Fluid–Structure Interaction Analysis of Perfusion Process of Vascularized Channels within Hydrogel Matrix Based on Three-Dimensional Printing
Abstract
:1. Introduction
2. Materials and Methods
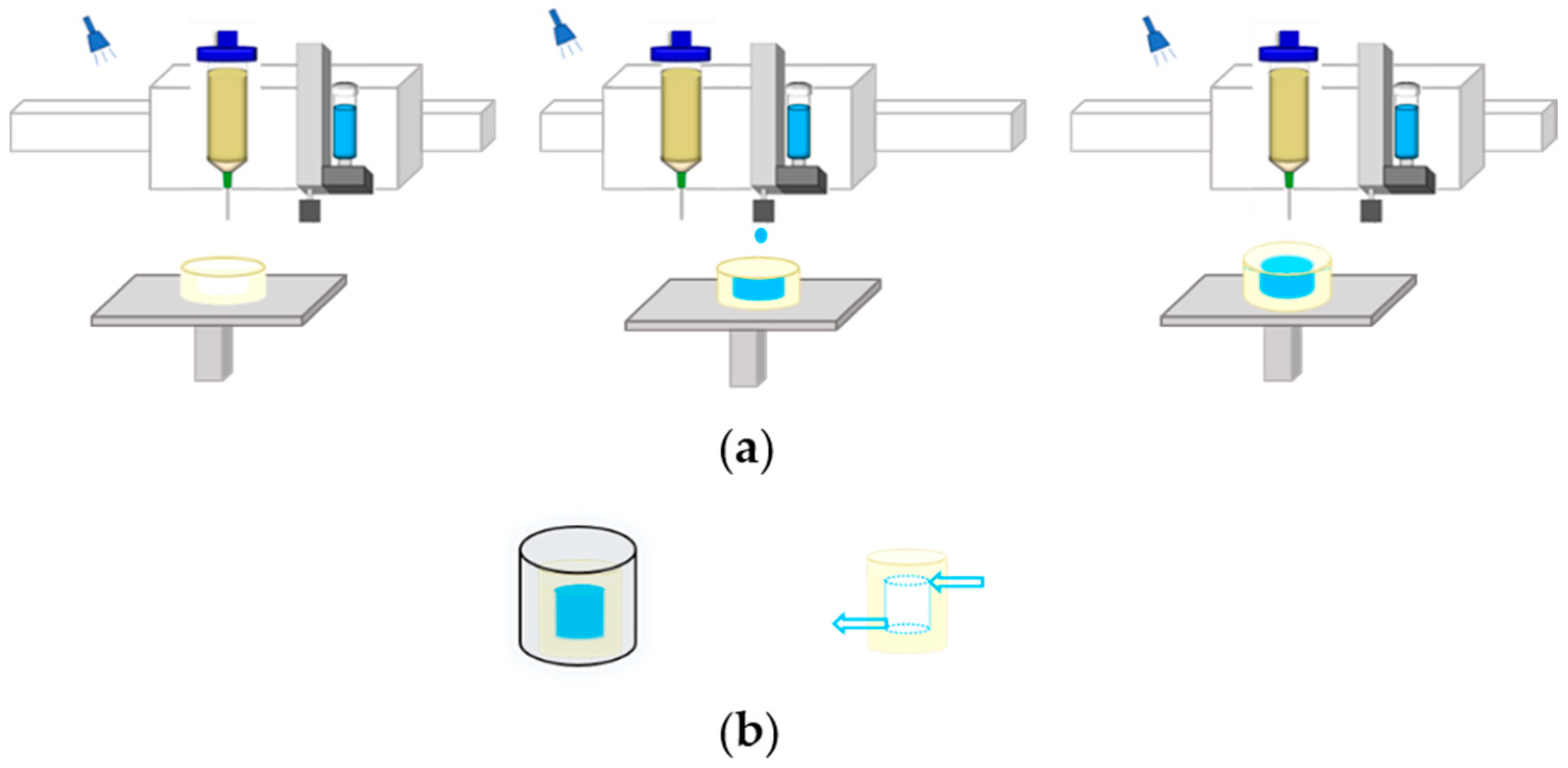
2.1. Model
2.2. Fluid Model
2.3. Solid Model
3. Results and Discussion
3.1. The Effect of Perfusion Pressure in the Perfusion Process
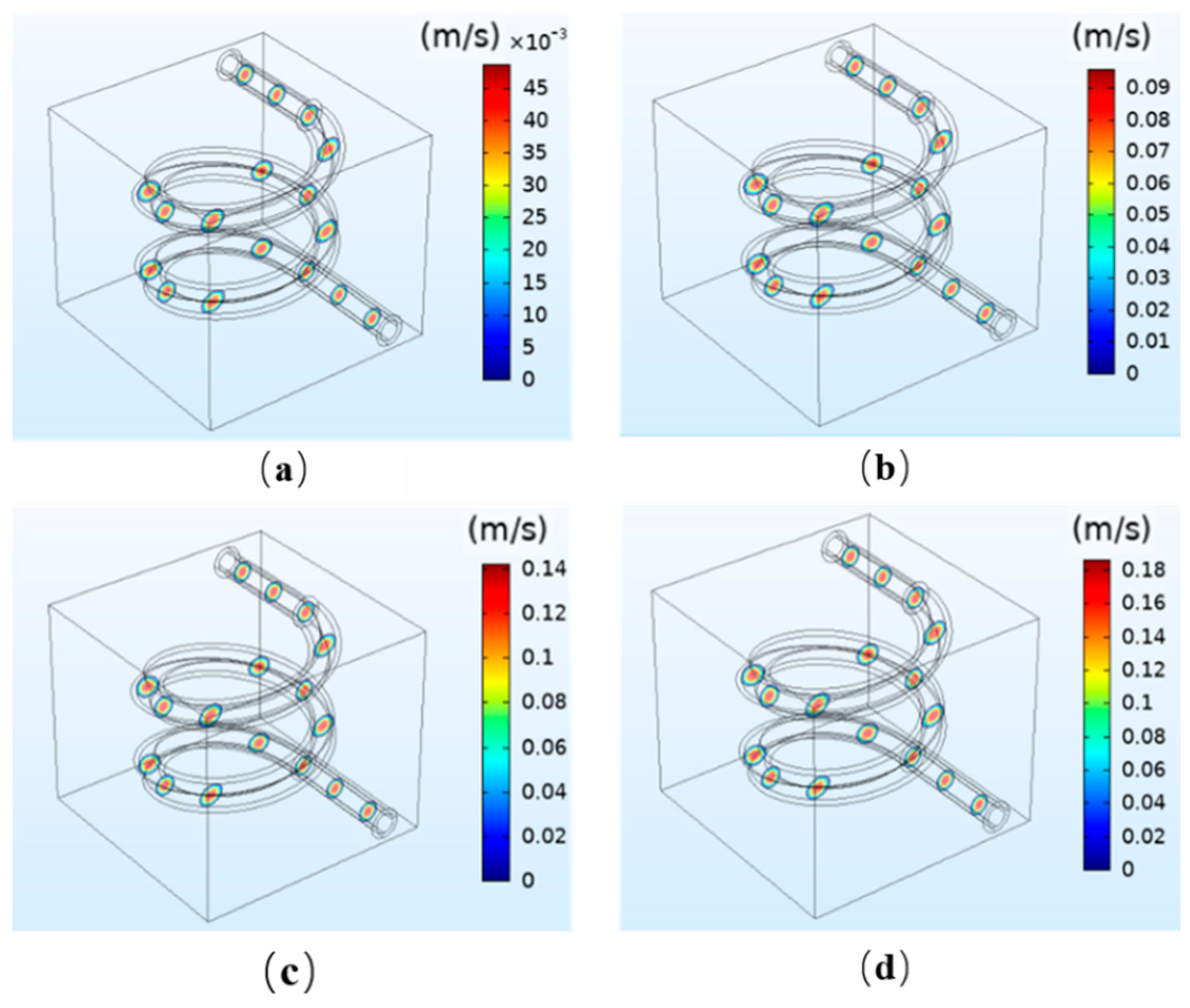
3.1.1. The Simulation Results of Fluid Flow Velocity
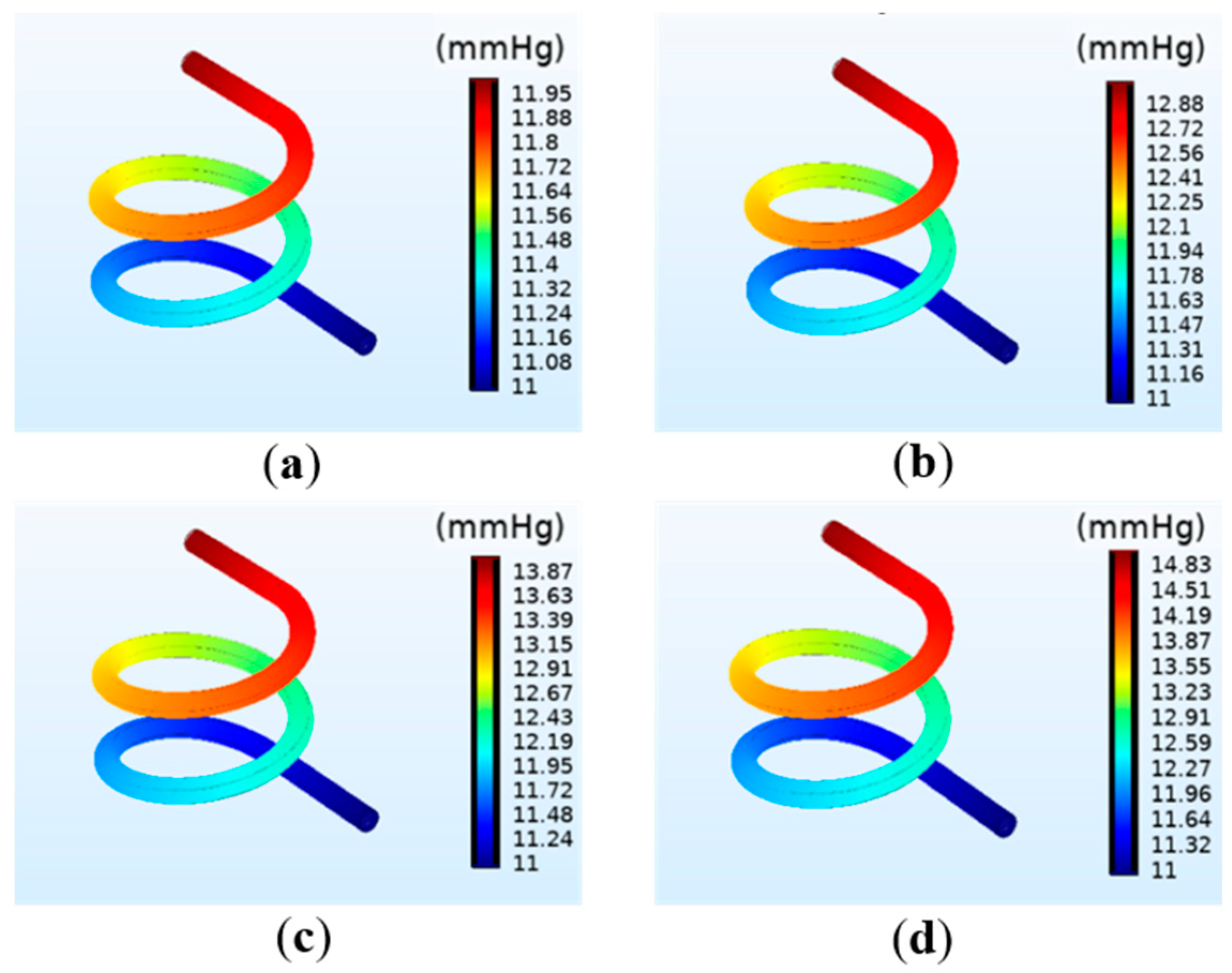
3.1.2. The Simulation Results of the Pressure on the Vessel
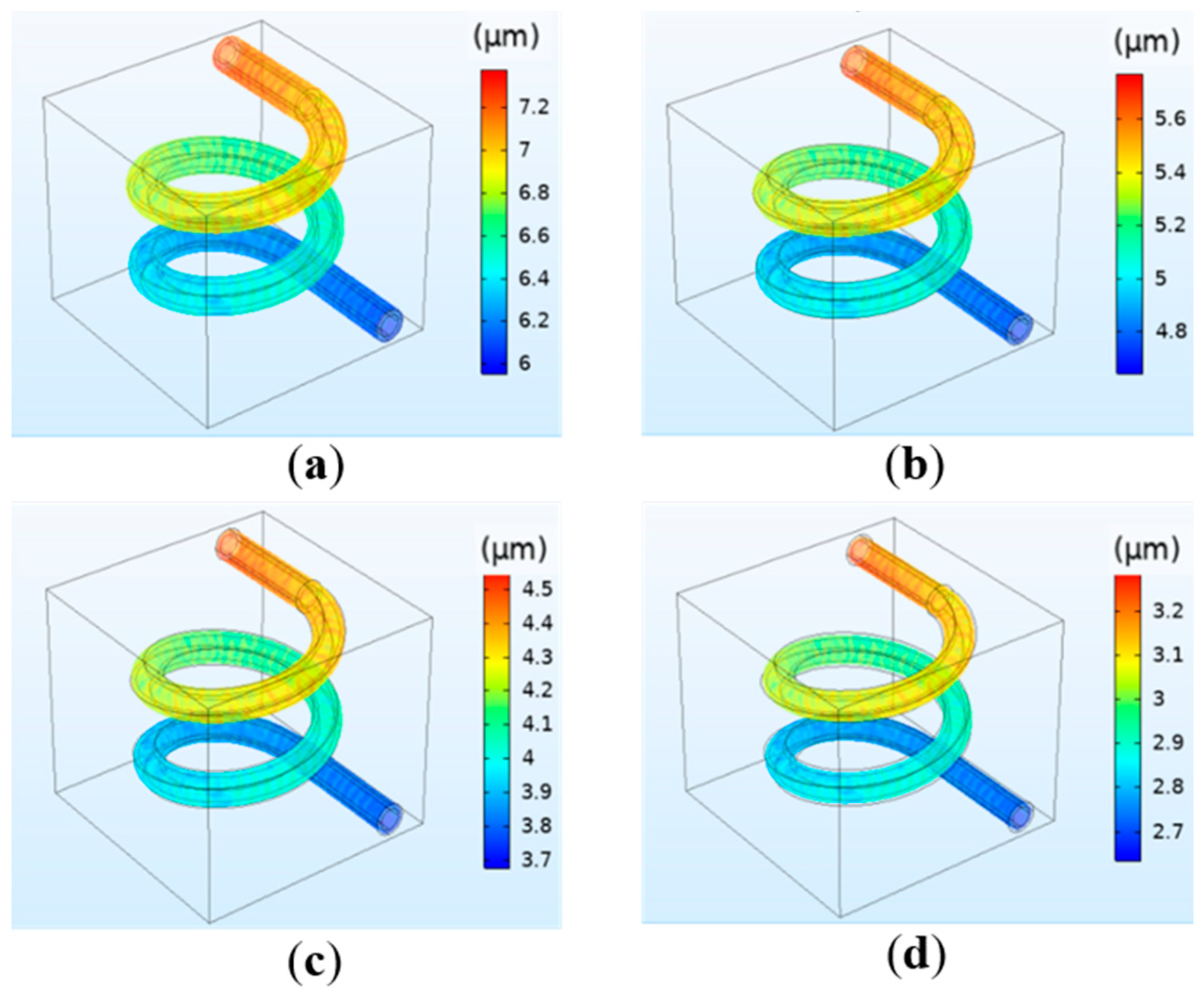
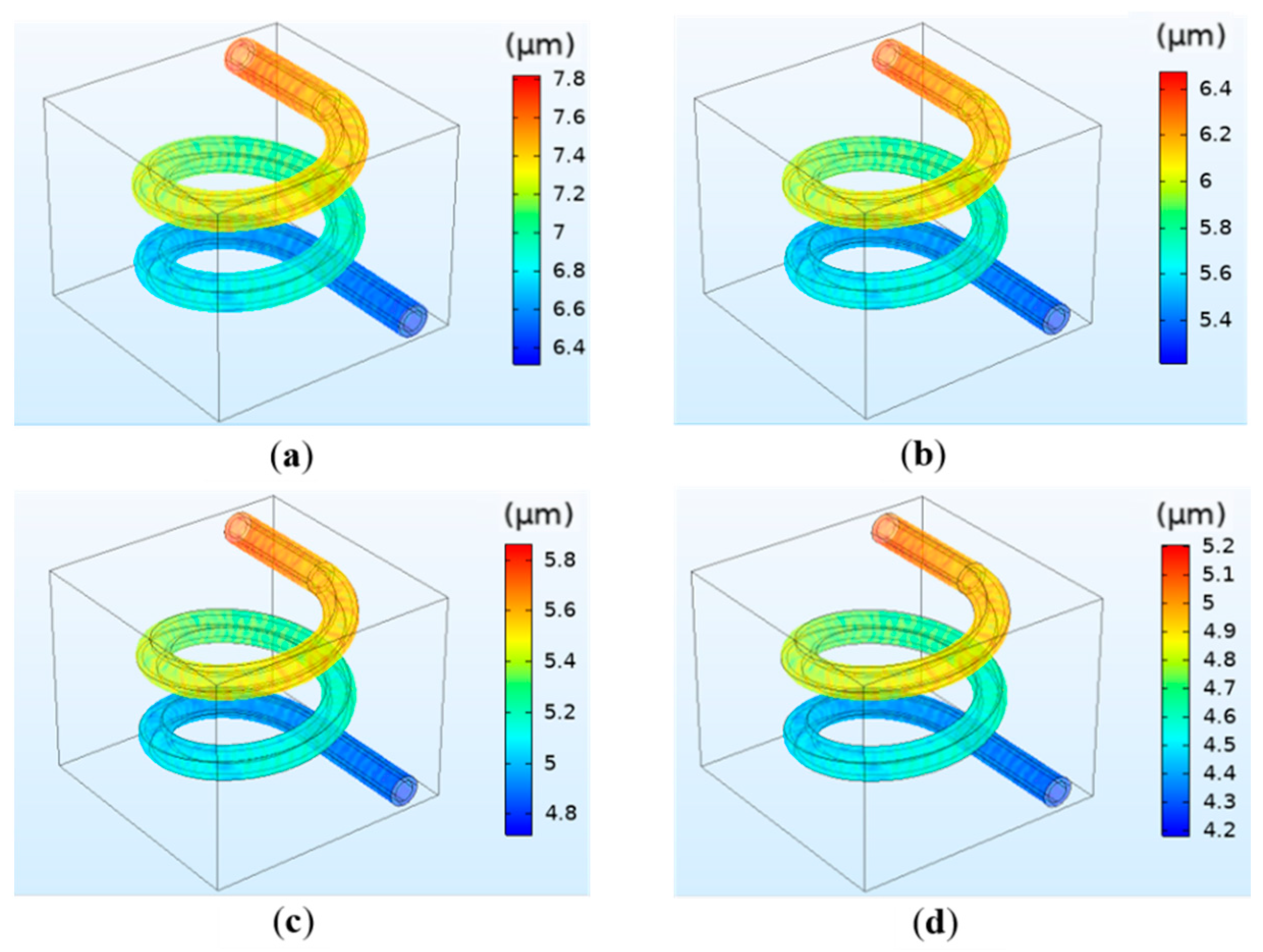
3.1.3. The Simulation Results of the Deformation of the Vessel
3.2. The Effect of Hydrogel Concentration in the Perfusion Process
3.3. The Effect of Crosslinking Density in the Perfusion Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wilson, W.C.; Boland, T. Cell and organ printing 1: Protein and cell printers. Anat. Rec. Adv. Integr. Anat. Evol. Boil. 2003, 272, 491–496. [Google Scholar] [CrossRef]
- Liaw, C.-Y.; Guvendiren, M. Current and emerging applications of 3D printing in medicine. Biofabrication 2017, 9, 024102. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Langer, R. A decade of progress in tissue engineering. Nat. Protoc. 2016, 11, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Debasitis, J.C.; Lee, V.K.; Lee, J.-H.; Fischer, K.; Edminster, K.; Park, J.-K.; Yoo, S.-S. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication. Biomaterials 2009, 30, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, L.; Wang, J.; Xu, Y.; Zhang, W.; Khang, G.; Wang, X. In vitrovascularization of a combined system based on a 3D printing technique. J. Tissue Eng. Regen. Med. 2014, 10, 833–842. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Zhang, R. Rapid prototyping as a tool for manufacturing bioartificial livers. Trends Biotechnol. 2007, 25, 505–513. [Google Scholar] [CrossRef]
- Nomi, M.; Atala, A.J.; De Coppi, P.; Soker, S. Principals of neovascularization for tissue engineering. Mol. Asp. Med. 2002, 23, 463–483. [Google Scholar] [CrossRef]
- Sarker, M.; Naghieh, S.; McInnes, A.; Schreyer, D.J.; Chen, X. Strategic Design and Fabrication of Nerve Guidance Conduits for Peripheral Nerve Regeneration. Biotechnol. J. 2018, 13, 1700635. [Google Scholar] [CrossRef]
- He, K.; Wang, X. Rapid prototyping of tubular polyurethane and cell/hydrogel constructs. J. Bioact. Compat. Polym. 2011, 26, 363–374. [Google Scholar] [CrossRef]
- Griffith, L.G.; Wu, B.; Cima, M.J.; Powers, M.J.; Chaignaud, B.; Vacanti, J.P. In Vitro Organogenesis of Liver Tissuea. Ann. N. Y. Acad. Sci. 2006, 831, 382–397. [Google Scholar] [CrossRef]
- Zein, N.N.; Hanouneh, I.A.; Bishop, P.D.; Samaan, M.; Eghtesad, B.; Quintini, C.; Miller, C.; Yerian, L.; Klatte, R.S. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transplant. 2013, 19, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Grix, T.; Ruppelt, A.; Thomas, A.; Amler, A.-K.; Noichl, B.P.; Lauster, R.; Kloke, L. Bioprinting Perfusion-Enabled Liver Equivalents for Advanced Organ-on-a-Chip Applications. Genes 2018, 9, 176. [Google Scholar] [CrossRef] [Green Version]
- Pillay, V.; Tsai, T.-S.; Choonara, Y.E.; Du Toit, L.C.; Kumar, P.; Modi, G.; Naidoo, D.; Tomar, L.K.; Tyagi, C.; Ndesendo, V.M.K. A review of integrating electroactive polymers as responsive systems for specialized drug delivery applications. J. Biomed. Mater. Res. Part A 2013, 102, 2039–2054. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tang, H.; Feng, C.; Shi, J.; Yang, J. The Research on Multi-Material 3D Vascularized Network Integrated Printing Technology. Micromachines 2020, 11, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Vunjak-Novakovic, G.; Tandon, N.; Godier, A.; Maidhof, R.; Marsano, A.; Martens, T.P.; Radisic, M. Challenges in Cardiac Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 169–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minuth, W.W.; Denk, L.; Glashauser, A. A modular culture system for the generation of multiple specialized tissues. Biomaterials 2010, 31, 2945–2954. [Google Scholar] [CrossRef]
- Hossain, S.; Bergstrom, D.; Chen, X. Modelling and simulation of the chondrocyte cell growth, glucose consumption and lactate production within a porous tissue scaffold inside a perfusion bioreactor. Biotechnol. Rep. 2014, 5, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.A.; Fernandes, T.G.; Diogo, M.M.; Da Silva, C.L.; Cabral, J.M.S. Stem cell cultivation in bioreactors. Biotechnol. Adv. 2011, 29, 815–829. [Google Scholar] [CrossRef]
- Lee, C.-J.; Zhang, Y.; Takao, H.; Murayama, Y.; Qian, Y. The influence of elastic upstream artery length on fluid–structure interaction modeling: A comparative study using patient-specific cerebral aneurysm. Med. Eng. Phys. 2013, 35, 1377–1384. [Google Scholar] [CrossRef]
- Ho, H.; Mithraratne, K.; Hunter, P. Numerical Simulation of Blood Flow in an Anatomically-Accurate Cerebral Venous Tree. IEEE Trans. Med. Imaging 2012, 32, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Du, J.; Lin, T. Preparation and characterization of PEGDA/SA hydrogel. J. Funct Mater. 2018, 49, 8086–8092. [Google Scholar] [CrossRef]
- Jeon, J.H.; Jeong, O.C. Effect of shear stress magnitude on intracellular calcium expression in bone cells. Microelectron Eng 2012, 97, 329–332. [Google Scholar] [CrossRef]
- Lu, L.; Liu, X.; Dai, A.L.; Tong, Z. Difference in Concentration Dependence of Relaxation Critical Exponentnfor Alginate Solutions at Sol−Gel Transition Induced by Calcium Cations. Biomacromolecules 2005, 6, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zheng, D.; Yu, G.; Wang, H.; Yang, L.; Shan, J. Preparation of poly (vinyl alcohol)/calcium alginate hydrogel and the study on mechanical property. New Chem. Mater. 2015, 118–120. Available online: http://www.cqvip.com/QK/92152X/201506/665211871.html (accessed on 21 August 2020).
- Tse, J.R.; Engler, A.J. Preparation of Hydrogel Substrates with Tunable Mechanical Properties. Curr. Protoc. Cell Boil. 2010, 47, 10.16.1–10.16.16. [Google Scholar] [CrossRef]
- Cesaretti, M. A 96-well assay for uronic acid carbazole reaction. Carbohydr. Polym. 2003, 54, 59–61. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Liang, Q.; Li, H.; Peng, L.; Wu, M.; Zhao, X.; Cui, X.; Ruan, C.; Liu, W. Osteochondral Regeneration with 3D-Printed Biodegradable High-Strength Supramolecular Polymer Reinforced-Gelatin Hydrogel Scaffolds. Adv. Sci. 2019, 6, 1900867. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Shi, X.; Wu, D.; Khshen, F.K.; Deng, L.; Dong, A.; Wangb, W.; Zhang, J. Injectable, Biodegradable, Thermosensitive Nanoparticles-Aggregated Hydrogel with Tumor-Specific Targeting, Penetration, and Release for Efficient Postsurgical Prevention of Tumor Recurrence. ACS Appl. Mater. Interfaces 2019, 11, 19700–19711. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a Photo-Crosslinking, Biodegradable GelMA/PEGDA Hydrogel for Guided Bone Regeneration Materials. Materials 2018, 11, 1345. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Shi, J.; Yang, J.; Feng, C.; Tang, H. Fluid–Structure Interaction Analysis of Perfusion Process of Vascularized Channels within Hydrogel Matrix Based on Three-Dimensional Printing. Polymers 2020, 12, 1898. https://doi.org/10.3390/polym12091898
Yang S, Shi J, Yang J, Feng C, Tang H. Fluid–Structure Interaction Analysis of Perfusion Process of Vascularized Channels within Hydrogel Matrix Based on Three-Dimensional Printing. Polymers. 2020; 12(9):1898. https://doi.org/10.3390/polym12091898
Chicago/Turabian StyleYang, Shuai, Jianping Shi, Jiquan Yang, Chunmei Feng, and Hao Tang. 2020. "Fluid–Structure Interaction Analysis of Perfusion Process of Vascularized Channels within Hydrogel Matrix Based on Three-Dimensional Printing" Polymers 12, no. 9: 1898. https://doi.org/10.3390/polym12091898
APA StyleYang, S., Shi, J., Yang, J., Feng, C., & Tang, H. (2020). Fluid–Structure Interaction Analysis of Perfusion Process of Vascularized Channels within Hydrogel Matrix Based on Three-Dimensional Printing. Polymers, 12(9), 1898. https://doi.org/10.3390/polym12091898




