencia, C.H.; Zuluaga, F.; Grande Tovar, C.D. Antimicrobial Films Based on Nanocomposites of Chitosan / Poly ( vinyl alcohol )/ Graphene Oxide for Biomedical Applications. Biomolecules 2019, 9, 109. [Google Scholar] [CrossRef]
- Safadi, F.F.; Barbe, M.F.; Abdelmagid, S.M.; Rico, M.C.; Aswad, R.A.; Litvin, J.; Popoff, S.N. Bone Structure, Development and Bone Biology. In Bone Pathology; Khurana, J.S., Ed.; Humana Press: New York, NY, USA, 2009; Volume 2, pp. 1–50. ISBN 9781588297662. [Google Scholar]
- Mirmusavi, M.H.; Zadehnajar, P.; Semnani, D.; Karbasi, S.; Fekrat, F.; Heidari, F. Evaluation of physical, mechanical and biological properties of poly 3-hydroxybutyrate-chitosan-multiwalled carbon nanotube/silk nano-micro composite scaffold for cartilage tissue engineering applications. Int. J. Biol. Macromol. 2019, 132, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Islas-Blancas, M.E.; Cervantes-Uc, J.M. Characterization of bone cements prepared with functionalized methacrylates and hydroxyapatite. J. Biomater. Sci. Polym. Ed. 2001, 12, 893–910. [Google Scholar] [CrossRef] [PubMed]


| Formulation | SP | |||
|---|---|---|---|---|
| PMMA | BaSO4 | BPO | CS | |
| ABC 0% CS | 88 | 10 | 2 | 0 |
| ABC 5% CS | 83 | 10 | 2 | 5 |
| ABC 10% CS | 78 | 10 | 2 | 10 |
| ABC 15% CS | 73 | 10 | 2 | 15 |
| ABC 20% CS | 68 | 10 | 2 | 20 |
| Formulation | Tmax (°C) | tset (s) | WCA (°) |
|---|---|---|---|
| ABC 0% CS | 59 ± 3 | 355 ± 30 | 64.9 ± 2.2 |
| ABC 5% CS | 58 ± 2 | 370 ± 15 | 65.1 ± 1.1 |
| ABC 10% CS | 54 ± 3 | 420 ± 15 | 66.4 ± 1.4 |
| ABC 15% CS | 54 ± 4 | 460 ± 20 * | 60.3 ± 2.1 ** |
| ABC 20% CS | 46 ± 2 * | 545 ± 25 * | 56.8 ± 1.9 ** |
| Formulation | RMC (%) | TgDSC (°C) | TgDMA (°C) |
|---|---|---|---|
| ABC 0% CS | 1.06 | 106 | 114 |
| ABC 5% CS | 1.83 | 108 | 116 |
| ABC 10% CS | 1.32 | 107 | 115 |
| ABC 15% CS | 1.57 | 105 | 110 |
| ABC 20% CS | 6.25 | 101 | 113 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia Zapata, M.E.; Mina Hernandez, J.H.; Grande Tovar, C.D. Acrylic Bone Cement Incorporated with Low Chitosan Loadings. Polymers 2020, 12, 1617. https://doi.org/10.3390/polym12071617
Valencia Zapata ME, Mina Hernandez JH, Grande Tovar CD. Acrylic Bone Cement Incorporated with Low Chitosan Loadings. Polymers. 2020; 12(7):1617. https://doi.org/10.3390/polym12071617
Chicago/Turabian StyleValencia Zapata, Mayra Eliana, José Herminsul Mina Hernandez, and Carlos David Grande Tovar. 2020. "Acrylic Bone Cement Incorporated with Low Chitosan Loadings" Polymers 12, no. 7: 1617. https://doi.org/10.3390/polym12071617
APA StyleValencia Zapata, M. E., Mina Hernandez, J. H., & Grande Tovar, C. D. (2020). Acrylic Bone Cement Incorporated with Low Chitosan Loadings. Polymers, 12(7), 1617. https://doi.org/10.3390/polym12071617







