In Situ Polymerization of Polypyrrole @ Aluminum Fumarate Metal–Organic Framework Hybrid Nanocomposites for the Application of Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
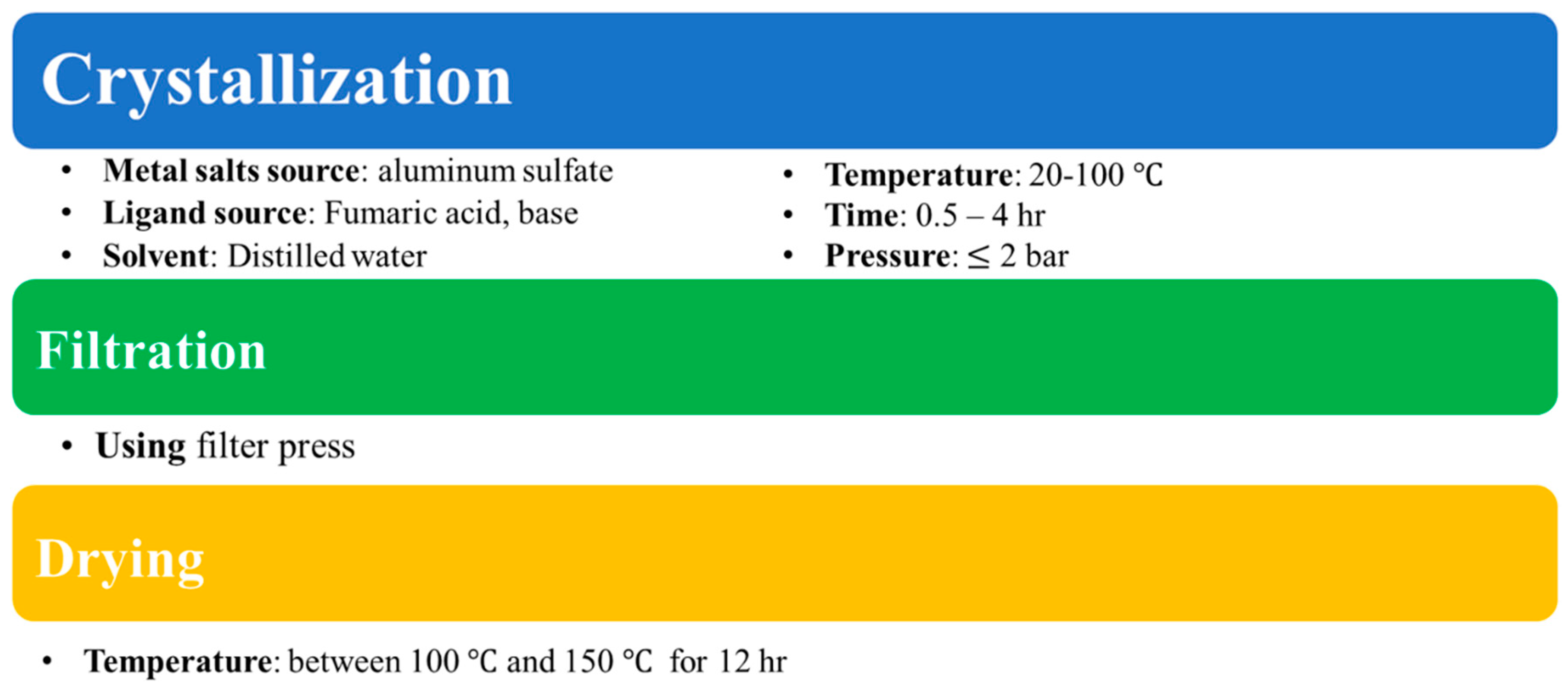
2.2. In Situ Polymerization of Pyrrole with Aluminum Fumarate MOF
2.3. Characterization
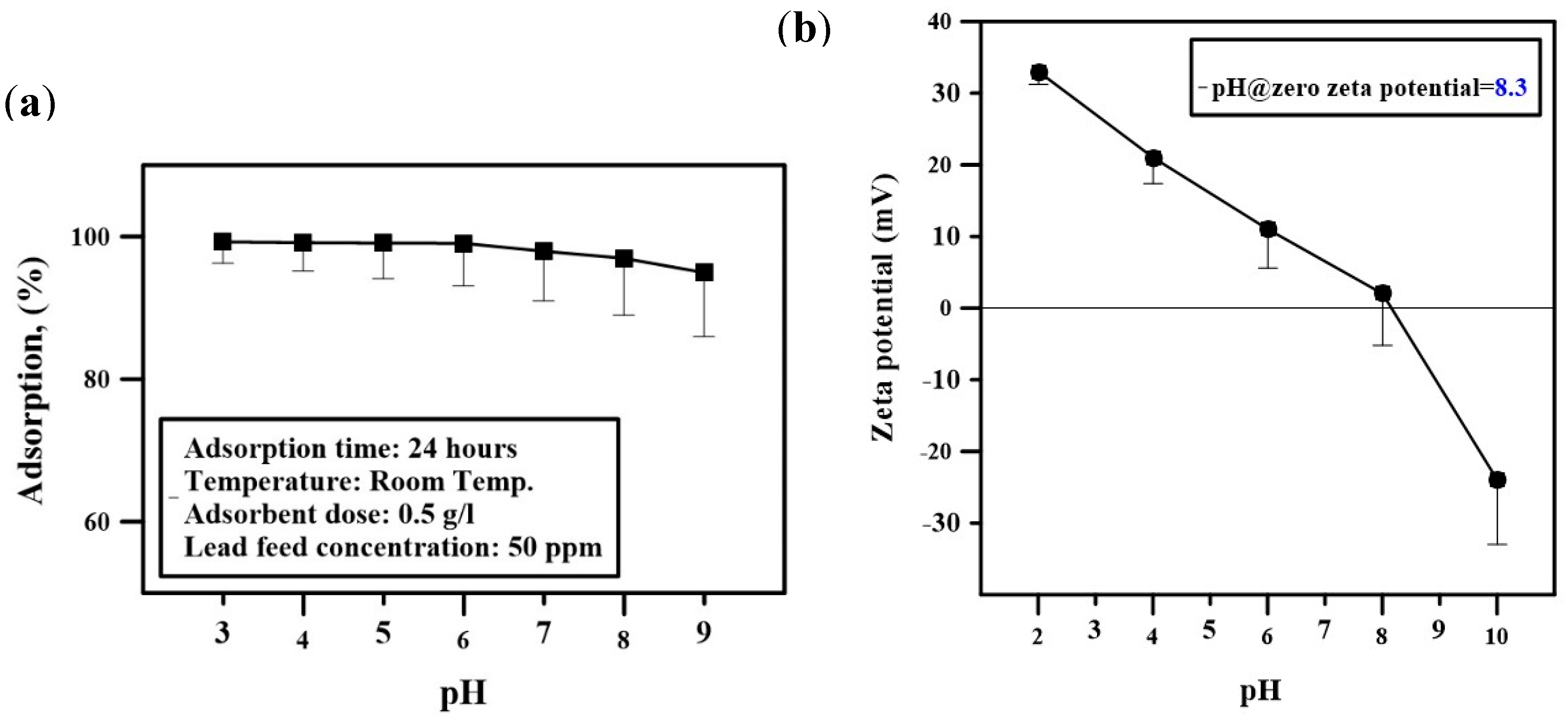
2.4. Determination of Optimum pH and Composite Dose
2.5. Adsorption Study
2.6. Lead Adsorption Isotherm
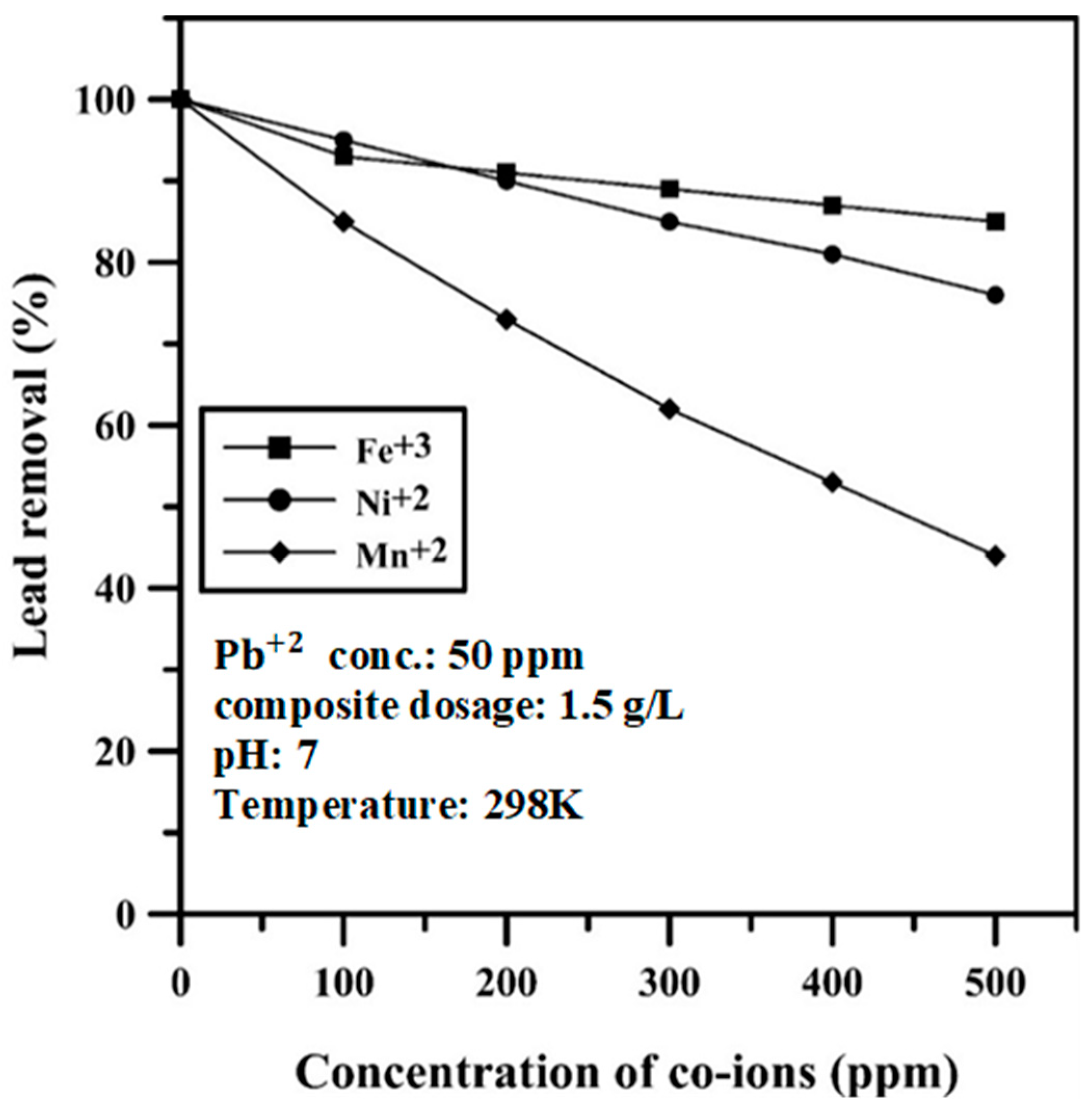
2.7. Effect of Coexisting Heavy Metals
2.8. Lead Adsorption of PPy, MOF, and Composite
2.9. Regeneration Study
3. Results and Discussion
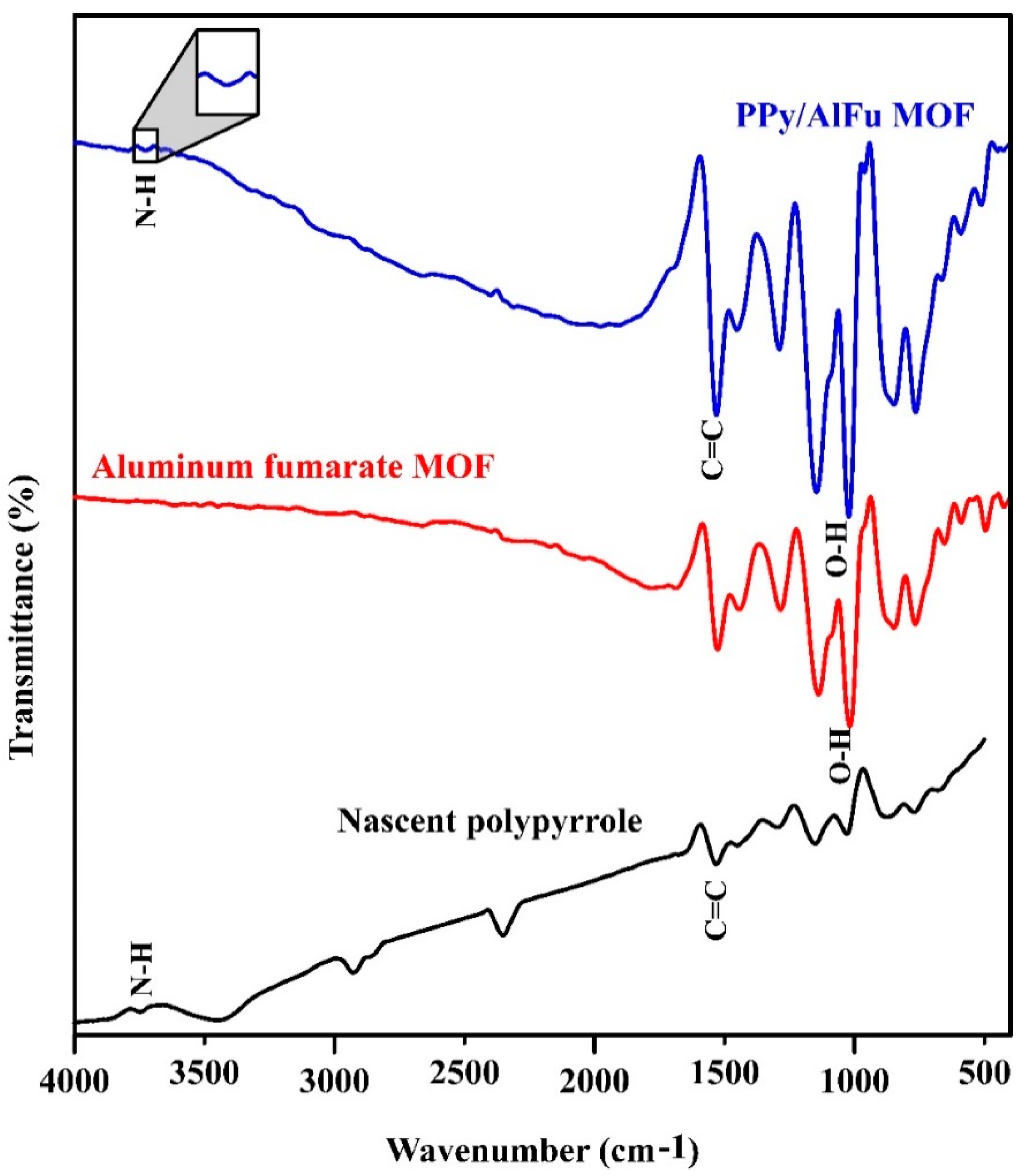
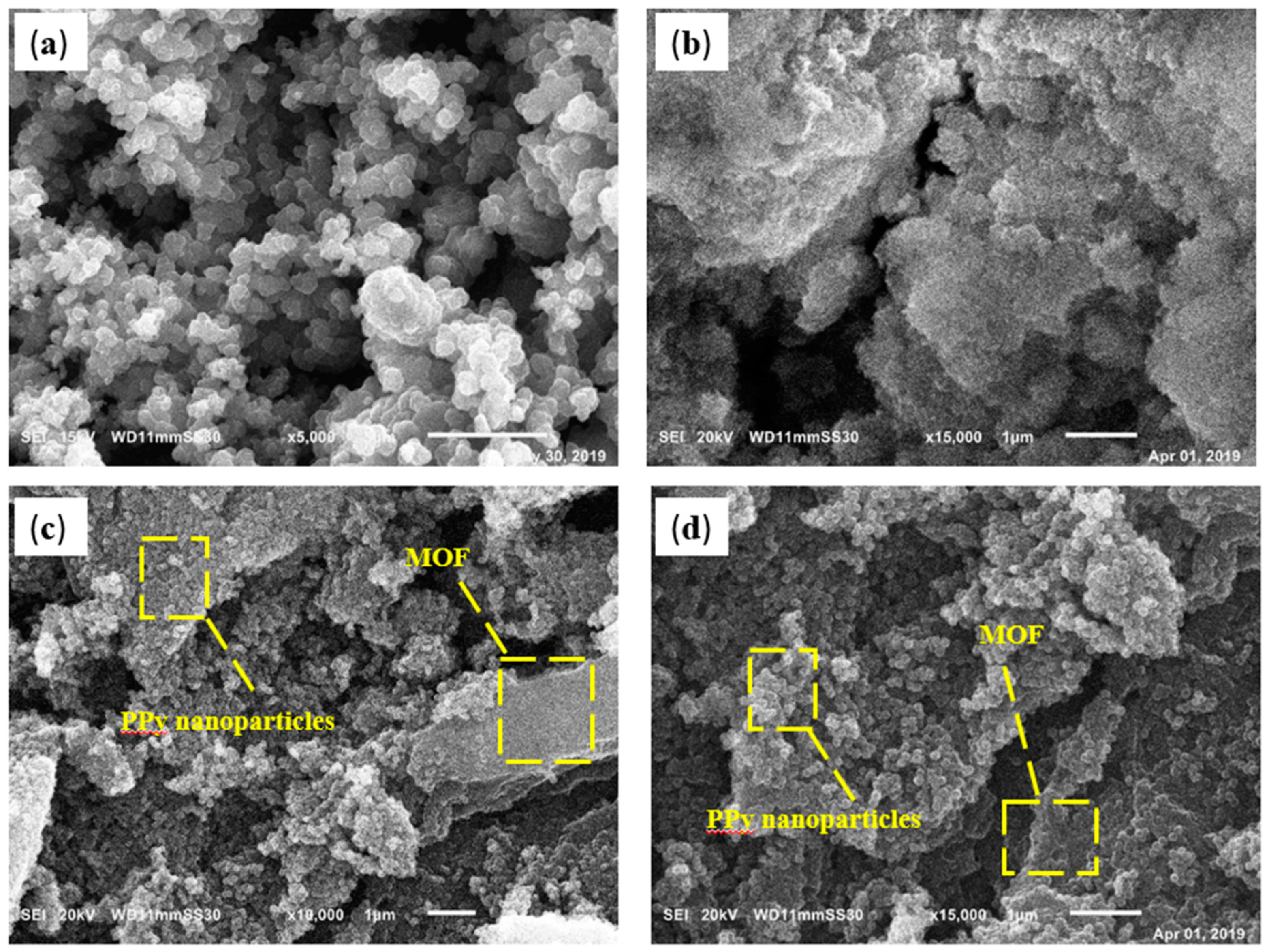
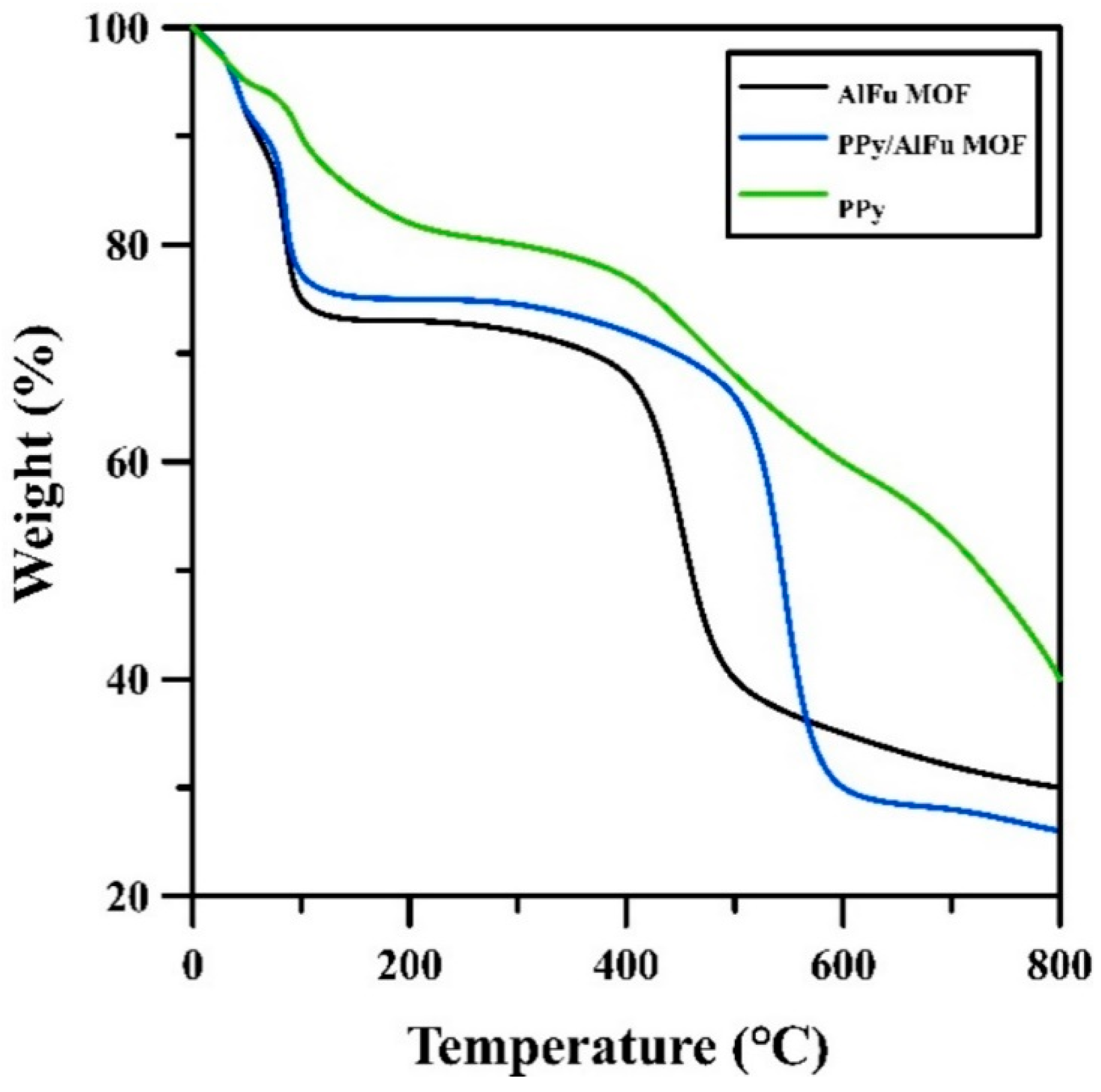
3.1. Characterization of Polypyrrole/Aluminum Fumarate MOF Composite
3.2. Adsorption Study
3.3. Adsorption Isotherms
3.4. Effect of Coexisting Heavy Metals
3.5. Lead Adsorption of PPy, MOF, and Composite
3.6. Desorption Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farha, O.K.; Yazaydın, A.Ö.; Eryazici, I.; Malliakas, C.D.; Hauser, B.; Kanatzidis, M.G.; Nguyen, S.T.; Snurr, R.Q.; Hupp, J.T. De novo synthesis of a metal-organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2010, 2, 944–948. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Kanga, J.R.R.; Caddy, J.S. Towards conducting metal-organic frameworks. Aust. J. Chem. 2011, 64, 718–722. [Google Scholar] [CrossRef]
- Bratby, J. Coagulation and flocculation in water and wastewater treatment. Water 2006, 21, 14–17. [Google Scholar] [CrossRef]
- Knappe, D.; Rossner, A.; Snyder, S.; Strickland, C. Alternative Adsorbents for the Removal of Polar Organic Contaminants. Water Intell. Online 2007, 1, 43–45. [Google Scholar]
- Yang, F.; Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C. Metal-Organic Frameworks Supported on Nanofiber for Desalination by Direct Contact Membrane Distillation. ACS Appl. Mater. Interfaces 2018, 10, 11251–11260. [Google Scholar] [CrossRef] [PubMed]
- Qadir, N.U.; Said, S.A.M.; Bahaidarah, H.M. Structural stability of metal organic frameworks in aqueous media—Controlling factors and methods to improve hydrostability and hydrothermal cyclic stability. Microp. Mesoporous Mater. 2015, 21, 61–90. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Yuan, X.; Zhang, J.; Chew, J.W. Metal-organic framework membranes for wastewater treatment and water regeneration. Coord. Chem. Rev. 2020, 404, 213116. [Google Scholar] [CrossRef]
- Shanahan, J.; Kissel, D.S.; Sullivan, E. PANI@UiO-66 and PANI@UiO-66-NH2 Polymer-MOF Hybrid Composites as Tunable Semiconducting Materials. ACS Omega 2020, 5, 6395–6404. [Google Scholar] [CrossRef]
- Ashour, R.M.; Abdel-Magied, A.F.; Wu, Q.; Olsson, R.T.; Forsberg, K. Green Synthesis of Metal-Organic Framework Bacterial Cellulose Nanocomposites for Separation Applications. Polymers 2020, 12, 1104. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, T.; Anshup. Noble metal nanoparticles for water purification: A critical review. Thin Solid Films 2009, 517, 6441–6478. [Google Scholar] [CrossRef]
- Sieve, Z.M. Zeolite—Structure and Properties. Theguardian 2013, 2–4. [Google Scholar]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Xue, M.; Zhu, G. Metal-organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef] [PubMed]
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal–organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal-organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef]
- Jun, B.M.; Heo, J.; Taheri-Qazvini, N.; Park, C.M.; Yoon, Y. Adsorption of selected dyes on Ti3C2Tx MXene and Al-based metal-organic framework. Ceram. Int. 2020, 46, 2960–2968. [Google Scholar] [CrossRef]
- Lian, X.; Yan, B. A lanthanide metal-organic framework (MOF-76) for adsorbing dyes and fluorescence detecting aromatic pollutants. RSC Adv. 2016, 6, 11570–11576. [Google Scholar] [CrossRef]
- Lee, T.B.; Kim, D.; Jung, D.H.; Choi, S.B.; Yoon, J.H.; Kim, J.; Choi, K.; Choi, S.-H. Understanding the mechanism of hydrogen adsorption into metal organic frameworks. Catal. Today 2007, 120, 330–335. [Google Scholar] [CrossRef]
- Jun, B.M.; Hwang, H.S.; Heo, J.; Han, J.; Jang, M.; Sohn, J.; Park, C.M.; Yoon, Y. Removal of selected endocrine-disrupting compounds using Al-based metal organic framework: Performance and mechanism of competitive adsorption. J. Ind. Eng. Chem. 2019, 79, 345–352. [Google Scholar] [CrossRef]
- Gaab, M.; Trukhan, N.; Maurer, S.; Gummaraju, R.; Müller, U. The progression of Al-based metal-organic frameworks—From academic research to industrial production and applications. Microp. Mesoporous Mater. 2012, 157, 131–136. [Google Scholar] [CrossRef]
- Shi, P.F.; Wang, L.X.; Yu, Z.P. A novel, interpenetrating metal-organic framework: Synthesis, structure, and luminescence detection of nitrobenzene. Aust. J. Chem. 2017, 70, 792–796. [Google Scholar] [CrossRef][Green Version]
- Derakhshani, M.; Hashamzadeh, A.; Amini, M.M. High surface area mesoporous alumina nanosheets and nanorolls from an aluminum based metal organic framework. Ceram. Int. 2016, 42, 17742–17748. [Google Scholar] [CrossRef]
- Inamdar, H.K.; Reddy, N.; Khader, S.A.; Naresh, P.; Prasad, M.V.N.A.; Prasad, G.S.D. Polypyrrole/Cr2O3 hybrid nanocomposites (NCs) prepared for their structural, morphological, optical and conductivity studies. Compos. Commun. 2019, 14, 21–28. [Google Scholar] [CrossRef]
- Kayal, S.; Chakraborty, A.; Teo, H.W.B. Green synthesis and characterization of aluminium fumarate metal-organic framework for heat transformation applications. Mater. Lett. 2018, 221, 165–167. [Google Scholar] [CrossRef]
- Zayan, S.E.; El-Shazly, A.H.; El-Kady, M.F. Assessment of polypyrrole nanoparticles synthesized in presence and absence of surfactant for heavy metals decontamination. AIP Conf. Proc. 2019, 2190, 20–25. [Google Scholar]
- Karmakar, S.; Dechnik, J.; Janiak, C.; De, S. Aluminium fumarate metal-organic framework: A super adsorbent for fluoride from water. J. Hazard. Mater. 2016, 303, 10–20. [Google Scholar] [CrossRef]
- D’Ischia, M.; Napolitano, A.; Pezzella, A. Pyrroles and their benzo derivatives: Applications. In Comprehensive Heterocyclic Chemistry III; Elsevier: Amsterdam, The Netherlands, 2008; Volume 3, pp. 353–388. [Google Scholar]
- Arora, K.; Chaubey, A.; Singhal, R.; Singh, R.P.; Pandey, M.; Samanta, S.; Malhotra, B.; Chand, S. Application of electrochemically prepared polypyrrole-polyvinyl sulphonate films to DNA biosensor. Biosens. Bioelectron. 2006, 21, 1777–1783. [Google Scholar] [CrossRef]
- Cheng, D.; Zhao, L.; Li, N.; Smith, S.J.; Wu, N.; Zhang, J.; Ng, D.; Wu, C.; Martinez, M.R.; Batten, M.P.; et al. Aluminum fumarate MOF/PVDF hollow fiber membrane for enhancement of water flux and thermal efficiency in direct contact membrane distillation. J. Memb. Sci. 2019, 588, 117204. [Google Scholar] [CrossRef]
- Luo, M.; Li, M.; Li, Y.; Chang, K.; Liu, K.; Liu, Q.; Wang, Y.; Lu, Z.; Liu, X.; Wang, D. In-situ polymerization of PPy/cellulose composite sponge with high elasticity and conductivity for the application of pressure sensor. Compos. Commun. 2017, 6, 68–72. [Google Scholar] [CrossRef]
- Jeon, S.S.; Lee, Y.W.; Im, S.S. Enhanced thermal stability of polypyrrole hexagonal microplates fabricated by organic crystal surface-induced polymerization. Polym. Degrad. Stab. 2011, 96, 778–783. [Google Scholar] [CrossRef]
- Shokry, H.; Elkady, M.; Salama, E. Eco-friendly magnetic activated carbon nano-hybrid for facile oil spills separation. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-S.; Malarvizhi, R.; Sulochana, N. Equilibrium isotherm studies of methylene blue adsorption onto activated carbon prepared from Delonix regia pods. J. Environ. Prot. Sci. 2009, 3, 111–116. [Google Scholar]
- Bulut, Y.; Aydin, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Bhattacharya, K.G.; Sharma, A. Kinetics and thermodynamics of Methylene Blue adsorption on Neem (Azadirachta indica) leaf powder. Dye. Pigment. 2005, 65, 51–59. [Google Scholar] [CrossRef]







| Langmuir Isotherm | Freundlich Isotherm | |||
|---|---|---|---|---|
| Temperature K | K | Qm | Kf | n |
| 298 | 0.015 | 600 | 43 | 2.63 |
| 308 | 0.012 | 527 | 38 | 2.7 |
| Adsorbent Material | Qe (mg/g) | Reference |
|---|---|---|
| PPy/MOF composite | 600 | Present study |
| Aluminum fumarate MOF | 431 | [26] |
| NMAC fabricated using water hyacinth roots | 30.20 | [32] |
| AC fabricated using Delonix regia pods | 24 | [33] |
| AC fabricated using wheat shells | 16.56 | [34] |
| AC fabricated from coconut husk | 5.87 | [35] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zayan, S.; Elshazly, A.; Elkady, M. In Situ Polymerization of Polypyrrole @ Aluminum Fumarate Metal–Organic Framework Hybrid Nanocomposites for the Application of Wastewater Treatment. Polymers 2020, 12, 1764. https://doi.org/10.3390/polym12081764
Zayan S, Elshazly A, Elkady M. In Situ Polymerization of Polypyrrole @ Aluminum Fumarate Metal–Organic Framework Hybrid Nanocomposites for the Application of Wastewater Treatment. Polymers. 2020; 12(8):1764. https://doi.org/10.3390/polym12081764
Chicago/Turabian StyleZayan, Sarah, Ahmed Elshazly, and Marwa Elkady. 2020. "In Situ Polymerization of Polypyrrole @ Aluminum Fumarate Metal–Organic Framework Hybrid Nanocomposites for the Application of Wastewater Treatment" Polymers 12, no. 8: 1764. https://doi.org/10.3390/polym12081764
APA StyleZayan, S., Elshazly, A., & Elkady, M. (2020). In Situ Polymerization of Polypyrrole @ Aluminum Fumarate Metal–Organic Framework Hybrid Nanocomposites for the Application of Wastewater Treatment. Polymers, 12(8), 1764. https://doi.org/10.3390/polym12081764








