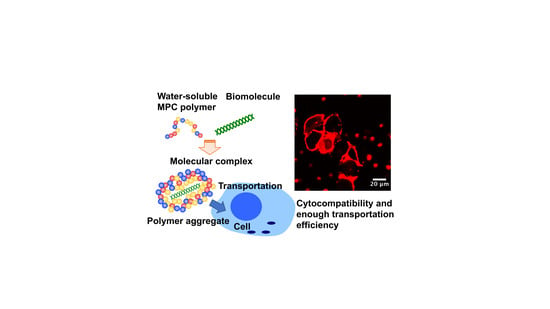
Water-Soluble and Cytocompatible Phospholipid Polymers for Molecular Complexation to Enhance Biomolecule Transportation to Cells In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymerization Procedure
2.3. Characterization of Polymers
2.4. Surface Tension Measurement
2.5. Fluorescence Spectrum Measurement
2.6. Cell Culture Procedure
2.7. Evaluation of Interaction of MPC Polymer with Cells
2.8. Preparation of siRNA/MPC Polymer Complex
2.9. Introduction of siRNA/MPC Polymer Complex into Cells
2.10. Evaluation of Functionality of siRNA/MPC Polymer Complex
2.11. Statistical Analysis
3. Results and Discussion
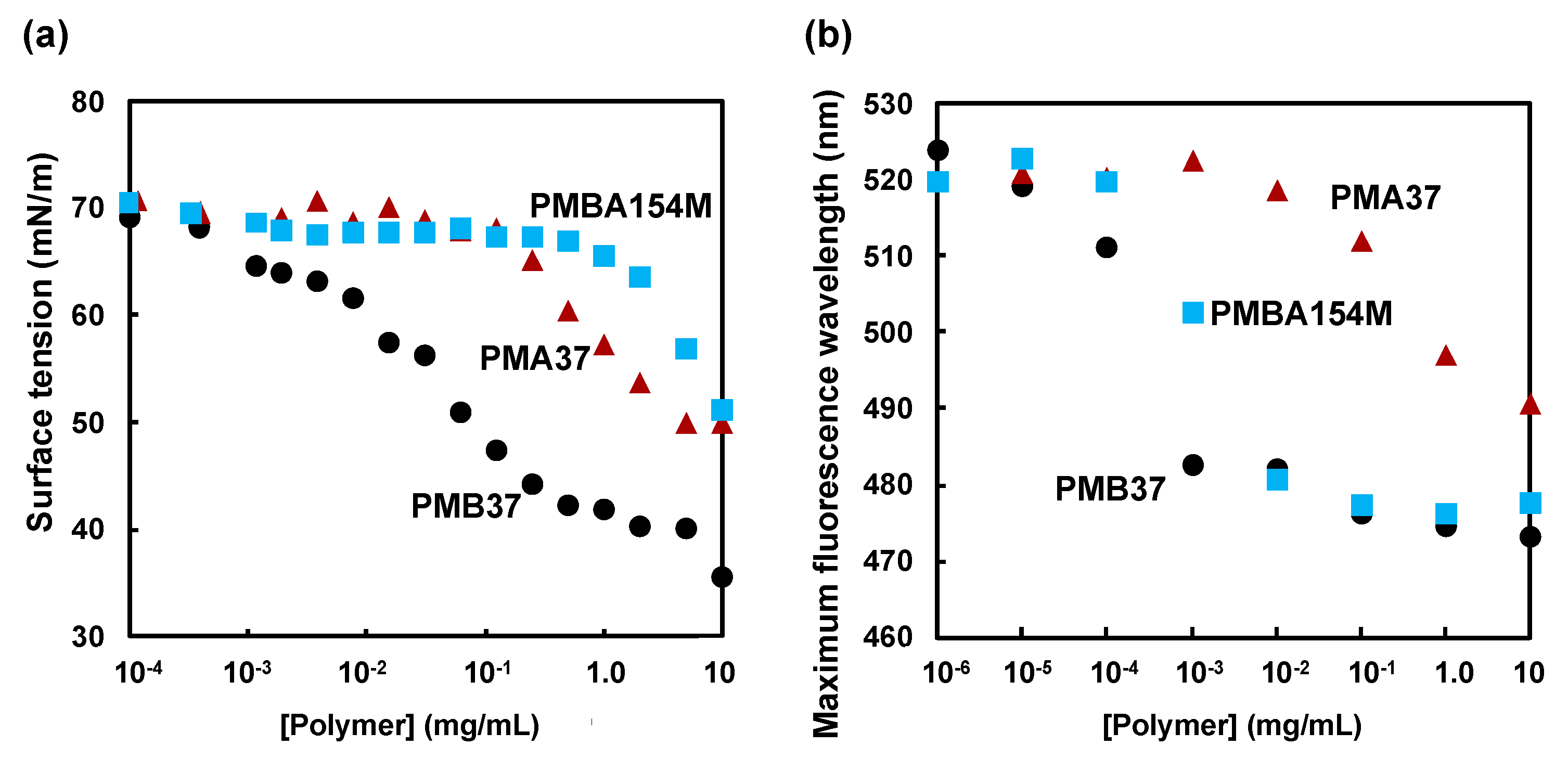
3.1. Characteristics of the MPC Polymers in Aqueous Medium
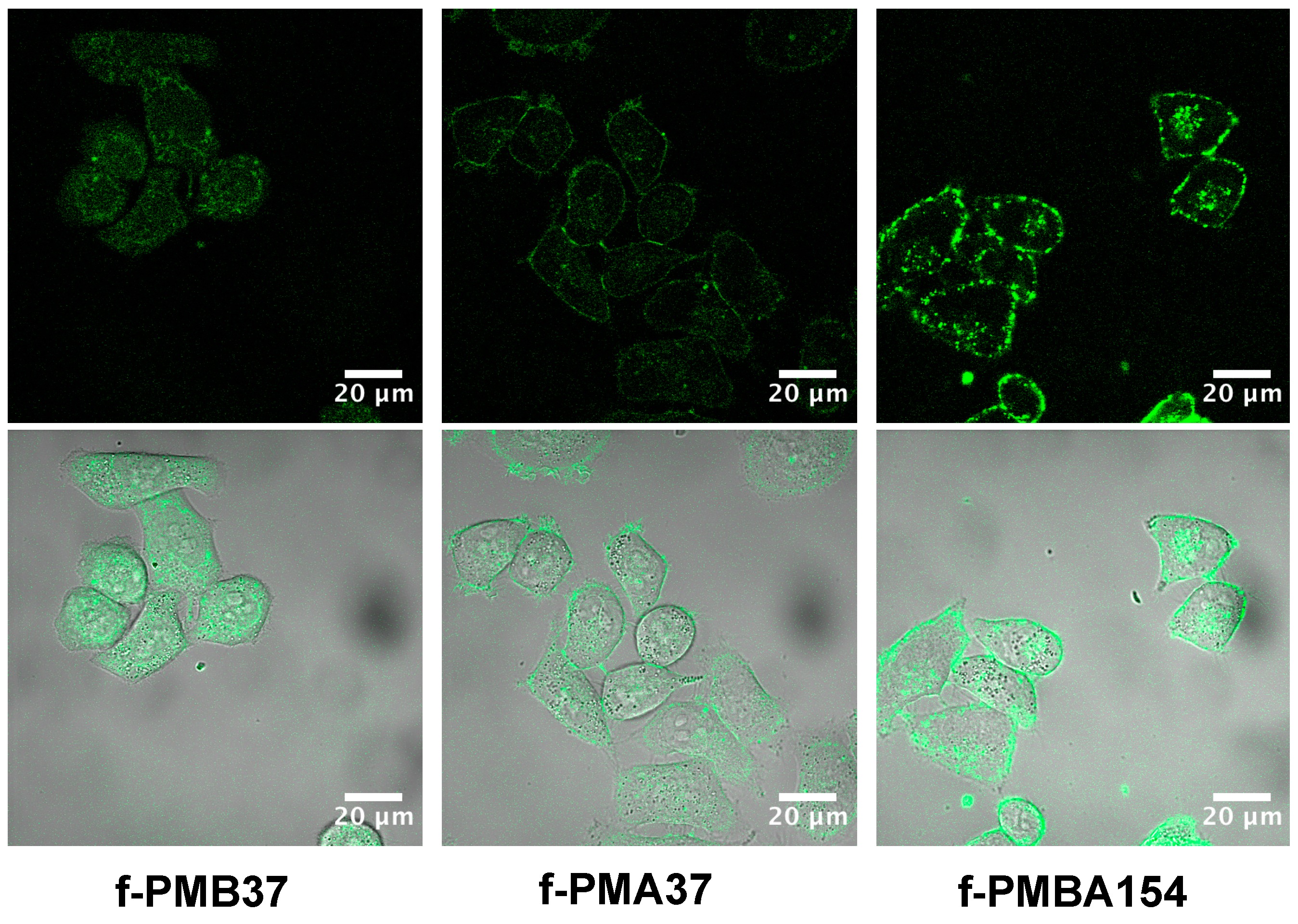
3.2. Internalization of the MPC Polymers to Cells
3.3. Complex Formation of the MPC Polymer with siRNA
3.4. Transportation of siRNA/MPC Polymer Complex into Cells
3.5. Effects of siRNA/MPC Polymer Complex on Functionality of Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, T.G.; Jeong, J.H.; Kim, S.W. Current status of polymeric gene delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 467–486. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.F.; Wong, W.T. Design of polymeric gene carriers for effective intracellular delivery. Trends Biotechnol. 2018, 36, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Dubruel, P.; Schacht, E. Vinyl polymers as non-viral gene delivery carriers: Current status and prospects. Macromol. Biosci. 2006, 6, 789–810. [Google Scholar] [CrossRef] [PubMed]
- O’Rorke, S.; Keeney, M.; Pandit, A. Non-viral polyplexes: Scaffold mediated delivery for gene therapy. Prog. Polym. Sci. 2010, 35, 441–458. [Google Scholar] [CrossRef]
- Kundu, P.P.; Sharma, V. Synthetic polymeric vectors in gene therapy. Curr. Opin. Solid State Mater. Sci. 2008, 12, 89–102. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Green, J.J.; Chan, J.M.; Langer, R.; Anderson, D.G. Polymeric materials for gene delivery and DNA vaccination. Adv. Mater. 2009, 21, 847–867. [Google Scholar] [CrossRef]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Lin, X.; Ishihara, K. Water-soluble polymers bearing phosphorylcholine group and other zwitterionic groups for carrying DNA derivatives. J. Biomater. Sci. Polym. Ed. 2014, 25, 1461–1478. [Google Scholar] [CrossRef]
- De Ilarduya, C.T.; Sun, Y.; Düzgüneş, N. Gene delivery by lipoplexes and polyplexes. Eur. J. Pharm. Sci. 2010, 40, 159–170. [Google Scholar] [CrossRef]
- Wang, B.; Brand-Miller, J. The role and potential of sialic acid in human nutrition. Eur. J. Clin. Nutr. 2003, 57, 1351–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, S. Sialic acid as a tumor marker. Ann. Clin. Lab. Sci. 1994, 24, 376–384. [Google Scholar] [PubMed]
- Chiba, N.; Ueda, M.; Shimada, T.; Jinno, H.; Watanabe, J.; Ishihara, K.; Kitajima, M. Development of gene vectors for pinpoint targeting to human hepatocytes by cationically modified polymer complexes. Eur. Surg. Res. 2007, 39, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, N. Cationic polymer optimization for efficient gene delivery. Mini Rev. Med. Chem. 2010, 10, 108–125. [Google Scholar] [CrossRef]
- Ishihara, K.; Ueda, T.; Nakabayashi, N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polym. J. 1990, 22, 355–360. [Google Scholar] [CrossRef]
- Ahmed, M.; Bhuchar, N.; Ishihara, K.; Narain, R. Well-controlled cationic water-soluble phospholipid polymer-DNA nanocomplexes for gene delivery. Bioconjug. Chem. 2011, 22, 1228–1238. [Google Scholar] [CrossRef]
- Ishihara, K. Revolutionary advances in 2-methacryloyloxyethyl phosphorylcholine polymers as biomaterials. J. Biomed. Mater. Res. A 2019, 107, 933–943. [Google Scholar] [CrossRef]
- Monge, S.; Canniccioni, B.; Graillot, A.; Robin, J.J. Phosphorus-containing polymers: A great opportunity for the biomedical field. Biomacromolecules 2011, 12, 1973–1982. [Google Scholar] [CrossRef]
- Fukazawa, K.; Ishihara, K. Enhanced stability of NADH/dehydrogenase mixture system by water-soluble phospholipid polymers. Biomater. Biomech. Bioeng. 2016, 3, 37–46. [Google Scholar] [CrossRef]
- Goda, T.; Goto, Y.; Ishihara, K. Cell-penetrating macromolecules: Direct penetration of amphipathic phospholipid polymers across plasma membrane of living cells. Biomaterials 2010, 31, 2380–2387. [Google Scholar] [CrossRef]
- Lin, X.; Konno, T.; Ishihara, K. Cell-membrane-permeable and cytocompatible phospholipid polymer nanoprobes conjugated with molecular beacons. Biomacromolecules 2014, 15, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Imaizumi, Y.; Hatano, H.; Matsumoto, A.; Ishihara, K.; Miyahara, Y. Translocation mechanisms of cell-penetrating polymers identified by induced proton dynamics. Langmuir 2019, 35, 8167–8173. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.R.; Lewis, A.L.; Kirkwood, L.C.; Rose, S.F.; Lloyd, A.W.; Vick, T.A.; Stratford, P.W. Biological evaluation and drug delivery application of cationically modified phospholipid polymers. Biomaterials 2004, 25, 4785–4796. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.K.; Ma, Y.; Armes, S.P.; Lewis, A.L.; Baldwin, T.; Stolnik, S. Phosphorylcholine-polycation diblock copolymers as synthetic vectors for gen delivery. J. Control. Release 2004, 100, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Sakaki, S.; Tsuchida, M.; Iwasaki, Y.; Ishihara, K. A water-soluble phospholipid polymer as a new biocompatible synthetic DNA carrier. Bull. Chem. Soc. Jpn. 2004, 77, 2283–2288. [Google Scholar] [CrossRef]
- Kitagawa, T.; Iwase, R.; Ishihara, K.; Yamaoka, T.; Murakami, A. Facilitated disassembly of polyplex composed of self-assembling amphiphilic polycations enhances the gene transfer efficiency. Chem. Lett. 2005, 34, 1478–1479. [Google Scholar] [CrossRef]
- Lam, J.K.; Armes, S.P.; Lewis, A.L.; Stolnik, S. Folate conjugated phosphorylcholine-based polycations for specific targeting in nucleic acids delivery. J. Drug Target. 2009, 17, 512–523. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, H.; Jiang, X.; Zhou, W.; Zhu, B.; Zeng, Y.; Yao, K.; Ren, C. Lipofectamine RNAiMAX: An efficient siRNA transfection reagent in human embryonic stem cells. Mol. Biotechnol. 2008, 40, 19–26. [Google Scholar] [CrossRef]
- Christie, R.J.; Matsumoto, Y.; Miyata, K.; Nomoto, T.; Fukushima, S.; Osada, K.; Halnaut, J.; Pittella, F.; Kim, H.J.; Nishiyama, N.; et al. Targeted polymeric micelles for siRNA treatment of experimental cancer by intravenous injection. ACS Nano 2012, 6, 5174–5189. [Google Scholar] [CrossRef]
- Ueda, T.; Oshida, H.; Kurita, K.; Ishihara, K.; Nakabayashi, N. Preparation of 2-methacryloyloxyethyl phosphorylcholine copolymers with alkyl methacrylates and their blood compatibility. Polym. J. 1992, 24, 1259–1269. [Google Scholar] [CrossRef]
- Ishihara, K.; Iwasaki, Y.; Nakabayashi, N. Polymeric lipid nanosphere consisting of water-soluble poly(2-methacryloyloxyethyl phosphorylcholine-co-n-butyl methacrylate). Polym. J. 1999, 31, 1231–1236. [Google Scholar] [CrossRef] [Green Version]
- Braun, K.; Stürzel, C.M.; Biskupek, J.; Kaiser, U.; Kirchhoff, F.; Lindén, M. Comparison of different cytotoxicity assays for in vitro evaluation of mesoporous silica nanoparticles. Toxicol. Vitr. 2018, 52, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Berardo, C.; Siciliano, V.; Di Pasqua, L.G.; Richelmi, P.; Vairetti, M.; Ferrigno, A. Comparison between Lipofectamine RNAiMAX and GenMute transfection agents in two cellular models of human hepatoma. Eur. J. Histochem. 2019, 63, 3048. [Google Scholar] [CrossRef] [PubMed]
- Yusa, S.; Fukuda, K.; Yamamoto, T.; Ishihara, K.; Morishima, Y. Synthesis of well-defined amphiphilic block copolymers having phospholipid polymer sequences as a novel biocompatible polymer micelle reagent. Biomacromolecules 2005, 6, 663–670. [Google Scholar] [CrossRef]
- Lee, S.B.; Russell, A.J.; Matyjaszewski, K. ATRP synthesis of amphiphilic random, gradient, and block copolymers of 2-(dimethylamino)ethyl methacrylate and n-butyl methacrylate in aqueous media. Biomacromolecules 2003, 4, 1386–1393. [Google Scholar] [CrossRef]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef] [Green Version]
- Kore, G.; Kolate, A.; Nej, A.; Misra, A. Polymeric micelle as multifunctional pharmaceutical carriers. J. Nanosci. Nanotechnol. 2014, 14, 288–307. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef]
- Lin, X.; Fukazawa, K.; Ishihara, K. Photoinduced inhibition of DNA unwinding in vitro with water-soluble polymers containing both phosphorylcholine and photoreactive groups. Acta Biomater. 2016, 40, 226–234. [Google Scholar] [CrossRef]
- Ishihara, K. Blood-compatible surfaces with phosphorylcholine-based polymers for cardiovascular medical devices. Langmuir 2019, 35, 1778–1787. [Google Scholar] [CrossRef]
- Sadkowski, P.J.; Fleming, G.R. The influence of solvent—Solute interaction on radiation less processes: Excited state dynamics of 1,8-anilinonaphthalene sulfonate and related molecules. Chem. Phys. 1980, 54, 79–89. [Google Scholar] [CrossRef]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.J.; Yang, W.T. Polymer vectors via controlled/living radical polymerization for gene delivery. Prog. Polym. Sci. 2011, 36, 1099–1131. [Google Scholar] [CrossRef]
- Ishihara, K.; Yanokuchi, S.; Teramura, Y.; Fukazawa, K. Combination of two antithrombogenic methodologies for preventing thrombus formation on a poly(ether ether ketone) substrate. Colloids Surf. B Biointerfaces 2020, 192, 111021. [Google Scholar] [CrossRef]
- Futaki, S.; Suzuki, T.; Ohashi, W.; Yagami, T.; Tanaka, S.; Ueda, K.; Sugiura, Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001, 276, 5836–5840. [Google Scholar] [CrossRef] [Green Version]
- De Wet, J.R.; Wood, K.V.; DeLuca, M.; Helinski, D.R.; Subramani, S. Firefly luciferase gene: Structure and expression in mammalian cells. Mol. Cell Biol. 1987, 7, 725–737. [Google Scholar] [CrossRef] [Green Version]
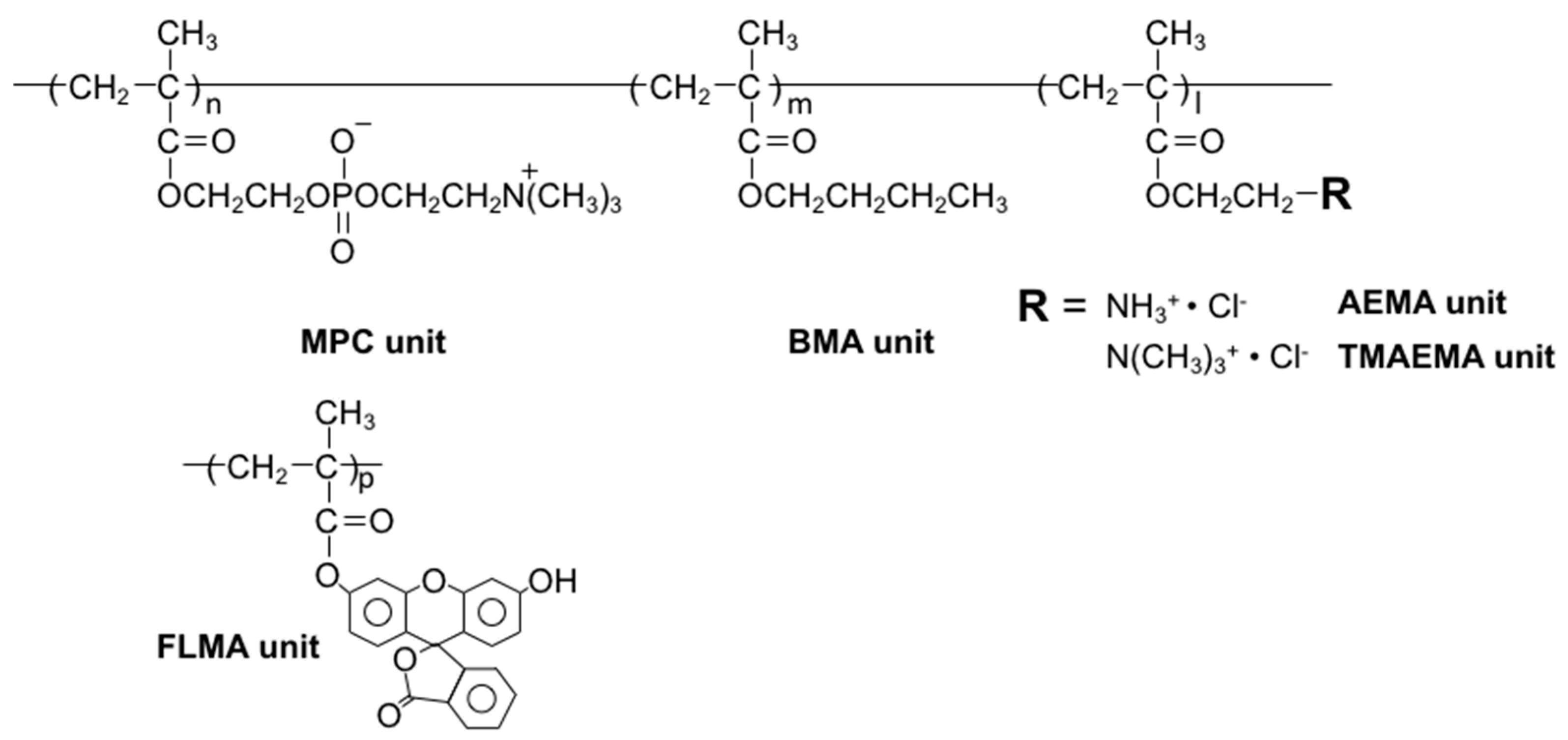




| Polymers | Composition (MPC/BMA/AEMA/TMAEMA) | Initiator | Solvent | [Monomer] (M) | [Initiator] (mM) | Time (h) | Molecular Weight | |||
|---|---|---|---|---|---|---|---|---|---|---|
| In Feed | In Polymer | Mw | Mn | Mw/Mn | ||||||
| PMB37 | 0.30/0.70/0/0 | 0.29/0.71/0/0 | PB-ND | EtOH | 1.6 | 55 | 4.0 | 1.0 × 104 | 7.4 × 103 | 1.4 |
| PMA37 | 0.30/0/0.70/0 | 0.29/0/0.71/0 | AIBN | MeOH/H2O (90/10) | 1.0 | 5.0 | 3.0 | 7.5 × 105 | 2.7 × 105 | 2.6 |
| PMBA154S | 0.10/0.50.0.40/0 | 0.18/0.38/0.44/0 | AIBN | MeOH/H2O (90/10) | 0.5 | 12.5 | 20 | 1.4 × 105 | 9.5 × 104 | 1.4 |
| PMBA154M | 0.10/0.50.0.40/0 | 0.12/0.53/0.35/0 | AIBN | MeOH/H2O (90/10) | 1.0 | 5.0 | 20 | 3.6 × 105 | 2.1 × 105 | 1.7 |
| PMBA154L | 0.10/0.50.0.40/0 | 0.18/0.36/0.46/0 | AIBN | MeOH/H2O (90/10) | 2.0 | 2.0 | 2.0 | 1.5 × 106 | 7.2 × 105 | 2.2 |
| PMBA127 | 0.10/0.20/0.70/0 | 0.06/0.21/0.73/0 | AIBN | MeOH/H2O (90/10) | 1.0 | 5.0 | 20 | 3.2 × 105 | 2.3 × 105 | 1.4 |
| PMT37 | 0.30/0/0/0.70 | 0.37/0/0/0.63 | AIBN | EtOH | 1.0 | 5.0 | 3.0 | 3.8 × 105 | 2.2 × 105 | 1.7 |
| PMBT154 | 0.10/0.50/0/0.40 | 0.12/0.55/0/0.33 | AIBN | EtOH | 1.0 | 5.0 | 6.0 | 4.1 × 105 | 2.5 × 105 | 1.6 |
| PMBT127 | 0.10/0.20/0/0.70 | 0.15/0.19/0/0.66 | AIBN | EtOH | 1.0 | 5.0 | 6.0 | 4.6 × 105 | 2.3 × 105 | 2.0 |
| PAEMA | 0/0/1.0/0 | 0/0/1.0/0 | AIBN | MeOH/H2O (90/10) | 1.0 | 5.0 | 3.0 | 3.3 × 105 | 2.3 × 105 | 1.4 |
| PTMAEMA | 0/0/0/1.0 | 0/0/0/1.0 | AIBN | EtOH | 1.0 | 5.0 | 3.0 | 6.5 × 105 | 3.5 × 105 | 1.9 |
| f-PMB37 | 0.30/0/70/0/0 0.1 ppm of FLMA | 0.27/0.73/0/0 + FLMA | PB-ND | EtOH | 1.0 | 55 | 18 | 1.1 × 104 | 5.7 × 103 | 2.0 |
| f-PMA37 | 0.30/0/0.70/0 0.1 ppm of FLMA | 0.28/0/0.72/0 + FLMA | AIBN | MeOH/H2O (90/10) | 1.0 | 5.0 | 3.0 | 4.4 × 105 | 2.8 × 105 | 1.6 |
| f-PMA154 | 0.10/0.50/0.40/0/ 1.0 ppm of FLMA | 0.18/0.36/0.46/0 + FLMA | AIBN | MeOH/H2O (90/10) | 1.0 | 5.0 | 20 | 3.2 × 105 | 2.0 × 105 | 1.6 |
| Polymers | Volume-Means Aggregate Size (nm)(PDI) | ζ-Potential (mV) | |
|---|---|---|---|
| Polymer | SiRNA/Polymer Complex | SiRNA/Polymer Complex | |
| PMB37 | 4.5 ± 0.2(0.31) | 4.2 ± 0.2(0.26) | −0.5 ± 0.1 |
| PMA37 | 520 ± 52(0.74) | 93 ± 26(0.55) | 38 ± 5 |
| PMBA154M | 61 ± 21(0.47) | 24 ± 6(0.31) | 25 ± 6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishihara, K.; Hachiya, S.; Inoue, Y.; Fukazawa, K.; Konno, T. Water-Soluble and Cytocompatible Phospholipid Polymers for Molecular Complexation to Enhance Biomolecule Transportation to Cells In Vitro. Polymers 2020, 12, 1762. https://doi.org/10.3390/polym12081762
Ishihara K, Hachiya S, Inoue Y, Fukazawa K, Konno T. Water-Soluble and Cytocompatible Phospholipid Polymers for Molecular Complexation to Enhance Biomolecule Transportation to Cells In Vitro. Polymers. 2020; 12(8):1762. https://doi.org/10.3390/polym12081762
Chicago/Turabian StyleIshihara, Kazuhiko, Shohei Hachiya, Yuuki Inoue, Kyoko Fukazawa, and Tomohiro Konno. 2020. "Water-Soluble and Cytocompatible Phospholipid Polymers for Molecular Complexation to Enhance Biomolecule Transportation to Cells In Vitro" Polymers 12, no. 8: 1762. https://doi.org/10.3390/polym12081762
APA StyleIshihara, K., Hachiya, S., Inoue, Y., Fukazawa, K., & Konno, T. (2020). Water-Soluble and Cytocompatible Phospholipid Polymers for Molecular Complexation to Enhance Biomolecule Transportation to Cells In Vitro. Polymers, 12(8), 1762. https://doi.org/10.3390/polym12081762