3D Printing of Polymer Waste for Improving People’s Awareness about Marine Litter
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of the Wire and 3D Print
- -
- PET pellets: PET_P.
- -
- PET flakes from bottles: PET_B
- -
- extruded wire, rapid cooling: PET_RC
- -
- extruded wire, slow cooling: PET_SC
- -
- 3D printed sample from extruded wire, rapid cooling: PET_3D
2.2. Analysis of the Degree of Crystallinity
2.3. Rheological and Thermogravimetric Analysis
2.4. Mechanical Characterization
2.5. Analysis of the Porosity Distribution
3. Results and Discussion
3.1. Analysis of the Degree of Crystallinity
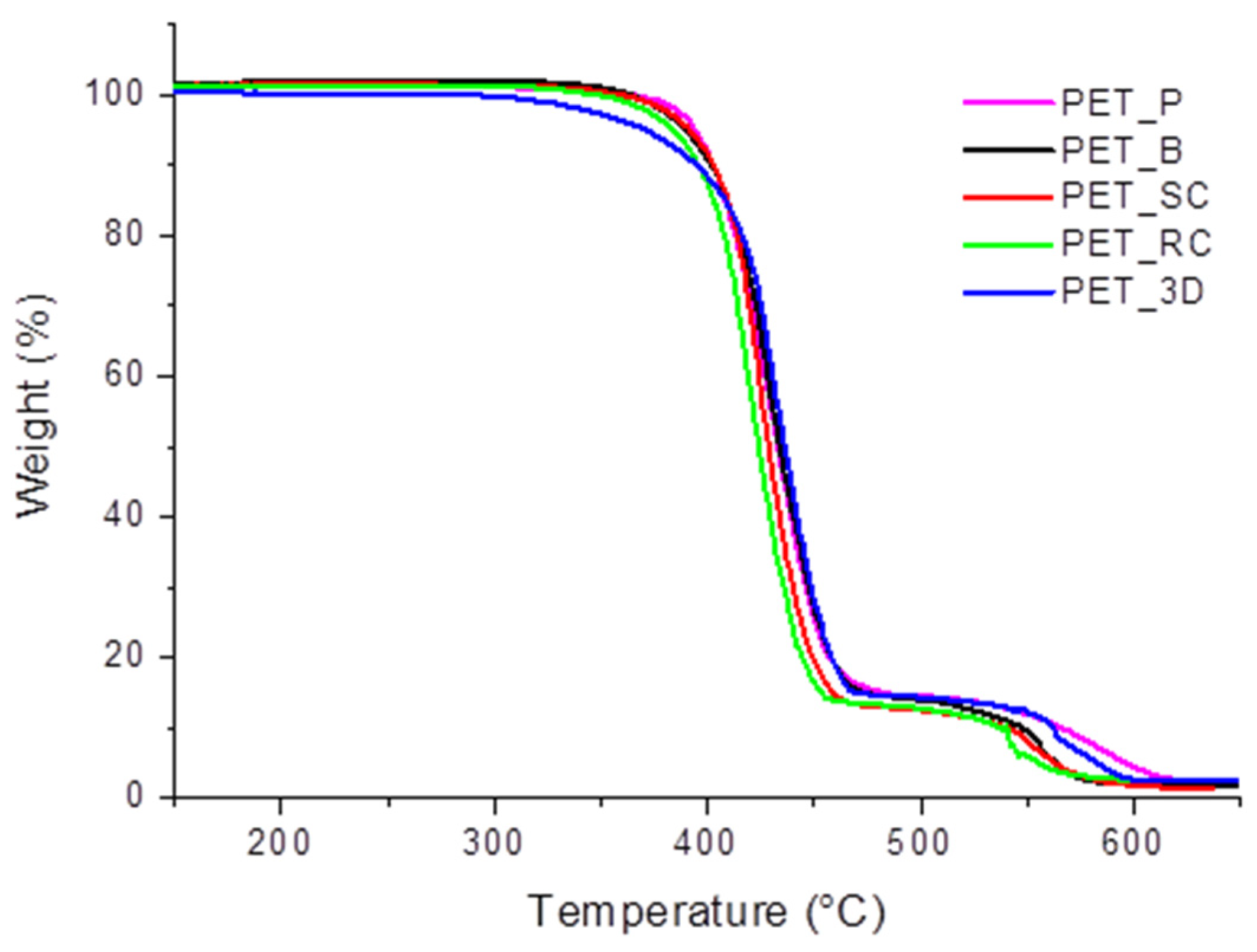
3.2. Rheological and Thermogravimetric Analysis
3.3. Mechanical Characterization
3.4. Micro CT Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferrari, F.; Striani, R.; Minosi, S.; De Fazio, R.; Visconti, P.; Patrono, L.; Catarinucci, L.; Esposito Corcione, C.; Greco, A. An innovative IOT-oriented prototype platform for the management and valorization of the organic fraction of municipal solid waste. J. Clean. Prod. 2020, 247, 119618. [Google Scholar] [CrossRef]
- Corcione, C.E.; Ferrari, F.; Striani, R.; Greco, A. An innovative green process for the stabilization and valorization of organic fraction of municipal solid waste (OFMSW): Optimization of the curing process, II part. Appl. Sci. 2019, 9, 3702. [Google Scholar] [CrossRef]
- Ferrari, F.; Striani, R.; Visconti, P.; Esposito Corcione, C.; Greco, A. Durability analysis of formaldehyde/solid urban waste blends. Polymers 2019, 11, 1838. [Google Scholar] [CrossRef] [PubMed]
- Beede, D.N.; Bloom, D.E. Economics of the Generation and Management of MSW; NBER Working Papers 5116; National Bureau of Economic Research, Inc.: Cambridge, MA, USA, 1995. [Google Scholar]
- Plastic Pollution. Available online: https://ourworldindata.org/plastic-pollution (accessed on 1 September 2018).
- Dewil, R.; Everaert, K.; Baeyens, J. The European plastic waste issue: Trends and toppers in its sustainable re-use. In Proceedings of the 17th International Congress of Chemical and Process Engineering, Prague, Czech Republic, 27–31 August 2006. [Google Scholar]
- Morreale, M.; Liga, A.; Mistretta, M.C.; Ascione, L.; La Mantia, F.P. Mechanical, Thermomechanical and Reprocessing Behavior of Green Composites from Biodegradable Polymer and Wood Flour. Materials 2015, 8, 7536–7548. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Mellinas, A.C.; Ramos, M.; Garrigós, M.C.; Jiménez, A. Natural additives and agricultural wastes in biopolymer formulations for food packaging. Front. Chem. 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Jiun, Y.L.; Tze, C.T.; Moosa, U.; Tawawneh, M.A. Effects of Recycling Cycle on Used Thermoplastic Polymer and Thermoplastic Elastomer Polymer. Polym. Polym. Compos. 2016, 24, 735–740. [Google Scholar] [CrossRef]
- Kumar, S.; Panda, A.K.; Singh, R. A review on tertiary recycling of high-density polyethylene to fuel. Resour. Conserv. Recycl. 2011, 55, 893–910. [Google Scholar] [CrossRef]
- La Mantia, F.; Morreale, M.; Botta, L.; Mistretta, M.; Ceraulo, M.; Scaffaro, R. Degradation of polymer blends: A brief review. Polym. Degrad. Stab. 2017, 145, 79–92. [Google Scholar] [CrossRef]
- Verma, R.; Vinoda, K.; Papireddy, M.; Gowda, A. Toxic Pollutants from Plastic Waste- A Review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Zarfl, C.; Matthies, M. Are marine plastic particles transport vectors for organic pollutants to the Arctic? Mar. Pollut. Bull. 2010, 60, 1810–1814. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B Boil. Sci. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; Mcgonigle, D.; Russell, A.E. Lost at Sea: Where Is All the Plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.J. Synthetic polymers in the marine environment: A rapidly increasing, long-term threat. Environ. Res. 2008, 108, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.R.; Andrady, A.L. Plastics in the Marine Environment. In Plastics and the Environment; Wiley Interscience: Hoboken, NJ, USA, 2004; pp. 379–401. [Google Scholar]
- Gigault, J.; Pedrono, B.; Ter Halle, A.; Maxit, B. Marine plastic litter: The unanalyzed nano-fraction. Environ. Sci. Nano 2016, 3, 346–350. [Google Scholar] [CrossRef]
- Zia, K.M.; Bhatti, H.N.; Bhatti, I.A. Methods for polyurethane and polyurethane composites, recycling and recovery: A review. React. Funct. Polym. 2007, 67, 675–692. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Liu, Y.-N. Separation of polyethylene terephthalate from municipal waste plastics by froth flotation for recycling industry. Waste Manag. 2015, 35, 42–47. [Google Scholar] [CrossRef]
- Bjorksten, J.; Tovey, H.; Harker, B.; Henning, J. Polyesters and Their Applications; Chapman and Hall: London, UK, 1956. [Google Scholar]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Raheem, A.B.; Noor, Z.Z.; Hassan, A.; Hamid, M.K.A.; Samsudin, S.A.; Sabeen, A.H. Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J. Clean. Prod. 2019, 225, 1052–1064. [Google Scholar] [CrossRef]
- Demirel, B.; Yaras, A.; Elçiçek, H. Crystallization behavior of PET materials. J. BAÜ Fen Bil. Enst. Dergisi Cilt 2011, 13, 26–35. [Google Scholar]
- Exconde, M.K.J.E.; Co, J.A.A.; Manapat, J.Z.; Magdaluyo, E.R. Materials Selection of 3D Printing Filament and Utilization of Recycled Polyethylene Terephthalate (PET) in a Redesigned Breadboard. Procedia CIRP 2019, 84, 28–32. [Google Scholar] [CrossRef]
- Garzon-Hernandez, S.; Garcia-Gonzalez, D.; Jérusalem, A.; Arias, A. Design of FDM 3D printed polymers: An experimental-modelling methodology for the prediction of mechanical properties. Mater. Des. 2020, 188, 108414. [Google Scholar] [CrossRef]
- Corcione, C.E.; Gervaso, F.; Scalera, F.; Montagna, F.; Maiullaro, T.; Sannino, A.; Maffezzoli, A. 3D printing of hydroxyapatite polymer-based composites for bone tissue engineering. J. Polym. Eng. 2017, 37, 741–746. [Google Scholar] [CrossRef]
- Prevorsek, D.C.; Tirpak, G.A.; Harget, P.J.; Reimschuessel, A.C. Effects of thermal contraction on structure and properties of PET fibers. J. Macromol. Sci. Part B 1974, 9, 733–759. [Google Scholar] [CrossRef]
- Rim, P.B.; Nelson, C.J. Properties of PET Fibers with High Modulus and low Shrinkage (HMLS). I. Yarn Properties and Morphology. J. Appl. Polym. Sci. 1991, 42, 1807–1813. [Google Scholar] [CrossRef]
- Ibrahim, Y.; Melenka, G.W.; Kempers, R. Fabrication and tensile testing of 3D printed continuous wire polymer composites. Rapid Prototyp. J. 2018, 24, 1131–1141. [Google Scholar] [CrossRef]
- Miller, M.L. The Structure of Polymers; Reinhold Publishing Corp: New York, NY, USA, 1966. [Google Scholar]
- Munk, P.; Aminabhavi, T.M. Introduction to Macromolecular Science; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Alves, N.M.; Mano, J.F.; Balaguer, E.; Meseguer Dueñas, J.M.; Gómez Ribelles, J.L. Glass transition and structural relaxation in semi-crystalline poly (ethylene terephthalate): A DSC study. Polymers 2002, 43, 4111–4122. [Google Scholar] [CrossRef]
- Groeninckx, G.; Berghmans, H.; Overbergh, N.; Smets, G. Crystallization of poly (ethylene terephthalate) induced by inorganic compounds. I. Crystallization behavior from the glassy state in a low-temperature region. J. Polym. Sci. Polym. Phys. 1974, 12, 303–316. [Google Scholar] [CrossRef]
- Jabarin, S.A. Optical properties of thermally crystallized poly (ethylene terephthalate). Polym. Eng. Sci. 1982, 22, 815–820. [Google Scholar] [CrossRef]
- Blaine, R.L. Determination of Polymer Crystallinity by DSC. TA123; TA Instrument Publication: New Castle, DE, USA, 2010. [Google Scholar]
- Maffezzoli, A.; Torre, L.; Kenny, J.M. Determination of the crystallization enthalpy of New-TPI. J. Appl. Polym. Sci. 1998, 67, 763–766. [Google Scholar] [CrossRef]
- Nunes, R.; Costab, V.; Caladoc, V.; Tavares Branco, J. Wear, Friction, and Microhardness of a Thermal Sprayed PET-Poly (Ethylene Terephthalate) Coating. Mater. Res. 2009, 12, 121–125. [Google Scholar] [CrossRef]
- Hermans, P.H.; Weidinger, A. On the determination of the crystalline fraction of polyethylene from X-ray diffraction. Macromol. Chem. Phys. 2003, 44, 24–36. [Google Scholar] [CrossRef]
- Bosley, D.E. X-Ray Determination of Crystallinity in Poly (ethylene Terephthalate). J. Appl. Polym. Sci. 1964, 8, 1521–1529. [Google Scholar] [CrossRef]
- Kulicke, W.; Kniewske, R. The shear viscosity dependence on concentration, molecular weight, and shear rate of polystyrene solutions. Rheol. Acta 1984, 23, 75–83. [Google Scholar] [CrossRef]
- Incarnato, L.; Scarfato, P.; Di Maio, L.; Acierno, D. Structure and rheology of recycled PET modified by reactive extrusion. Polymers 2000, 41, 6825–6831. [Google Scholar] [CrossRef]
- Maddah, H.A. Polypropylene as a Promising Plastic: A Review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Greco, A.; Ferrari, F.; Maffezzoli, A. Processing of Super Tough Plasticized PLA by Rotational Molding. Adv. Polym. Technol. 2019, 2019. [Google Scholar] [CrossRef]
- Farhoodi, M.; Dadashi, S.; Mousavi, M.; Sotudeh-Gharebagh, R.; Emam-Djomeh, Z.; Oromiehie, A.; Hemati, F. Influence of TiO2 Nanoparticle Filler on the Properties of PET and PLA Nanocomposites. Polymers 2012, 36. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Ying Hsi Fuh, J.; Lee, H.P. Effect of Porosity on Mechanical Properties of 3D Printed Polymers: Experiments and Micromechanical Modeling Based on X-ray Computed Tomography Analysis. Polymers 2019, 11, 1154. [Google Scholar] [CrossRef]



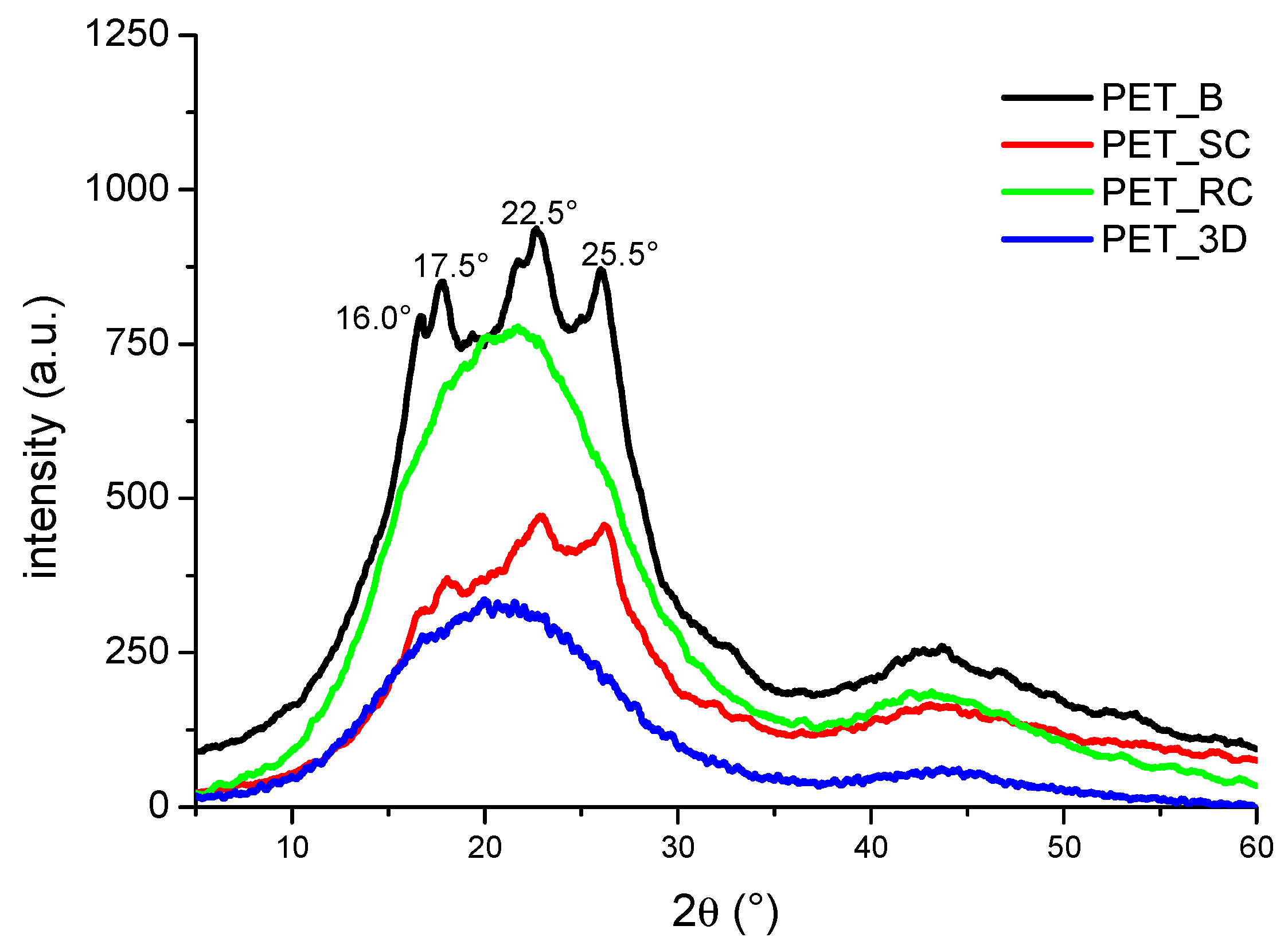



| Sample | Tg (°C) | Tm (°C) | HC (J/g) | HM (J/g) | xcDSC | xcT | xcXRD |
|---|---|---|---|---|---|---|---|
| PET_P | 81 | 261.2 | -- | 50.67 | 1 | 0.36 | -- |
| PET_B | 81 | 249.8 | -- | 52.82 | 1 | 0.38 | 0.28 |
| PET_SC | 84 | 253.9 | -- | 41.67 | 1 | 0.30 | 0.22 |
| PET_RC | 71 | 252.8 | 29.92 | 48.87 | 0.43 | 0.13 | 0 |
| PET_3D | 71 | 253.9 | 30.75 | 65.11 | 0.53 | 0.24 | 0 |
| SAMPLE | n |
|---|---|
| PET_P | 0.69 |
| PET_B | 0.88 |
| PET_SC | 0.97 |
| PET_RC | 0.92 |
| PET_3D | 0.94 |
| SAMPLE | Tonset (°C) | T95% weight loss (°C) | Solid Residue (%) |
|---|---|---|---|
| PET_P | 369.43 | 596.96 | 2.34 ± 0.62 |
| PET_B | 359.74 | 564.57 | 1.92 ± 0.88 |
| PET_SC | 353.83 | 564.52 | 1.56 ± 0.91 |
| PET_RC | 351.56 | 555.32 | 2.56 ± 0.47 |
| PET_3D | 329.44 | 584.08 | 2.67 ± 0.33 |
| SAMPLE | σ (MPa) | ε (%) | E (GPa) |
|---|---|---|---|
| NEAT PET [46] | 59.62 ± 2.9 | 5.22 ± 1.60 | 2.47 ± 1.98 |
| PET_B | 89.2 ± 9.16 | 3.12 ± 0.81 | 2.71 ± 0.23 |
| PET_SC | 63.0 ± 6.79 | 3.73 ± 0.78 | 2.19 ± 0.32 |
| PET_RC | 39.1 ± 5.21 | 4.20 ± 0.74 | 1.14 ± 0.22 |
| PET_3D | 40.9 ± 6.18 | 5.15 ± 0.89 | 1.01 ± 0.36 |
| PLA [42] | 63.5 ± 4.61 | 4.21 ± 0.44 | 2.40 ± 0.21 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, F.; Esposito Corcione, C.; Montagna, F.; Maffezzoli, A. 3D Printing of Polymer Waste for Improving People’s Awareness about Marine Litter. Polymers 2020, 12, 1738. https://doi.org/10.3390/polym12081738
Ferrari F, Esposito Corcione C, Montagna F, Maffezzoli A. 3D Printing of Polymer Waste for Improving People’s Awareness about Marine Litter. Polymers. 2020; 12(8):1738. https://doi.org/10.3390/polym12081738
Chicago/Turabian StyleFerrari, Francesca, Carola Esposito Corcione, Francesco Montagna, and Alfonso Maffezzoli. 2020. "3D Printing of Polymer Waste for Improving People’s Awareness about Marine Litter" Polymers 12, no. 8: 1738. https://doi.org/10.3390/polym12081738
APA StyleFerrari, F., Esposito Corcione, C., Montagna, F., & Maffezzoli, A. (2020). 3D Printing of Polymer Waste for Improving People’s Awareness about Marine Litter. Polymers, 12(8), 1738. https://doi.org/10.3390/polym12081738









