Preparation and pH Controlled Release of Fe3O4/Anthocyanin Magnetic Biocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Magnetic Fe3O4 Nanoparticles
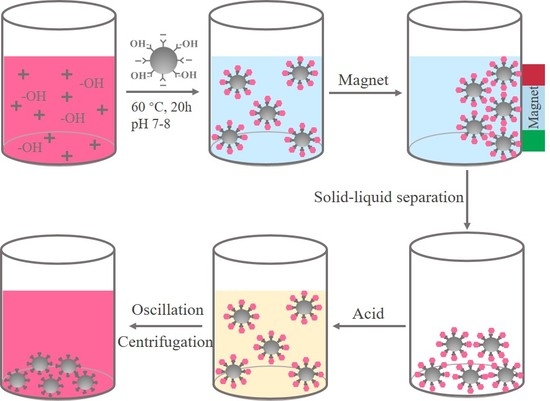
2.3. Preparation of Fe3O4/Anthocyanin Magnetic Biocomposite
2.4. Release Research
2.5. Characterizations
3. Results
3.1. Preparation of Fe3O4/Anthocyanin Magnetic Biocomposite
3.2. Properties of Fe3O4/Anthocyanin Magnetic Biocomposites
3.3. Release of Anthocyanins from Fe3O4/Anthocyanin Magnetic Biocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, H.S.; Zhou, Q.; Chang, J.; Wu, C.T. Grape seed-inspired smart hydrogel scaffolds for melanoma therapy and wound healing. ACS Nano 2019, 13, 4302–4311. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Teng, H.; Xie, Z.L.; Cao, H.; Cheang, W.S.; Skalicka-Woniak, K.; Georgiev, M.I.; Xiao, J.B. Modifications of dietary flavonoids towards improved bioactivity: An update on structure-activity relationship. Crit. Rev. Food Sci. Nutr. 2018, 58, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Teng, H.; Zhang, K.Y.; Skalicka-Wozniak, K.; Georgiev, M.I.; Xiao, J.B. Agrimonolide and desmethylagrimonolide induced HO-1 expression in HepG2 cells through Nrf2-transduction and p38 inactivation. Front. Pharmacol. 2017, 7, 513. [Google Scholar] [CrossRef]
- Teng, H.; Fang, T.; Lin, Q.Y.; Song, H.B.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Sun, X.H.; Zhou, T.T.; Wei, C.H.; Lan, W.Q.; Zhao, Y.; Pan, Y.J.; Wu, V.C.H. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control 2018, 94, 155–161. [Google Scholar] [CrossRef]
- Gowd, V.; Jia, Z.Q.; Chen, W. Anthocyanins as promising molecules and dietary bioactive components against diabetes—A review of recent advances. Trends Food Sci. Technol. 2017, 68, 1–13. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, T.; Zhang, B.L.; Zhao, H.F. Addition of sucrose during the blueberry heating process is good or bad? Evaluating the changes of anthocyanins/anthocyanidins and the anticancer ability in HepG-2 cells. Food Res. Int. 2018, 107, 509–517. [Google Scholar] [CrossRef]
- Su, C.C.; Wang, C.J.; Huang, K.H.; Lee, Y.J.; Chan, W.M.; Chang, Y.C. Anthocyanins from hibiscus sabdariffa calyx attenuate in vitro and in vivo melanoma cancer metastasis. J. Funct. Foods 2018, 48, 614–631. [Google Scholar] [CrossRef]
- Carrillo, C.; Buve, C.; Panozzo, A.; Grauwet, T.; Hendrickx, M. Role of structural barriers in the in vitro bioaccessibility of anthocyanins in comparison with carotenoids. Food Chem. 2017, 227, 271–279. [Google Scholar] [CrossRef]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef]
- Pina, F.; Melo, M.J.; Laia, C.A.T.; Parola, A.J.; Lima, J.C. Chemistry and applications of flavylium compounds: A handful of colours. Chem. Soc. Rev. 2012, 41, 869–908. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Hanh, P.T.; Pham-Hoang, B.N.; Ho, P.T.; Tran, T.T.T.; Wache, Y. Encapsulation of hibiscus sabdariffa l. anthocyanins as natural colours in yeast. Food Res. Int. 2018, 107, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.S.; Temelli, F. Preparation of anthocyanin-loaded liposomes using an improved supercritical carbon dioxide method. Innov. Food Sci. Emerg. Technol. 2017, 39, 119–128. [Google Scholar] [CrossRef]
- Zhao, L.S.; Temelli, F.; Chen, L.Y. Encapsulation of anthocyanin in liposomes using supercritical carbon dioxide: Effects of anthocyanin and sterol concentrations. J. Funct. Foods 2017, 34, 159–167. [Google Scholar] [CrossRef]
- Rabelo, C.A.S.; Taarji, N.; Khalid, N.; Kobayashi, I.; Nakajima, M.; Neves, M.A. Formulation and characterization of water-in-oil nanoemulsions loaded with acai berry anthocyanins: Insights of degradation kinetics and stability evaluation of anthocyanins and nanoemulsions. Food Res. Int. 2018, 106, 542–548. [Google Scholar] [CrossRef]
- Klimaviciute, R.; Navikaite, V.; Jakstas, V.; Ivanauskas, L. Complexes of dextran sulfate and anthocyanins from vaccinium myrtillus: Formation and stability. Carbohydr. Polym. 2015, 129, 70–78. [Google Scholar] [CrossRef]
- Sui, X.N.; Sun, H.N.; Qi, B.K.; Zhang, M.; Li, Y.; Jiang, L.Z. Functional and conformational changes to soy proteins accompanying anthocyanins: Focus on covalent and non-covalent interactions. Food Chem. 2018, 245, 871–878. [Google Scholar] [CrossRef]
- Tan, C.; Selig, M.J.; Abbaspourrad, A. Anthocyanin stabilization by chitosan-chondroitin sulfate polyelectrolyte complexation integrating catechin co-pigmentation. Carbohydr. Polym. 2018, 181, 124–131. [Google Scholar] [CrossRef]
- Ge, J.; Yue, P.X.; Chi, J.P.; Liang, J.; Gao, X.L. Formation and stability of anthocyanins-loaded nanocomplexes prepared with chitosan hydrochloride and carboxymethyl chitosan. Food Hydrocoll. 2018, 74, 23–31. [Google Scholar] [CrossRef]
- Cruz, L.; Benohoud, M.; Rayner, C.M.; Mateus, N.; de Freitas, V.; Blackburn, R.S. Selective enzymatic lipophilization of anthocyanin glucosides from blackcurrant (Ribes nigrum L.) skin extract and characterization of esterified anthocyanins. Food Chem. 2018, 266, 415–419. [Google Scholar] [CrossRef]
- Du, H.; Wu, J.; Ji, K.X.; Zeng, Q.Y.; Bhuiya, M.W.; Su, S.; Shu, Q.Y.; Ren, H.X.; Liu, Z.A.; Wang, L.S. Methylation mediated by an anthocyanin, o-methyltransferase, is involved in purple flower coloration in paeonia. J. Exp. Bot. 2015, 66, 6563–6577. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.H.; Hsieh, J.F. The conversion and deglycosylation of isoflavones and anthocyanins in black soymilk process. Food Chem. 2018, 261, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Fang, S.; Li, Y.H.; Zhang, F.; Shao, Z.P.; Zeng, Y.T.; Chen, J.; Meng, Y.C. Effects of low acyl and high acyl gellan gum on the thermal stability of purple sweet potato anthocyanins in the presence of ascorbic acid. Food Hydrocoll. 2019, 86, 116–123. [Google Scholar] [CrossRef]
- Zhao, C.L.; Yu, Y.Q.; Chen, Z.J.; Wen, G.S.; Wei, F.G.; Zheng, Q.; Wang, C.D.; Xiao, X.L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef]
- Barreto, A.C.H.; Santiago, V.R.; Mazzetto, S.E.; Denardin, J.C.; Lavin, R.; Mele, G.; Ribeiro, M.; Vieira, I.G.P.; Goncalves, T.; Ricardo, N.; et al. Magnetic nanoparticles for a new drug delivery system to control quercetin releasing for cancer chemotherapy. J. Nanopart. Res. 2011, 13, 6545–6553. [Google Scholar] [CrossRef]
- Qin, X.G.; Yuan, D.; Wang, Q.; Hu, Z.Z.; Wu, Y.; Cai, J.; Huang, Q.R.; Li, S.Y.; Liu, G. Maillard-reacted whey protein isolates enhance thermal stability of anthocyanins over a wide pH range. J. Agric. Food Chem. 2018, 66, 9556–9564. [Google Scholar] [CrossRef]
- Xu, C.N.; Wang, Y.B.; Yu, H.Y.; Tian, H.Y.; Chen, X.S. Multifunctional theranostic nanoparticles derived from fruit-extracted anthocyanins with dynamic disassembly and elimination abilities. ACS Nano 2018, 12, 8255–8265. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, Z.G.; Wang, Y.; Chen, W.J.; Sun, C.J.; Cui, B.; Cui, J.H.; Yu, M.L.; Zeng, Z.H.; Guo, S.D.; et al. Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nat. Plants 2017, 3, 956–964. [Google Scholar] [CrossRef]
- Pan, Y.; Du, X.W.; Zhao, F.; Xu, B. Magnetic nanoparticles for the manipulation of proteins and cells. Chem. Soc. Rev. 2012, 41, 2912–2942. [Google Scholar] [CrossRef]
- Rubia, G.G.; Peigneux, A.; Puerma, J.; Oltolina, F.; Elert, K.; Colangelo, D.; Morales, J.G.; Prat, M.; Jimenez-Lopez, C. pH-Dependent adsorption release of doxorubicin on MamC-biomimetic magnetite nanoparticles. Langmuir 2018, 34, 13713–13724. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Alipour, S.; Zohreh, N. Delivery of doxorubicin using double-layered core-shell nanocarrier based on magnetic Fe3O4 core and salep shells. Langmuir 2018, 34, 13735–13744. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, J.X.; Hu, Y.Y.; Hu, C.M.; Pan, Y.; Yu, Q.; Chen, H. Using porous magnetic iron oxide nanomaterials as a facile photoporation nanoplatform for macromolecular delivery. J. Mater. Chem. B 2018, 6, 4427–4436. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zou, X.B.; Zhai, X.D.; Huang, X.W.; Jiang, C.P.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Guzel, E.; Arslan, B.S.; Durmaz, V.; Cesur, M.; Tutar, O.F.; Sari, T.; Isleyen, M.; Nebioglu, M.; Sisman, I. Photovoltaic performance and photostability of anthocyanins, isoquinoline alkaloids and betalains as natural sensitizers for DSSCs. Sol. Energy 2018, 173, 34–41. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Zhao, H.T.; Yang, X.; Zhang, H.; Dong, A.J.; Wang, J.; Li, B. Selective recognition and fast enrichment of anthocyanins by dummy molecularly imprinted magnetic nanoparticles. J. Chromatogr. A 2018, 1572, 9–19. [Google Scholar] [CrossRef]
- Uranga, J.; Etxabide, A.; Guerrero, P.; de la Caba, K. Development of active fish gelatin films with anthocyanins by compression molding. Food Hydrocoll. 2018, 84, 313–320. [Google Scholar] [CrossRef]
- Wojdylo, A.; Nowicka, P. Anticholinergic effects of Actinidia arguta fruits and their polyphenol content determined by liquid chromatography-photodiode array detector-quadrupole/time of flight-mass spectrometry (LC-MS-PDA-Q/TOF). Food Chem. 2019, 271, 216–223. [Google Scholar] [CrossRef]
- Zeng, Y.J.; Xu, P.; Yang, H.R.; Zong, M.H.; Lou, W.Y. Purification of anthocyanins from saskatoon berries and their microencapsulation in deep eutectic solvents. LWT Food Sci. Technol. 2018, 95, 316–325. [Google Scholar] [CrossRef]






| Materials | Zeta Potential (mV) | Average Value (mV) | Particle Size (nm) | Average Value (nm) | ||||
|---|---|---|---|---|---|---|---|---|
| Anthocyanin | +10.90 | +11.10 | +10.70 | +10.90 | \ | \ | \ | \ |
| Fe3O4 | −46.38 | −42.42 | −47.97 | −45.59 | 192.3 | 203.6 | 184.1 | 193.3 |
| Fe3O4/anthocyanin | −37.25 | −38.55 | −37.59 | −37.70 | 225.9 | 230.2 | 210.5 | 222.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Guan, Q.; Feng, M.; Wang, M.; Yan, N.; Wang, M.; Xu, L.; Gui, Z. Preparation and pH Controlled Release of Fe3O4/Anthocyanin Magnetic Biocomposites. Polymers 2019, 11, 2077. https://doi.org/10.3390/polym11122077
Jiang X, Guan Q, Feng M, Wang M, Yan N, Wang M, Xu L, Gui Z. Preparation and pH Controlled Release of Fe3O4/Anthocyanin Magnetic Biocomposites. Polymers. 2019; 11(12):2077. https://doi.org/10.3390/polym11122077
Chicago/Turabian StyleJiang, Xizhi, Qingbao Guan, Min Feng, Mengyang Wang, Nina Yan, Min Wang, Lei Xu, and Zhongzheng Gui. 2019. "Preparation and pH Controlled Release of Fe3O4/Anthocyanin Magnetic Biocomposites" Polymers 11, no. 12: 2077. https://doi.org/10.3390/polym11122077
APA StyleJiang, X., Guan, Q., Feng, M., Wang, M., Yan, N., Wang, M., Xu, L., & Gui, Z. (2019). Preparation and pH Controlled Release of Fe3O4/Anthocyanin Magnetic Biocomposites. Polymers, 11(12), 2077. https://doi.org/10.3390/polym11122077