Application of Artificial Neural Networks to Predict the Catalytic Pyrolysis of HDPE Using Non-Isothermal TGA Data
Abstract
1. Introduction
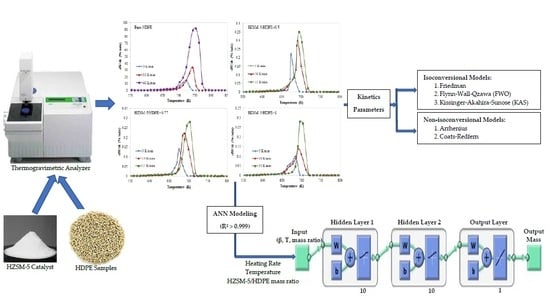
2. Materials and Methods
2.1. Experimental Procedure
2.2. Kinetic Theory
- wo: is the weight of the sample at t = 0,
- w: is the weight of the sample at t = t,
- wf: is the weight of the sample at the experiment end.
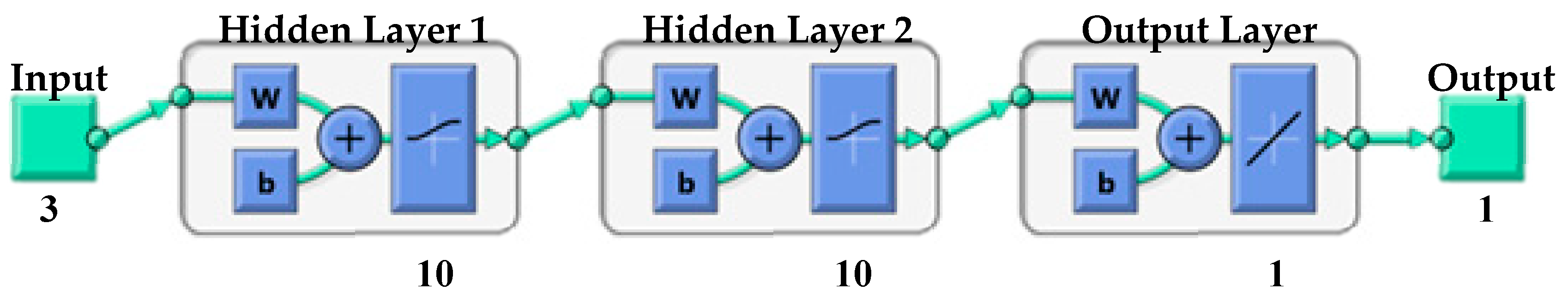
2.3. Artificial Neural Network (ANN) Modeling
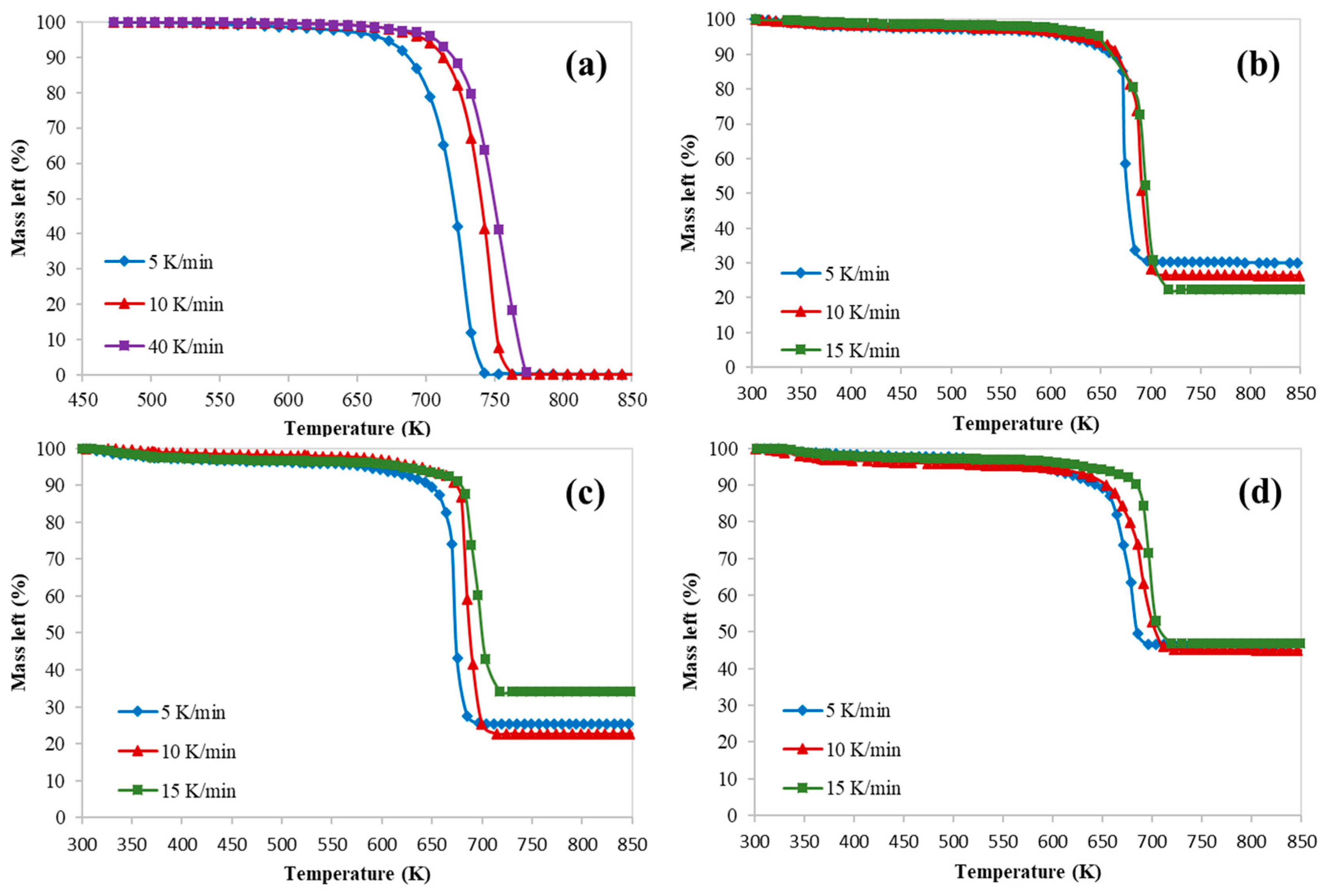
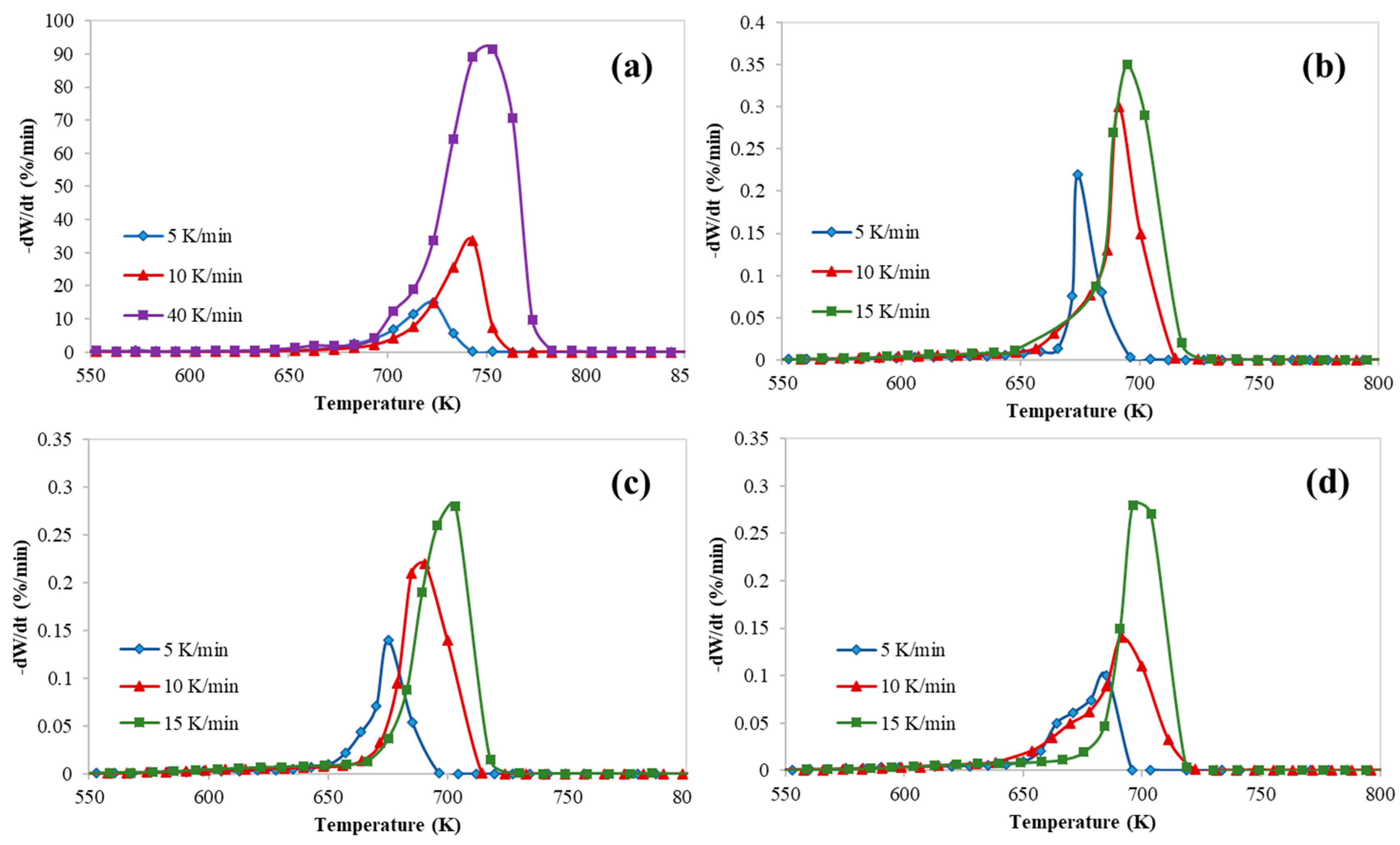
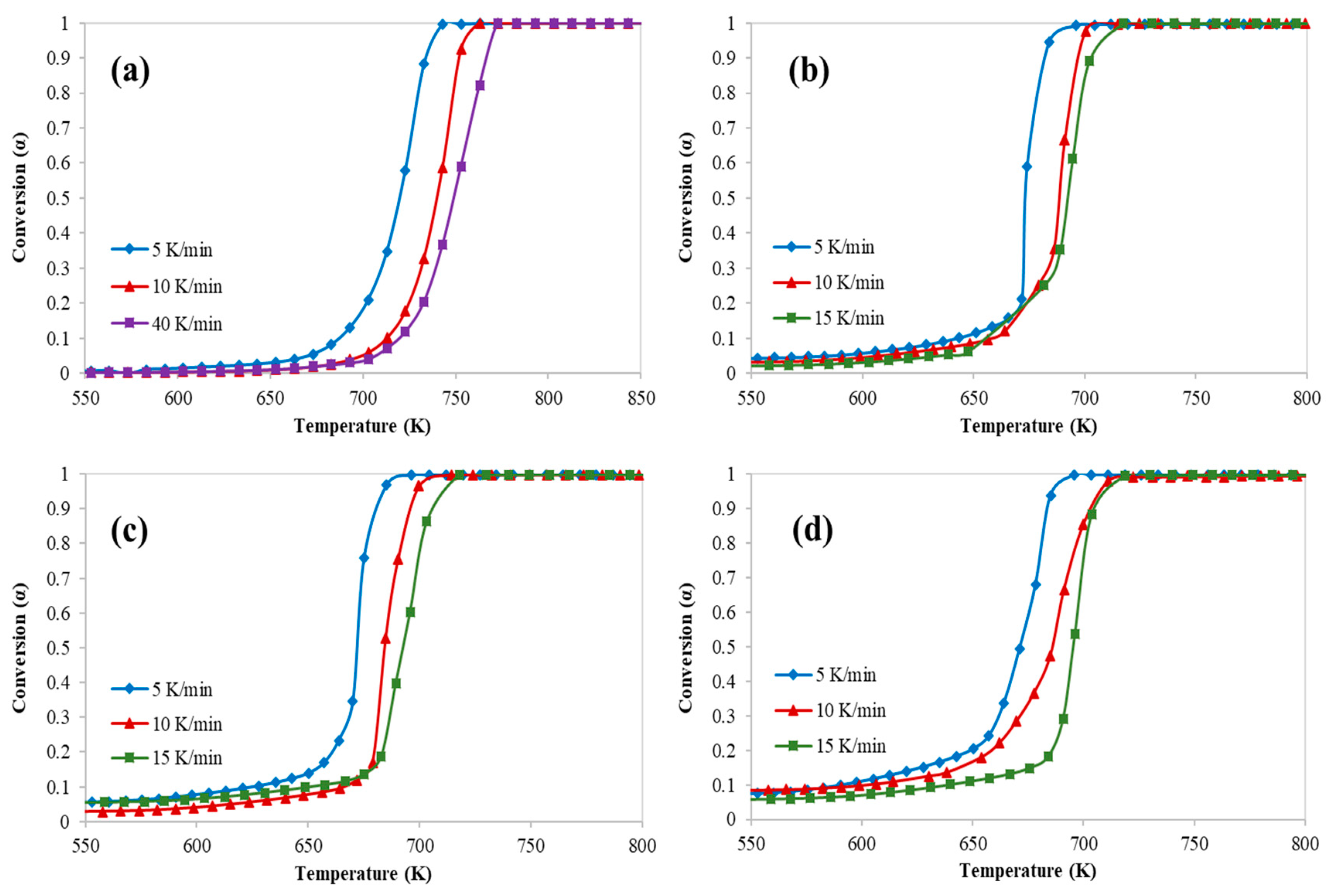
3. Results and Discussion
3.1. Kinetics Study of Catalytic Pyrolysis of HDPE
3.2. Prediction of Catalyst Pyrolysis by ANN Model
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cardona, S.C.; Corma, A. Tertiary recycling of polypropylene by catalytic cracking in a semi-batch stirred reactor. Appl. Catal. B 2000, 25, 151–162. [Google Scholar] [CrossRef]
- Miskolczi, N.; Bartha, L.; Dea’k, G. Thermal degradation of polyethylene and polystyrene from the packaging industry over different catalysts into fuel-like feedstocks. Polym. Degrad. Stabil. 2006, 91, 517–526. [Google Scholar] [CrossRef]
- Arandes, J.; Abajo, I.; Lopez-Valerio, D.; Fernandez, I.; Azkoiti, M.J.; Olazar, M.; Bilbao, J. Transformation of several plastic wastes into fuels by catalytic cracking. Ind. Eng. Chem. Res. 1997, 36, 4523–4529. [Google Scholar] [CrossRef]
- Chen, D.; Yin, L.; Wang, H.; He, P. Pyrolysis technologies for municipal solid waste: A review. Waste Manag. 2014, 34, 2466–2486. [Google Scholar] [CrossRef]
- Kaminsky, W.; Schlesselmann, B.; Simon, C.M. Thermal degradation of mixed plastic waste to aromatic and gas. Poly. Degrad. Stab. 1996, 53, 189–197. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Miguel, G.S.; Castro, M.C.; Madrid, S. Feedstock recycling of polyethylene in a two-step thermo-catalytic reaction system. J. Anal. Appl. Pyrolysis 2007, 79, 415–423. [Google Scholar] [CrossRef]
- Jan, M.R.; Shah, J.; Gulab, H. Catalytic conversion of waste high-density polyethylene into useful hydrocarbons. Fuel 2013, 105, 595–602. [Google Scholar] [CrossRef]
- Conesa, J.A.; Marcilla, A.; Font, R.; Caballero, J.A. Thermogravimetric studies on the thermal decomposition of polyethylene. J. Anal. Appl. Pyrolysis 1996, 36, 1–15. [Google Scholar] [CrossRef]
- Aboulkas, A.; el Harfi, K.; Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I Pyrolysis Kinet. Mech. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Chin, B.L.F.; Yusup, S.; Al Shoaibi, A.; Kannan, P.; Srinivasakannan, C.; Sulaiman, S.A. Kinetic studies of co-pyrolysis of rubber seed shell with high-density polyethylene. Energy Convers. Manag. 2014, 87, 746–753. [Google Scholar] [CrossRef]
- Silvarrey, L.S.D.; Phan, A.N. Kinetic study of municipal plastic waste. Int. J. Hydrog. Energy 2016, 41, 16352–16364. [Google Scholar] [CrossRef]
- Khedri, S.; Elyasi, S. Kinetic analysis for thermal cracking of HDPE: A new isoconversional approach. Polym. Degrad. Stabil. 2016, 129, 306–318. [Google Scholar] [CrossRef]
- Chan, J.H.; Balke, S.T. The thermal degradation kinetics of polypropylene: Part III. Thermogravimetric analyses. Polym. Degrad. Stab. 1997, 57, 135–149. [Google Scholar] [CrossRef]
- Dubdub, I.; Al-Yaari, M. Pyrolysis of low-density polyethylene: Kinetic study using TGA data and ANN prediction. Polymers 2020, 12, 891. [Google Scholar] [CrossRef] [PubMed]
- Quantrille, T.E.; Liu, Y.A. Artificial Intelligence in Chemical Engineering; Elsevier Science: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Govindan, B.; Jakka, S.C.B.; Radhakrishnan, T.K.; Tiwari, A.K.; Sudhakar, T.M.; Shanmugavelu, P.; Kalburgi, A.K.; Sanyal, A.; Sarkar, S. Investigation on kinetic parameters of combustion and oxy-combustion of calcined pet coke employing thermogravimetric analysis coupled to artificial neural network modeling. Energy Fuels 2018, 32, 3995–4007. [Google Scholar] [CrossRef]
- Conesa, J.A.; Caballero, J.A.; Reyes-Labarta, A.J. Artificial neural network for modeling thermal decompositions. J. Anal. Appl. Pyrolysis 2004, 71, 343–352. [Google Scholar] [CrossRef]
- Yıldız, Z.; Uzun, H.; Ceylan, S.; Topcu, Y. Application of artificial neural networks to co-combustion of hazelnut husk–lignite coal blends. Bioresour. Technol. 2016, 200, 42–47. [Google Scholar] [CrossRef]
- Çepelioğullar, Ö.; Mutlu, I.; Yaman, S.; Haykiri-Acma, H. A study to predict pyrolytic behaviors of refuse-derived fuel (RDF): Artificial neural network application. J. Anal. Appl. Pyrolysis 2016, 122, 84–94. [Google Scholar] [CrossRef]
- Charde, S.J.; Sonawane, S.S.; Sonawane, S.H.; Shimpi, N.G. Degradation kinetics of polycarbonate composites: Kinetic parameters and artificial neural network. Chem. Biochem. Eng. Q. 2018, 32, 151–165. [Google Scholar] [CrossRef]
- Chen, J.; Xie, C.; Liu, J.; He, Y.; Xie, W.; Zhang, X.; Chang, K.; Kuo, J.; Sun, J.; Zheng, L.; et al. Co-combustion of sewage sludge and coffee grounds under increased O2/CO2 atmospheres: Thermodynamic characteristics, kinetics, and artificial neural network modeling. Bioresour. Technol. 2018, 250, 230–238. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Tariq, R.; Hameed, Z.; Ali, I.; Taqvi, S.A.; Naqvi, M.; Niazi, M.B.K.; Noor, T.; Farooq, W. Pyrolysis of high-ash sewage sludge: Thermo-kinetic study using TGA and artificial neural networks. Fuel 2018, 233, 529–538. [Google Scholar] [CrossRef]
- Halali, M.A.; Azari, V.; Arabloo, M.; Mohammadi, A.H.; Bahadori, A. Application of a radial basis function neural network to estimate pressure gradient in water–oil pipelines. J. Taiwan Inst. Chem. Eng. 2016, 58, 189–202. [Google Scholar] [CrossRef]
- Baloch, M.K.; Khurram, M.J.Z.; Durrani, G.F. Application of different methods for the thermogravimetric analysis of polyethylene samples. J. Appl. Polym. Sci. 2011, 120, 3511–3518. [Google Scholar] [CrossRef]
- García, R.A.; Serrano, D.P.; Otero, D. Catalytic cracking of HDPE over hybrid zeolitic-mesoporous materials. J. Anal. Appl. Pyrolysis 2005, 74, 379–386. [Google Scholar] [CrossRef]
- Gobin, K.; Manos, G. Thermogravimetric study of polymer catalytic degradation over microporous materials. Polym. Degrad. Stab. 2004, 86, 225–231. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Li, Y.; Sun, Y. Effect of heating rate on pyrolysis behavior and kinetic characteristics of siderite. Materials 2017, 7, 211. [Google Scholar] [CrossRef]
- Chandrasekaran, S.R.; Kunwar, B.; Moser, B.R.; Rajagopalan, N.; Sharma, B.K. Catalytic thermal cracking of postconsumer waste plastics to fuels. 1. Kinetics and optimization. Energy Fuels 2015, 29, 6068–6077. [Google Scholar] [CrossRef]
- Al-Wahaibi, T.; Mjalli, F. Prediction of horizontal oil-water flow pressure gradient using artificial intelligence techniques. Chem. Eng. Commun. 2014, 201, 209–224. [Google Scholar] [CrossRef]
- Qinghua, W.; Honglan, Z.; Wei, L.; Junzheng, Y.; Xiaohong, W.; Wang, Y. Experimental study of horizontal gas-liquid two-phase flow in two medium-diameter pipes and prediction of pressure drop through BP neural networks. Int. J. Fluid Mach. Syst. 2018, 11, 255–264. [Google Scholar] [CrossRef]

| Waste Plastic | Proximate Analysis, wt % | Ultimate Analysis, wt % | |||||
|---|---|---|---|---|---|---|---|
| Moisture | Volatile | Ash | C | H | N | S | |
| HDPE | 4.504 | 94.278 | 1.218 | 78.33 | 20.71 | 0.00 | 0.96 |
| Name | SiO2/Al2O3 Mole Ratio | Nominal Cation Form | Na2O, wt% | Surface Area, m2/g |
|---|---|---|---|---|
| HZSM-5 (CBV3024E) | 30 | Hydrogen | 0.05 | 400 |
| Run No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| HZSM-5/HDPE (mass Ratio) | 0.5 | 0.77 | 1.0 | 0.5 | 0.77 | 1.0 | 0.5 | 0.77 | 1.0 |
| Heating Rate (K/min) | 5 | 5 | 5 | 10 | 10 | 10 | 15 | 15 | 15 |
| Model | Equation |
|---|---|
| Isoconversional | |
| Friedman | |
| Flynn–Wall–Qzawa (FWO) | |
| Kissinger–Akahira–Sunose (KAS) | |
| Non-isoconversional | |
| Arrhenius | |
| Coats–Redfern |
| HZSM-5/HDPE (Mass Ratio) | Heating Rate K/min | Tonset (K) | T5% (K) | Tpeak (K) | Tendset (K) | Mass Loss (%) | Residue (%) |
|---|---|---|---|---|---|---|---|
| 0.0 (Pure HDPE) | 5 | 473 | 668 | 723 | 743 | 99 | 1 |
| 10 | 473 | 698 | 743 | 763 | 99 | 1 | |
| 15 | 473 | 696 | 753 | 773 | 99 | 1 | |
| 0.5 | 5 | 310 | 589 | 674 | 696 | 70 | 30 |
| 10 | 304 | 615 | 691 | 712 | 74 | 26 | |
| 15 | 336 | 635 | 695 | 718 | 78 | 22 | |
| 0.77 | 5 | 301 | 512 | 675 | 696 | 75 | 25 |
| 10 | 334 | 622 | 696 | 712 | 77 | 23 | |
| 15 | 310 | 505 | 703 | 718 | 66 | 34 | |
| 1.0 | 5 | 325 | 516 | 685 | 696 | 54 | 46 |
| 10 | 316 | 396 | 691 | 713 | 55 | 45 | |
| 15 | 311 | 464 | 696 | 718 | 53 | 47 |
| Conversion | HZSM-5/HDPE = 0.5 | HZSM-5/HDPE = 0.77 | HZSM-5/HDPE = 1.0 | |||
|---|---|---|---|---|---|---|
| E (kJ/mol) | R2 | E (kJ/mol) | R2 | E (kJ/mol) | R2 | |
| Friedman Model | ||||||
| 0.5 | 120 | 0.9749 | 99 | 0.9949 | 263 | 0.9815 |
| 0.6 | 219 | 0.9811 | 147 | 0.9922 | 211 | 0.877 |
| 0.7 | 225 | 0.9977 | 154 | 0.9995 | 141 | 0.673 |
| 0.9 | 180 | 0.9552 | 142 | 0.9505 | 100 | 0.9085 |
| Average | 186 | 0.9772 | 135.5 | 0.9843 | 178.7 | 0.8600 |
| FWO Model | ||||||
| 0.5 | 202 | 0.9633 | 196 | 0.9995 | 332 | 0.9997 |
| 0.6 | 191 | 0.97 | 181 | 0.9991 | 183 | 1 |
| 0.7 | 198 | 0.9797 | 169 | 0.9997 | 196 | 0.9973 |
| 0.9 | 193 | 0.9824 | 166 | 0.9989 | 168 | 0.9136 |
| Average | 196 | 0.97385 | 178 | 0.9993 | 219.75 | 0.97765 |
| KSA Model | ||||||
| 0.5 | 201 | 0.9593 | 198 | 0.9787 | 170 | 0.9991 |
| 0.6 | 189 | 0.9665 | 179 | 0.999 | 182 | 1 |
| 0.7 | 196 | 0.9774 | 167 | 0.9997 | 195 | 0.997 |
| 0.9 | 192 | 0.9803 | 165 | 0.9931 | 191 | 0.9919 |
| Average | 194.5 | 0.970875 | 177.25 | 0.992625 | 184.5 | 0.997 |
| Run | Heating Rate (K/min) | HZSM-5/HDPE Mass Ratio | Coats–Redfern | Arrhenius | ||
|---|---|---|---|---|---|---|
| E (kJ/mol) | R2 | E (kJ/mol) | R2 | |||
| 1 | 5 | 0.5 | 126 | 0.9399 | 125 | 0.9995 |
| 4 | 10 | 0.5 | 140 | 0.9631 | 142 | 0.958 |
| 7 | 15 | 0.5 | 168 | 0.9942 | 168 | 0.8222 |
| Average | 144.7 | 0.9657 | 145 | 0.9266 | ||
| 2 | 5 | 0.77 | 140 | 0.9682 | 157 | 0.9813 |
| 5 | 10 | 0.77 | 145 | 0.9723 | 148 | 0.9103 |
| 8 | 15 | 0.77 | 110 | 0.9414 | 112 | 0.9047 |
| Average | 131.7 | 0.9606 | 139 | 0.9321 | ||
| 3 | 5 | 1 | 140 | 0.9476 | 129 | 0.9799 |
| 6 | 10 | 1 | 132 | 0.9997 | 135 | 0.9575 |
| 9 | 15 | 1 | 123 | 0.8387 | 156 | 0.9423 |
| Average | 131.7 | 0.9287 | 140 | 0.9599 | ||
| Model No. | Network Topology | 1st Transfer Function | 2nd Transfer Function | R2 | MSE for Training |
|---|---|---|---|---|---|
| ANN1 | NN-3-10-1 | TANSIG | - | 0.99920 | 1.48 |
| ANN2 | NN-3-15-1 | TANSIG | - | 0.99935 | 0.904 |
| ANN3 | NN-3-5-1 | TANSIG | - | 0.99722 | 5.18 |
| ANN4 | NN-3-10-1 | LOGSIG | - | 0.99878 | 2.64 |
| ANN5 | NN-3-15-1 | LOGSIG | - | 0.99888 | 1.41 |
| ANN6 | NN-3-5-1 | LOGSIG | - | 0.99824 | 2.46 |
| ANN7 | NN-3-15-15-1 | TANSIG | TANSIG | 0.99981 | 0.0635 |
| ANN8 | NN-3-15-15-1 | LOGSIG | TANSIG | 0.99962 | 0.0646 |
| ANN9 | NN-3-15-15-1 | TANSIG | LOGSIG | 0.99972 | 0.0571 |
| ANN10 | NN-3-10-15-1 | TANSIG | LOGSIG | 0.99983 | 0.0422 |
| ANN11 | NN-3-10-10-1 | TANSIG | LOGSIG | 0.99985 | 0.0860 |
| ANN12 | NN-3-10-10-1 | LOGSIG | LOGSIG | 0.99970 | 0.227 |
| ANN13 | NN-3-15-15-1 | LOGSIG | LOGSIG | 0.99904 | 1.08 |
| ANN14 | NN-3-10-15-1 | LOGSIG | LOGSIG | 0.99960 | 0.176 |
| ANN15 | NN-3-15-10-1 | LOGSIG | LOGSIG | 0.99970 | 0.0504 |
| ANN16 | NN-3-20-20-1 | LOGSIG | LOGSIG | 0.99974 | 0.0479 |
| ANN17 | NN-3-20-20-1 | TANSIG | LOGSIG | 0.99956 | 0.274 |
| ANN18 | NN-3-9-9-1 | TANSIG | LOGSIG | 0.99884 | 1.28 |
| ANN19 | NN-3-11-11-1 | TANSIG | LOGSIG | 0.99968 | 0.276 |
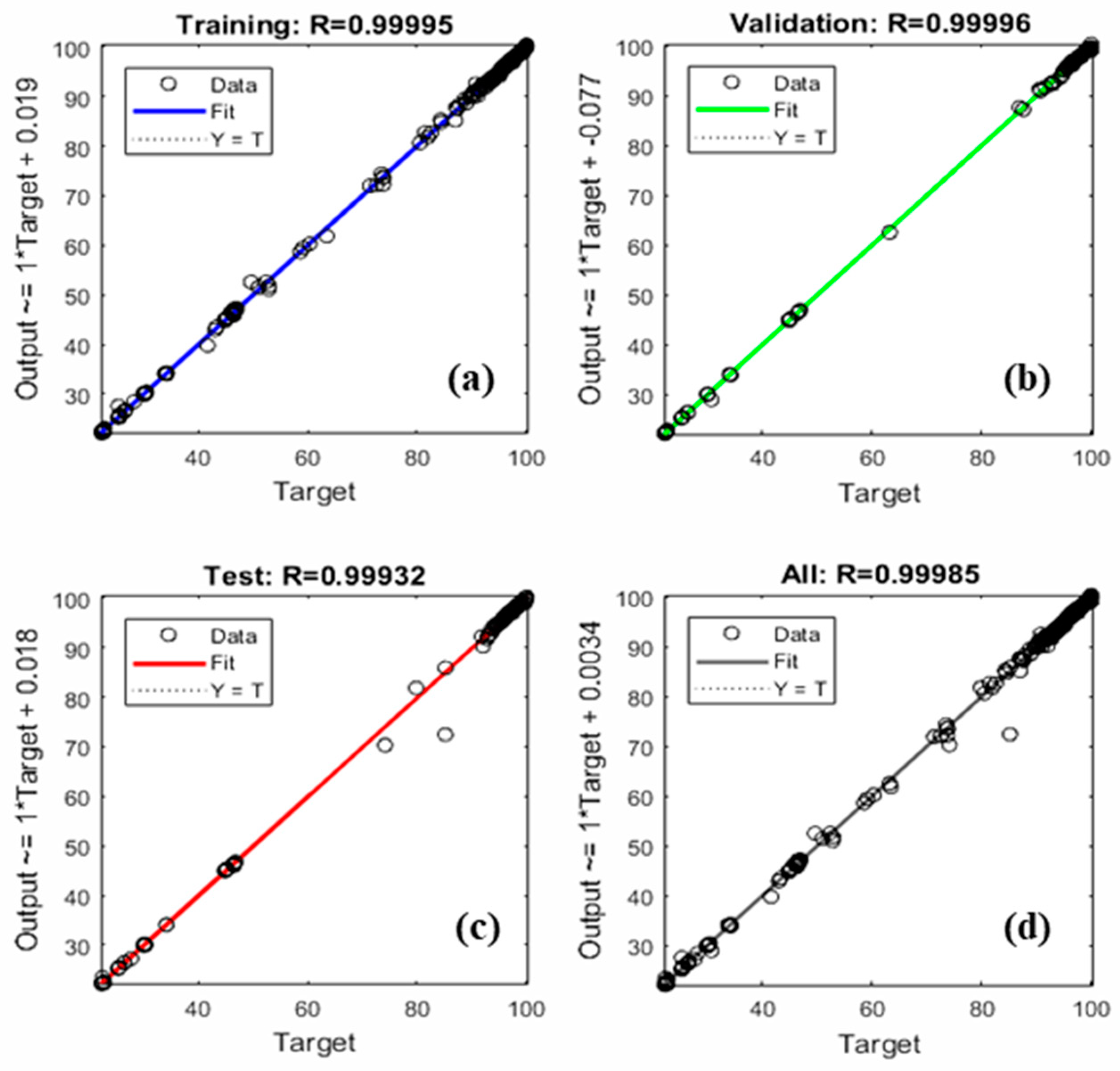
| Set | Statistical Parameters | |||
|---|---|---|---|---|
| R2 | RMSE | MAE | MBE | |
| Training | 0.99995 | 0.30721 | 0.16388 | 0.01954 |
| Validation | 0.99996 | 0.27855 | 0.17212 | −0.00186 |
| Test | 0.99932 | 1.19340 | 0.30865 | −0.15220 |
| All | 0.99985 | 0.53975 | 0.18683 | −0.00943 |
| No. | Input Data | Output Data | ||
|---|---|---|---|---|
| Heating Rate (K/min) | Temperature (K) | HZSM-5/HDPE (Mass Ratio) | Mass Left (%) | |
| 1 | 5 | 402.59 | 0.5 | 98.03 |
| 2 | 5 | 502.6 | 0.5 | 97.32 |
| 3 | 5 | 605.73 | 0.5 | 95.68 |
| 4 | 5 | 704.33 | 0.5 | 30.28 |
| 5 | 5 | 801.36 | 0.5 | 30.09 |
| 6 | 5 | 411.03 | 0.75 | 97.04 |
| 7 | 5 | 510.32 | 0.75 | 96.29 |
| 8 | 5 | 612.96 | 0.75 | 93.43 |
| 9 | 5 | 711.87 | 0.75 | 25.45 |
| 10 | 5 | 809.3 | 0.75 | 25.36 |
| 11 | 5 | 419.81 | 1 | 98.02 |
| 12 | 5 | 523.09 | 1 | 96.26 |
| 13 | 5 | 620.13 | 1 | 92.56 |
| 14 | 5 | 726.16 | 1 | 46.45 |
| 15 | 5 | 823.51 | 1 | 46.39 |
| 16 | 10 | 433.07 | 0.5 | 98.32 |
| 17 | 10 | 533.25 | 0.5 | 97.67 |
| 18 | 10 | 631.75 | 0.5 | 94.95 |
| 19 | 10 | 733 | 0.5 | 26.49 |
| 20 | 10 | 832.17 | 0.5 | 26.44 |
| 21 | 10 | 441.34 | 0.75 | 98.4 |
| 22 | 10 | 541.26 | 0.75 | 97.87 |
| 23 | 10 | 648.03 | 0.75 | 94.17 |
| 24 | 10 | 740.81 | 0.75 | 22.74 |
| 25 | 10 | 847.67 | 0.75 | 22.69 |
| 26 | 10 | 457.84 | 1 | 96.09 |
| 27 | 10 | 557.59 | 1 | 95.32 |
| 28 | 10 | 653.87 | 1 | 90.08 |
| 29 | 10 | 755.36 | 1 | 45.2 |
| 30 | 10 | 853.97 | 1 | 45.03 |
| 31 | 15 | 469.55 | 0.5 | 98.66 |
| 32 | 15 | 566.83 | 0.5 | 98.17 |
| 33 | 15 | 665.32 | 0.5 | 88.83 |
| 34 | 15 | 768.01 | 0.5 | 22.29 |
| 35 | 15 | 867.59 | 0.5 | 22.26 |
| 36 | 15 | 479.36 | 0.75 | 96.78 |
| 37 | 15 | 576.52 | 0.75 | 96.22 |
| 38 | 15 | 675.12 | 0.75 | 91.02 |
| 39 | 15 | 776.31 | 0.75 | 34.14 |
| 40 | 15 | 875.47 | 0.75 | 34.11 |
| 41 | 15 | 488.8 | 1 | 97.24 |
| 42 | 15 | 585.83 | 1 | 96.59 |
| 43 | 15 | 690.8 | 1 | 84.45 |
| 44 | 15 | 785.3 | 1 | 46.84 |
| 45 | 15 | 884.5 | 1 | 46.79 |
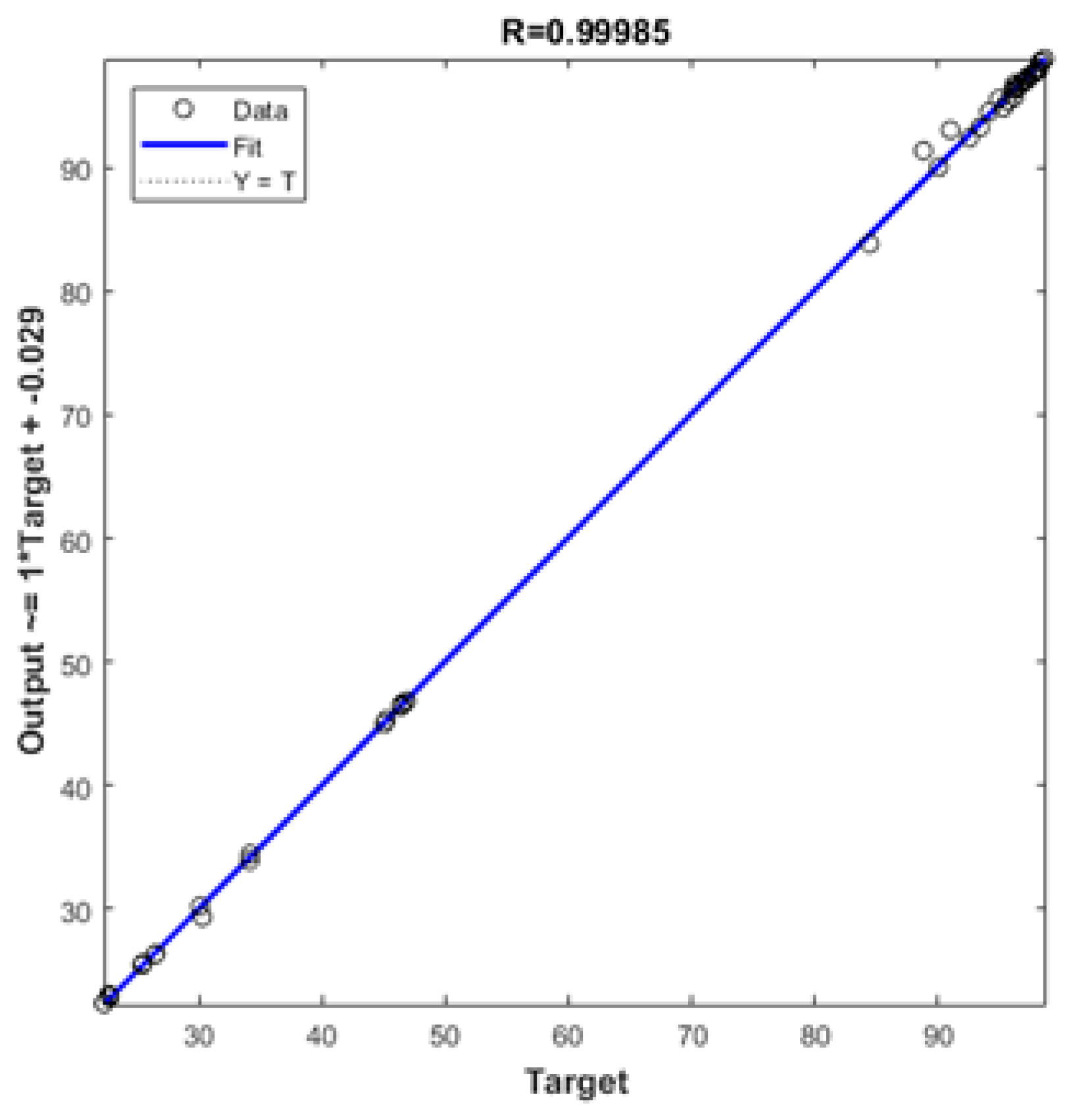
| Set | Statistical Parameters | |||
|---|---|---|---|---|
| R2 | RMSE | MAE | MBE | |
| New Tested Data | 0.99984 | 0.55767 | 0.29725 | 0.08688 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Yaari, M.; Dubdub, I. Application of Artificial Neural Networks to Predict the Catalytic Pyrolysis of HDPE Using Non-Isothermal TGA Data. Polymers 2020, 12, 1813. https://doi.org/10.3390/polym12081813
Al-Yaari M, Dubdub I. Application of Artificial Neural Networks to Predict the Catalytic Pyrolysis of HDPE Using Non-Isothermal TGA Data. Polymers. 2020; 12(8):1813. https://doi.org/10.3390/polym12081813
Chicago/Turabian StyleAl-Yaari, Mohammed, and Ibrahim Dubdub. 2020. "Application of Artificial Neural Networks to Predict the Catalytic Pyrolysis of HDPE Using Non-Isothermal TGA Data" Polymers 12, no. 8: 1813. https://doi.org/10.3390/polym12081813
APA StyleAl-Yaari, M., & Dubdub, I. (2020). Application of Artificial Neural Networks to Predict the Catalytic Pyrolysis of HDPE Using Non-Isothermal TGA Data. Polymers, 12(8), 1813. https://doi.org/10.3390/polym12081813