Physicochemical Studies on the Surface of Polyamide 6.6 Fabrics Functionalized by DBD Plasmas Operated at Atmospheric and Sub-Atmospheric Pressures
Abstract
1. Introduction
2. Materials and Methods
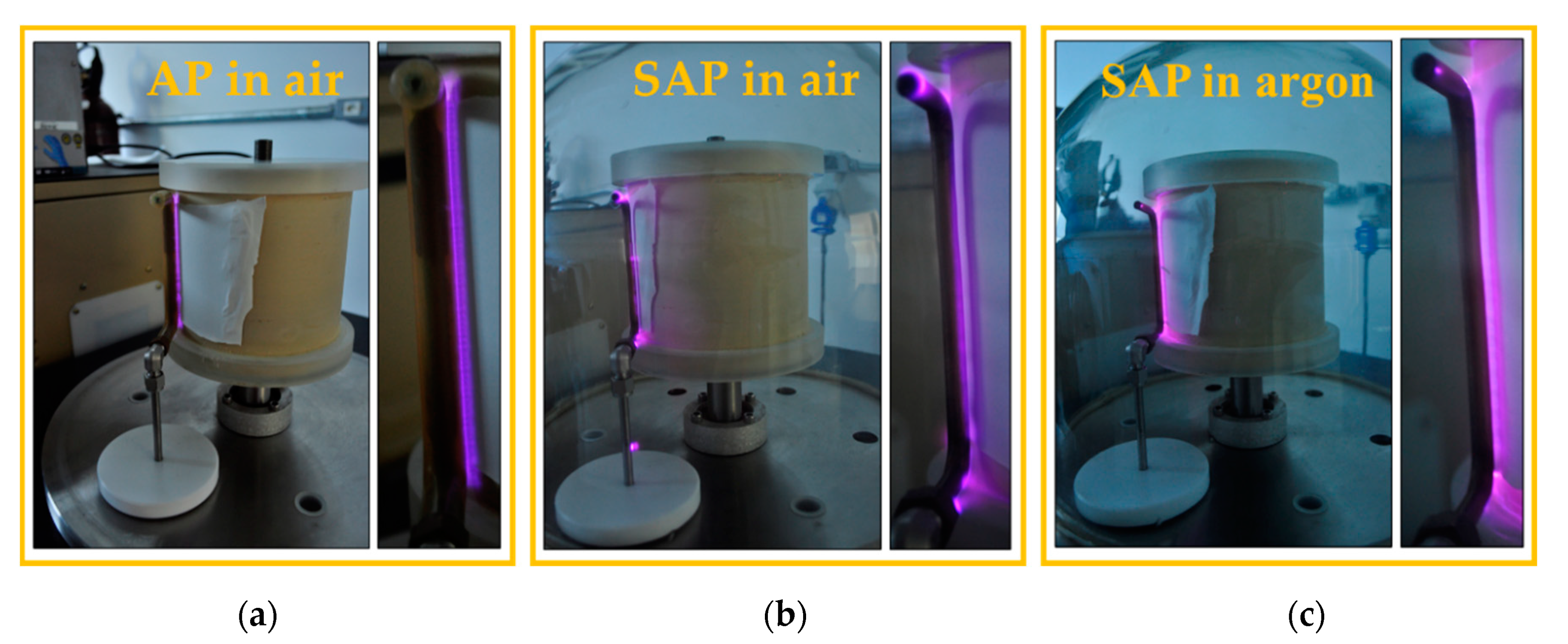
2.1. Polyamide Sample Preparation and Plasma Treatment
2.2. Polyamide Surface Characterizations
3. Results and Discussion
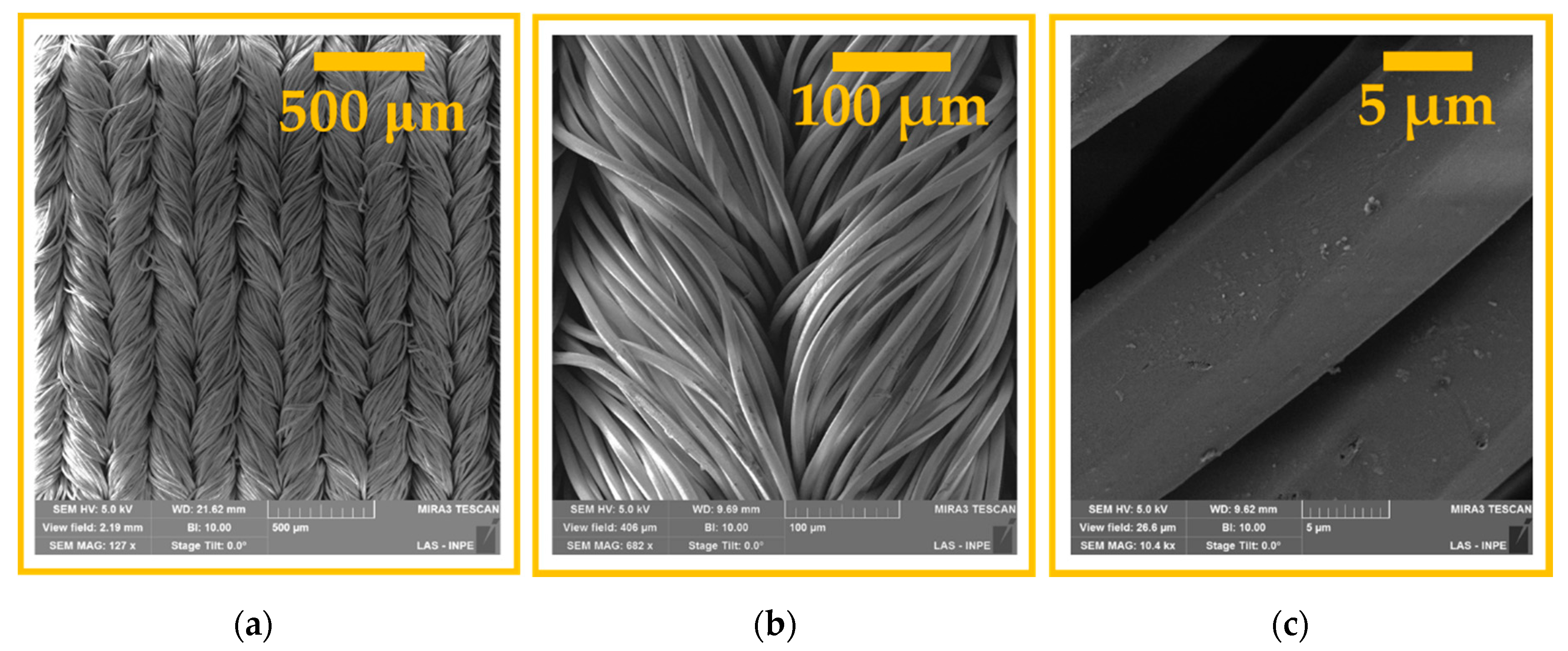
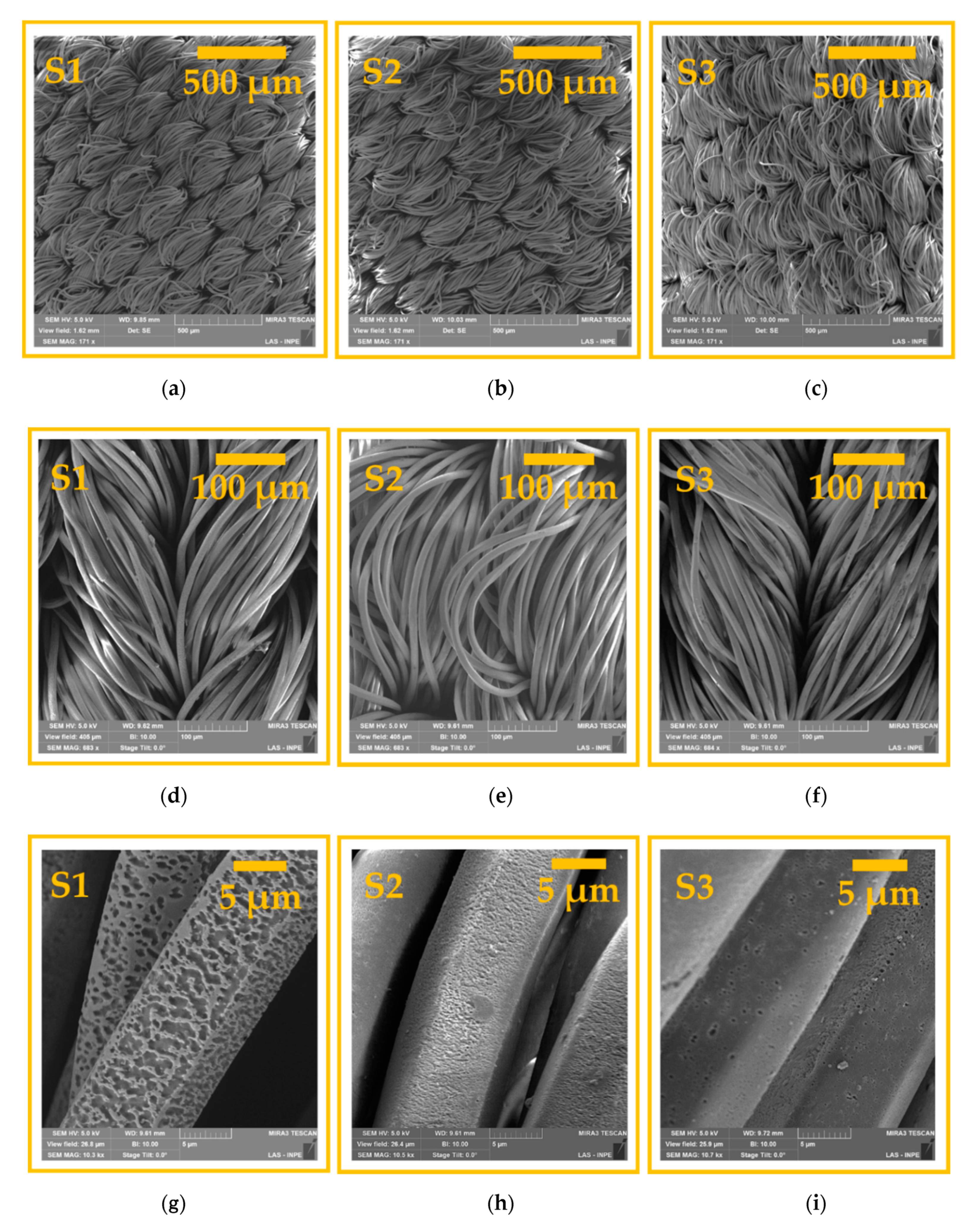
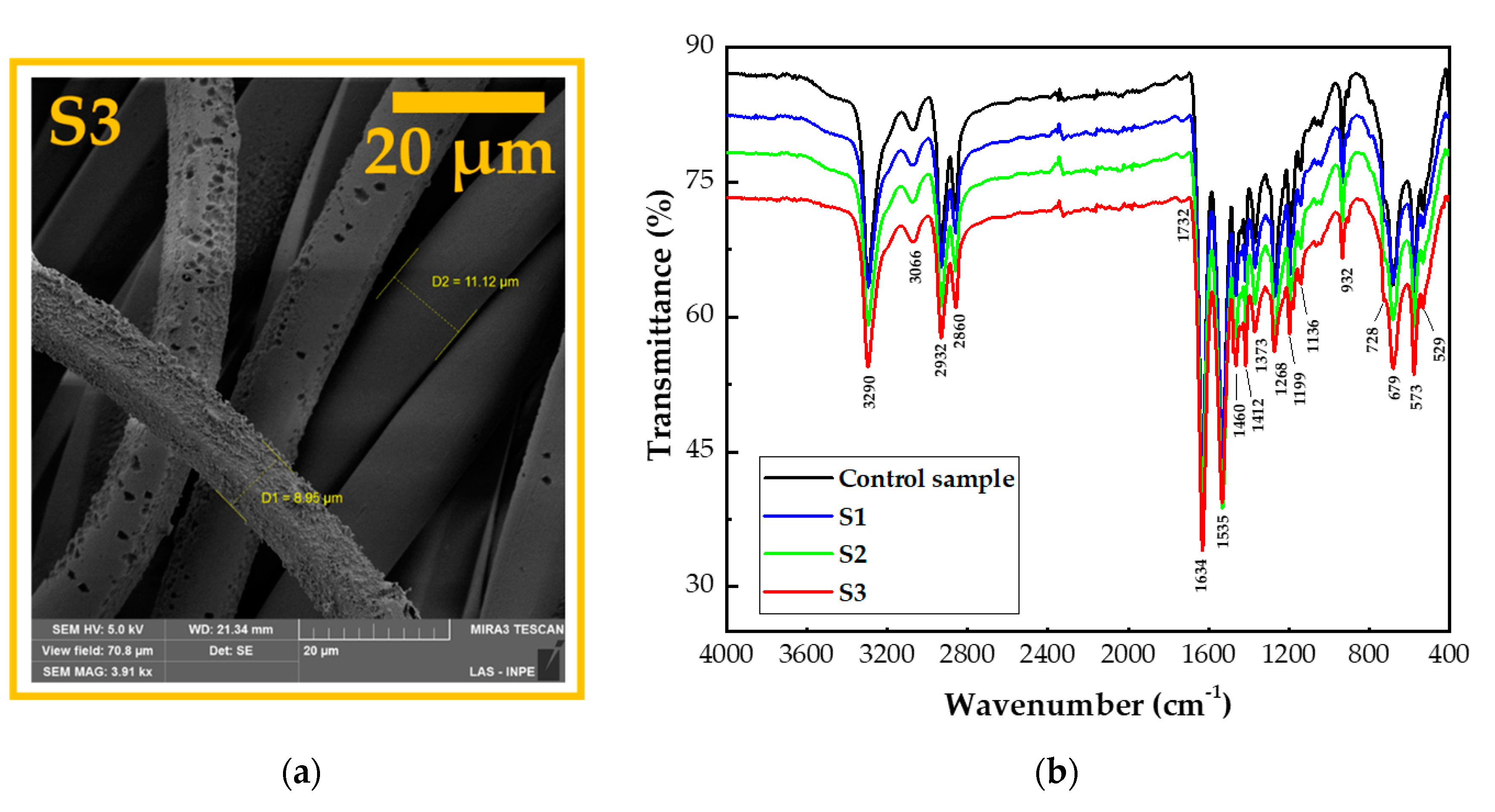
3.1. Surface Morphological Analysis and Chemical Analysis of the Inner Part of the Fibers
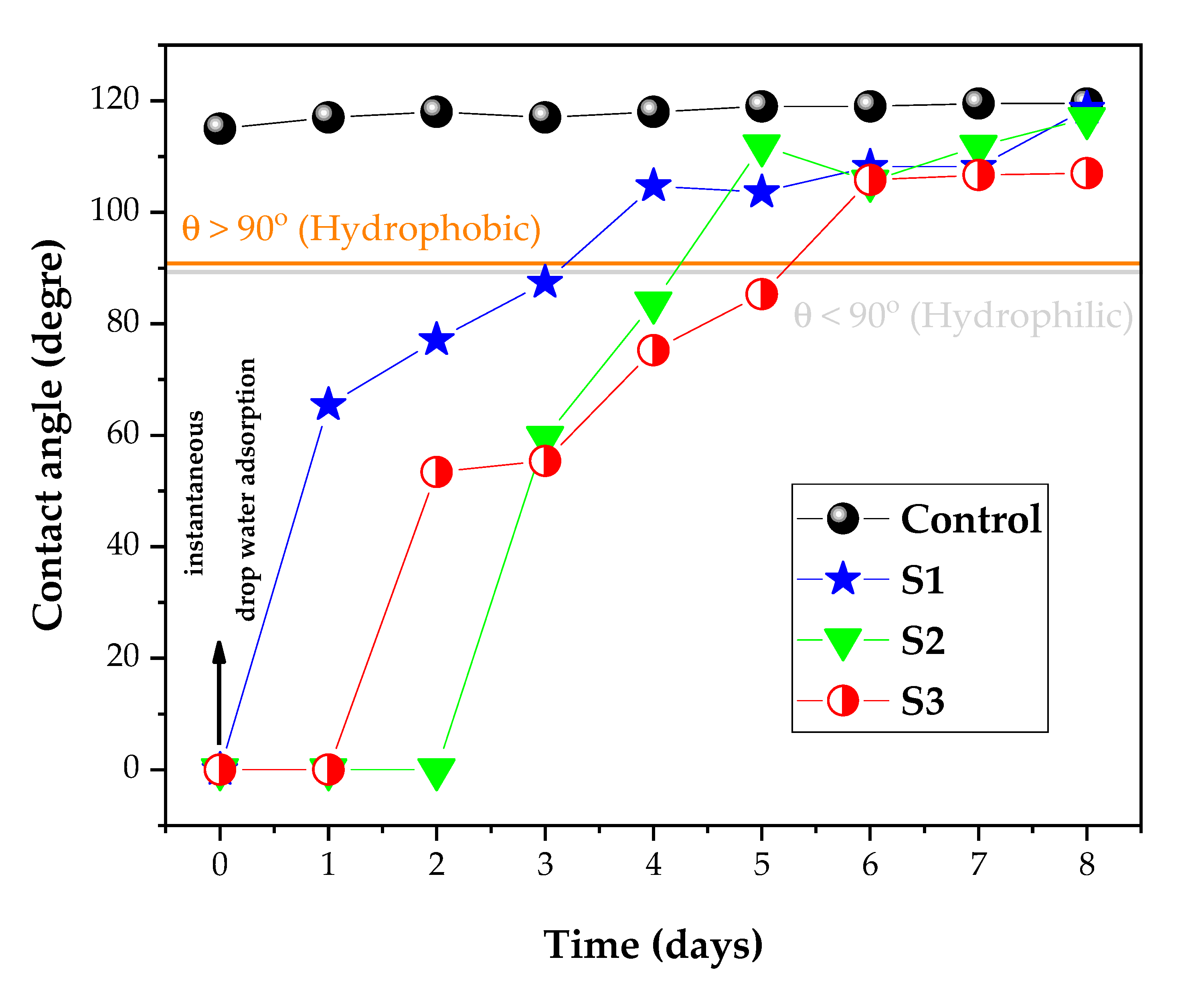
3.2. Wettability and Whiteness Analysis of the Fabrics
3.3. Surface Chemical Analysis of the Fabrics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heikkilä, P.; Taipale, A.; Harlin, A. Electrospinning of polyamides with different chain compositions for filtration applications. Polym. Eng. Sci. 2008, 48, 1168–1176. [Google Scholar] [CrossRef]
- Ryu, J.B.; Lyu, M.-Y. A study on the mechanical property and 3D fiber distribution in injection molded glass fiber reinforced PA66. Intern. Polym. Process. 2014, 29, 389–401. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, Z.; Schlarb, A.K. On the sliding wear of nanoparticle filled polyamide 66 composites. Compos. Sci. Technol. 2006, 66, 3188–3198. [Google Scholar] [CrossRef]
- Byett, J.H.; Allen, C. Dry sliding wear behavior of polyamide 66 and polycarbonate composites. Tribol. Int. 1992, 25, 237–246. [Google Scholar] [CrossRef]
- Apichartpattanasiri, S.; Hay, J.N.; Kukureka, S.N. A study of the tribological behavior of polyamide 66 with varying injection-moulding parameters. Wear 2001, 251, 1557–1566. [Google Scholar] [CrossRef]
- Matulevicius, J.; Kliucininkas, L.; Martuzevicius, D.; Krugly, E.; Tichonovas, M.; Baltrusaitis, J. Design and characterization of electrospun polyamide nanofiber media for air filtration applications. J. Nanomater. 2014, 2014, 1–13. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.; Yu, J.; Sun, G. Tunable fabrication of three-dimensional polyamide-66 nano-fiber/nets for high efficiency fine particulate filtration. J. Mater. Chem. 2012, 22, 1445–1452. [Google Scholar] [CrossRef]
- Suresha, B.; Kumar, B.N.R.; Venkataramareddy, M.; Jayaraju, T. Role of micro/nano fillers on mechanical and tribological properties of polyamide66/polypropylene composites. Mater. Des. 2010, 31, 1993–2000. [Google Scholar] [CrossRef]
- Kisner, A.; Rainert, K.T.; Ferrari, F.; Nau, C.T.; Barcellos, I.O.; Pezzin, S.H.; Andreaus, J. Chemical functionalization of polyamide 6.6 fabrics. React. Funct. Polym. 2013, 73, 1349–1356. [Google Scholar] [CrossRef]
- Li, L.; Peng, M.-y.; Teng, Y.; Gao, G. Diffuse plasma treatment of polyamide 66 fabric in atmospheric pressure air. Appl. Surf. Sci. 2016, 362, 348–354. [Google Scholar] [CrossRef]
- Bismarck, A.; Richter, D.; Wuertz, C.; Springer, J. Basic and acidic surface oxides on carbon fiber and their influence on the expected adhesion to polyamide. Colloids Surf. A Physicochem. Eng. Asp. 1999, 159, 341–350. [Google Scholar] [CrossRef]
- Peran, J.; Razic, S.E. Application of atmospheric pressure plasma technology for textile surface. Text. Res. J. 2019, 90, 1–24. [Google Scholar] [CrossRef]
- Acero, E.H.; Ribitsch, D.; Rodriguez, R.D.; Dellacher, A.; Zitzenbacher, S.; Marold, A.; Greimel, K.J.; Schroeder, M.; Kandelbauer, A.; Heumann, S.; et al. Two-step enzymatic functionalization of polyamide with phenolics. J. Mol. Catal. B Enzym. 2012, 79, 54–60. [Google Scholar] [CrossRef]
- Ren, C.S.; Wang, K.; Nie, Q.Y.; Wang, D.Z.; Guo, S.H. Surface modification of PE film by DBD plasma in air. Appl. Surf. Sci. 2008, 255, 3421–3425. [Google Scholar] [CrossRef]
- Carneiro, N.; Souto, A.P.; Silva, E.; Marimba, A.; Tena, B.; Ferreira, H.; Magalhães, V. Dyeability of corona-treated fabrics. Color. Technol. 2001, 117, 298–302. [Google Scholar] [CrossRef]
- Dumitrascu, N.; Borcia, C. Adhesion properties of polyamide-6 fibres treated by dielectric barrier discharge. Surf. Coat. Technol. 2006, 201, 1117–1123. [Google Scholar] [CrossRef]
- Masaeli, E.; Morshed, M.; Tavanai, H. Study of the wettability properties of polypropylene nonwoven mats by low-pressure oxygen plasma treatment. Surf. Interface Anal. 2007, 39, 770–774. [Google Scholar] [CrossRef]
- Molina, J.; Oliveira, F.R.; Souto, A.P.; Esteves, M.F.; Bonastre, J.; Cases, F. Enhanced adhesion of polypyrrole/PW12O hybrid coatings on polyester fabrics. J. Appl. Polym. 2013, 129, 422–433. [Google Scholar] [CrossRef]
- Károly, Z.; Kaláscska, G.; Sukumaran, J.; Fauconnier, D.; Kaláscska, Á.; Mohai, M.; Klébert, S. Effect of atmospheric cold plasma treatment on the adhesion and tribological properties of polyamide 66 and poly(tetrafluoroethylene). Materials 2019, 12, 658. [Google Scholar] [CrossRef]
- Katsuhiro, R.Y.; Pessoa, R.S.; Massi, M.; Grigorov, K.G.; Rubio, M.R.G.; Maciel, H.S. Surface modification of polyethylene by medium pressure microplasma generator. Surf. Eng. 2011, 27, 80–85. [Google Scholar]
- Shao, T.; Zhou, Y.; Zhang, C.; Yang, W.; Niu, Z.; Ren, C. Surface modification of polymethyl-methacrylate using atmospheric pressure argon plasma jets to improve surface flashover performance in vacuum. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 1747–1754. [Google Scholar] [CrossRef]
- Meiners, S.; Salge, J.G.H.; Prinz, E.; Förster, F. Surface modification of polymer materials by transient gas discharges at atmospheric pressure. Surf. Coat. Technol. 1998, 98, 1121–1127. [Google Scholar] [CrossRef]
- Akishev, Y.; Grushin, M.; Napartovich, A.; Trushkin, N. Novel AC and DC non-thermal plasma sources for cold surface treatment of polymer films and fabrics at atmospheric pressure. Plasma Polym. 2002, 7, 261–289. [Google Scholar] [CrossRef]
- Akishev, Y.S.; Grushin, M.E.; Monich, A.E.; Napartovich, A.P.; Trushkin, N.I. One-atmosphere argon dielectric-barrier corona discharge as an effective source of cold plasma for the treatment of polymer films and fabrics. High Energy Chem. 2003, 37, 286–291. [Google Scholar] [CrossRef]
- Hebert, T. Atmospheric-pressure cold plasma processing technology. In Plasma Technologies for Textiles, 1st ed.; Shishoo, R., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2007; pp. 79–128. [Google Scholar]
- Jelil, R.A. A review of low-temperature plasma treatment of textile materials. J. Mater. Sci. 2015, 50, 5913–5943. [Google Scholar] [CrossRef]
- Gasi, F.; Petraconi, G.; Bittencourt, E.; Lourenço, S.R.; Castro, A.H.R.; Miranda, F.S.; Essiptchouk, A.M.; Nascimento, L.; Petraconi, A.; Fraga, M.A.; et al. Plasma Treatment of Polyamide Fabric Surface by Hybrid Corona-Dielectric Barrier Discharge: Material Characterization and Dyeing/Washing Processes. Mater. Res. 2020, 23, e20190255. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Zille, A.; Souto, A.P. Dyeing mechanism and optimization of polyamide 6.6 functionalized with double barrier discharge (DBD) plasma in air. Appl. Surf. Sci. 2014, 293, 177–186. [Google Scholar] [CrossRef]
- Souza, P.R.F.; Souza, G.C.C.; Pinto, J.V.F.A.; Doria, A.C.O.C.; Nascimento, L.M.; Gomes, M.C.; Sobrinho, A.S.D.S.; Petraconi, G.; Sagas, J.C.; Rodrigues, B.V.M.; et al. Effect of ozone exposure on water uptake and germination of lentil (Lens culinaris) seeds. Ozone Sci. Eng. 2020, 42, 1–12. [Google Scholar] [CrossRef]
- Xi, M.; Li, Y.-L.; Shang, S.-Y.; Li, D.-H.; Yin, Y.-X.; Dai, X.-Y. Surface modification of aramid fiber by air DBD plasma at atmospheric pressure with continuous on-line processing. Surf. Coat. Technol. 2008, 202, 6029–6033. [Google Scholar] [CrossRef]
- Borcia, G.; Anderson, C.A.; Brown, N.M.D. Surface treatment of natural and synthetic textiles using a dielectric barrier discharge. Surf. Coat. Technol. 2006, 201, 3074–3081. [Google Scholar] [CrossRef]
- Pappas, D.; Bujanda, A.; Demaree, J.D.; Hirvonen, J.K.; Kosik, W.; Jensen, R.; McKnight, S. Surface modification of polyamide fibers and films using atmospheric plasmas. Surf. Coat. Technol. 2006, 201, 4384–4388. [Google Scholar] [CrossRef]
- Smirnov, S.A.; Rybkin, V.V.; Kholodkov, I.V. Simulation of the processes of formation and dissociation of neutral particles in air plasma: Vibrational kinectics of ground states of molecules. High Temp. 2002, 40, 161–165. [Google Scholar] [CrossRef]
- Karahan, H.A.; Özdogan, E. Improvements of surface functionality of cotton fibers by atmospheric plasma treatment. Fibers Polym. 2008, 9, 21–26. [Google Scholar] [CrossRef]
- Pessoa, R.S.; Bogos, N.S.; Gomes, M.P.; Maciel, H.S.; Medeiros, H.S.; Sagas, J.C.; Roberto, M.; Petraconi, G. Chemistry Studies of Low Pressure Argon Discharges: Experiments and Simulation. In Argon: Production, Characteristics and Applications, 1st ed.; Pessoa, R.S., Sismanoglu, B.N., Maciel, H.S., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; Volume 1, pp. 189–222. [Google Scholar]
- Maillo, J.; Pages, P.; Vallejo, E.; Lacorte, T.; Gacén, J. FTIR spectroscopy study of the interaction between fibre of polyamide 6 and iodine. Eur. Polym. J. 2005, 41, 753–759. [Google Scholar] [CrossRef]
- Vasanthan, N.; Salem, D.R. FTIR spectroscopic characterization of structural changes in polyamide-6 fibers during annealing and drawing. J. Polym. Sci. Pol. Phys. 2001, 39, 536–547. [Google Scholar] [CrossRef]
- Pandey, K.K.; Theagarajan, K.S. Analysis of wood surfaces and ground wood by diffuse reflectance (DRIFT) and photoacoustic (PAS) Fourier transform infrared spectroscopic techniques. Holz Roh Werkst. 1997, 55, 383–390. [Google Scholar] [CrossRef]
- Tusek, L.; Nitschke, M.; Werner, C.; Stana-Kleinschek, K.; Ribitsch, V. Surface characterization of NH3 plasma treated polyamide 6 foils. Colloids Surf. A 2001, 195, 81–95. [Google Scholar] [CrossRef]
- Levchik, S.V.; Costa, L.; Camino, G. Effect of the fire-retardant, ammonium polyphosphate, on the thermal decomposition of aliphatic polyamides: Part II—polyamide 6. Polym. Degrad. Stabil. 1992, 36, 229–237. [Google Scholar] [CrossRef]
- Ahlblad, G.; Forssttröm, D.; Stenberg, B.; Terselius, B.; Reitberger, T.; Svensson, L.-G. Oxidation profiles of polyamide 6,6 studied by imaging chemiluminescence and FTIR. Polym. Degrad. Stabil. 1997, 55, 287–293. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, C.; Guo, X.; Ren, Z. FTIR and molecular mechanics studies of H-bonds in aliphatic polyurethane and polyamide-66 model molecules. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 69, 407–412. [Google Scholar] [CrossRef]
- Vasanthan, N. Orientation and structure development in polyamide 6 fibers upon drawing. J. Polym. Sci. Pol. Phys. 2003, 41, 2870–2877. [Google Scholar] [CrossRef]
- Buyle, G. Nanoscale finishing of textiles via plasma treatment Materials Technology. Mater. Technol. 2009, 24, 46–51. [Google Scholar] [CrossRef]
- Wang, C.X.; Du, M.; Qui, Y.P. Uniformity and penetration of surface modification effects of atmospheric pressure plasma jet into textile materials. Adv. Mater. Res. 2011, 331, 356–359. [Google Scholar] [CrossRef]
- Wang, C.X.; Ren, Y.; Qiu, Y.P. Penetration depth of atmospheric pressure plasma surface modification into multiple layers of polyester fabrics. Surf. Coat. Technol. 2007, 202, 77–83. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Steffens, F.; Holanda, P.S.B.; Nascimento, J.H.O.; Matsui, K.N.; Souto, A.P. Physical, chemical and morphological characterization of polyamide fabrics treated with plasma discharge. Mater. Res. 2017, 20, 60–68. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, A. Modification of wool by air plasma and enzymes as a cleaner and environ mentally friendly process. J. Clean. Prod. 2015, 87, 961–965. [Google Scholar] [CrossRef]
- Elabid, A.E.A.; Zhang, J.; Shi, J.; Guo, Y.; Ding, K.; Zhang, J. Improving the low temperature dyeability of polyethylene terephthalate fabric with dispersive dyes by atmospheric pressure plasma discharge. Appl. Surf. Sci. 2016, 375, 26–34. [Google Scholar] [CrossRef]
- Zille, A.; Oliveira, F.R.; Souto, A.P. Plasma treatment in textile industry. Plasma Process. Polym. 2015, 12, 98–131. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Fernades, M.; Carneiro, N.; Souto, A.P. Functionalization of wool fabric with phase-change materials microcapsules after plasma surface modification. J. Appl. Polym. 2013, 128, 2638–2647. [Google Scholar] [CrossRef]
- Nakamatsu, J.; Delgado-Aparicio, L.F.; Da Silva, R.; Soberon, F. Ageing of plasma-treated poly(tetrafluoroethylene) surfaces. J. Adhes. Sci. Technol. 1999, 13, 753–761. [Google Scholar] [CrossRef]
- Van Der Mei, H.C.; Stokroos, I.; Schakenraad, J.M.; Busscher, H.J. Aging effects of repeatedly glow-discharged polyethylene: Influence on contact angle, infrared absorption, elemental surface composition, and surface topography. J. Adhes. Sci. Technol. 1991, 5, 757–769. [Google Scholar] [CrossRef]
- Canal, C.; Molina, R.; Bertran, E.; Erra, P. Wettability, ageing and recovery process of plasma-treated polyamide 6. J. Adhes. Sci. Technol. 2004, 18, 1077–1089. [Google Scholar] [CrossRef]
- Feitor, M.C.; Alves Junior, C.; Bezerra, C.M.; Sousa, R.R.M.; Costa, T.H.C. Evaluation of aging in air of poly(ethylene terephthalat) in oxygen plasma. Mater. Res. 2015, 18, 891–896. [Google Scholar] [CrossRef]
- CIE-International Commission on Illumination. Available online: http://cie.co.at/publications/colorimetry-part-4-cie-1976-lab-colour-space-0 (accessed on 21 August 2020).
- Pozo-Antonio, J.S.; Noya, D.; Montojo, C. Aesthetic effects on granite of adding nanoparticle TiO2 to Si-based consolidants (ethyl silicate or nano-sized silica). Coatings 2020, 10, 215. [Google Scholar] [CrossRef]
- Matusiak, M.; Walawska, A. Important aspects of cotton colour measurements. Fibres Tex. East. Eur. 2010, 18, 17–23. [Google Scholar]
- Brzezinski, S.; Kaleta, A.; Kowalczyk, D.; Malinnowska, G.; Gajdzicki, B. Effect of changes in the nanostructure of the outer layer of synthetics fibers on their dyeing properties. Fibres Tex. East. Eur. 2010, 18, 92–98. [Google Scholar]
- Joshi, M.; Bhattacharyya, A.; Ali, S.W. Characterization techniques for nanotechnology applications in textiles. Indian J. Fibre Text. Res. 2008, 33, 304–307. [Google Scholar]
- Oliveira, F.R.; Erkens, L.; Fangueiro, R.; Souto, A.P. Modification of banan fibers by DBD plasma treatment. Plasma Chem. Plasma Process. 2012, 32, 259–273. [Google Scholar] [CrossRef]
- Fu, R.K.Y.; Cheung, I.T.L.; Mei, Y.F.; Shek, C.H.; Siu, G.G.; Chu, P.K.; Yang, W.M.; Leng, Y.X.; Huang, Y.X.; Tian, X.B.; et al. Surface modification of polymeric materials by plasma immersion ion implantation. Nucl. Instrum. Methods Phys. Res. B. 2005, 237, 417–421. [Google Scholar] [CrossRef]
- Fu, R.K.Y.; Mei, Y.F.; Wan, G.J.; Siu, G.G.; Chu, P.K.; Huang, Y.X.; Tian, X.B.; Yang, S.Q.; Yang, S.Q.; Chen, J.Y. Surface composition and surface energy of Teflon treated by metal plasma immersion ion implantation. Surf. Sci. 2004, 573, 426–432. [Google Scholar] [CrossRef]
- Inagaki, N.; Tasaka, S.; Kawai, H. Surface modification of aromatic polyamide film by oxygen plasma. J. Polym. Sci. A: Polym. Chem. 1995, 33, 2001–2011. [Google Scholar] [CrossRef]
- Oh, K.W.; Kim, S.H.; Kim, E. Improved surface characteristics and the conductivity of polyaniline-nylon 6 fabrics by plasma treatment. J. Appl. Polym. Sci. 2001, 81, 684–694. [Google Scholar] [CrossRef]
- Dong, S.-S.-; Shao, W.-S.; Yang, L.; Ye, H.-J.; Zhen, L. Surface characterization and degradation behavior of polyimide films induced by coupling irradiation treatment. RSC Adv. 2018, 8, 28152. [Google Scholar] [CrossRef]
- Ektessaabi, A.; Hakamata, S. XPS study of ion beam modified polyimide films. Thin Solid Film. 2000, 377–378, 621–625. [Google Scholar] [CrossRef]
- Pezzotti, G.; Camara, C.; Marin, E.; Zhu, W.; Green, D.; Collins, A.; Putterman, S. Physical chemistry insights into surface charge phenomena during frictional coupling in triboelectric X-ray sources. J. Mater. Chem. C 2019, 7, 7708. [Google Scholar] [CrossRef]


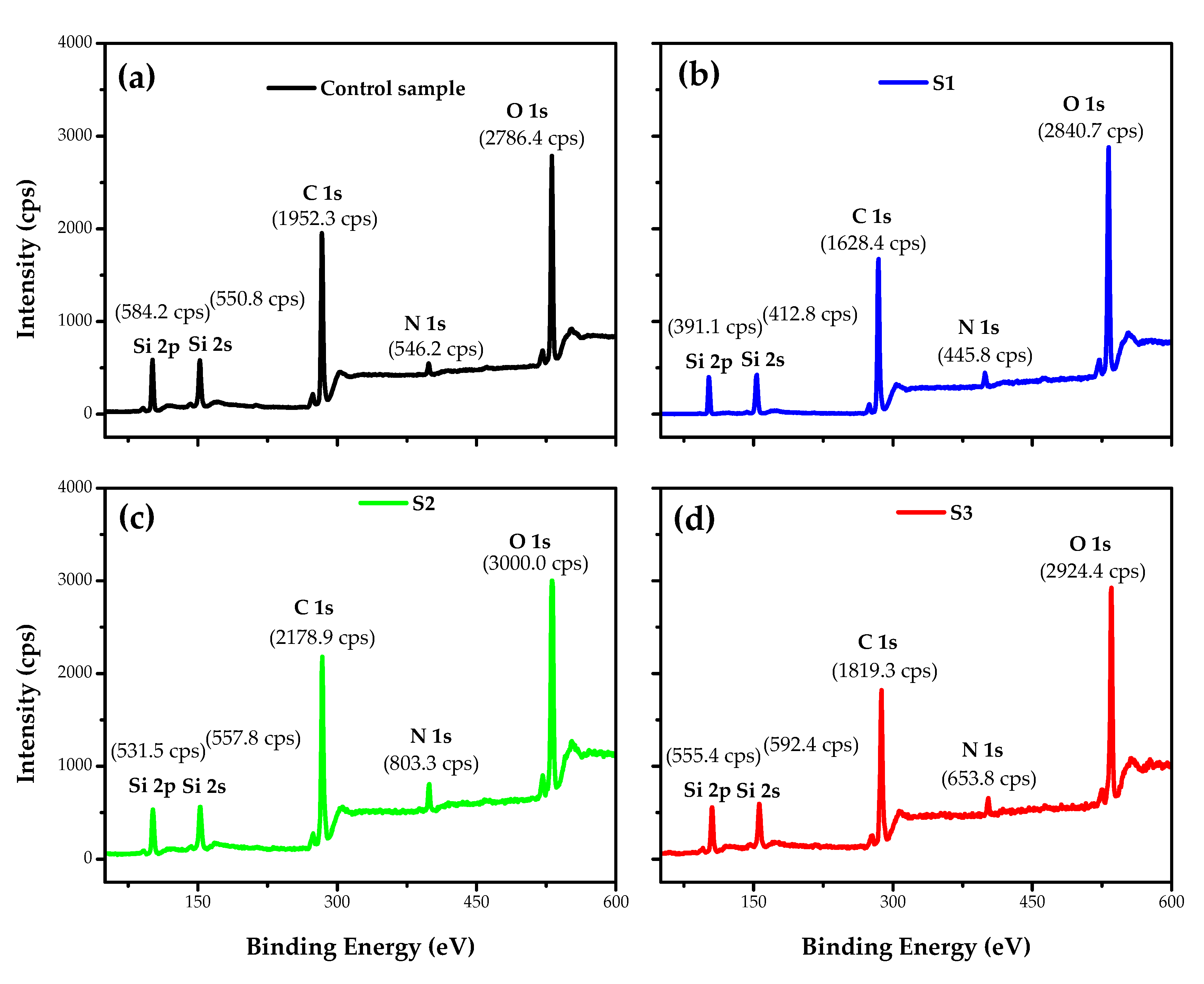

| Atomic Composition and Ratio | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Analysis | Samples | C (%) | O (%) | O/C | N (%) | N/C | Si (%) | Si/C | (O+N)/C |
| EDS | Control | 46.40 | 39.40 | 0.85 | 6.62 | 0.14 | 7.58 | 0.16 | 0.99 |
| S1 | 33.40 | 52.80 | 1.58 | 3.80 | 0.13 | 10.00 | 0.30 | 1.69 | |
| S2 | 35.05 | 51.60 | 1.47 | 4.06 | 0.12 | 9.29 | 0.27 | 1.58 | |
| S3 | 37.90 | 48.80 | 1.29 | 3.93 | 0.10 | 9.37 | 0.25 | 1.39 | |
| XPS | Control | 52.20 | 33.13 | 0.63 | 5.56 | 0.11 | 8.09 | 0.15 | 0.74 |
| S1 | 31.04 | 55.95 | 1.80 | 3.54 | 0.11 | 8.05 | 0.26 | 1.92 | |
| S2 | 32.33 | 54.37 | 1.68 | 3.85 | 0.12 | 9.06 | 0.28 | 1.80 | |
| S3 | 34.55 | 51.93 | 1.50 | 3.64 | 0.11 | 9.88 | 0.29 | 1.61 | |
| Sample | Carbon Bond Groups (%) | |||||
|---|---|---|---|---|---|---|
| COOH | C=O | C–O | C–N | C=C | C–Si | |
| Control | 0 | 8.87 | 9.80 | 3.72 | 73.00 | 4.61 |
| S1 | 1.06 | 6.80 | 9.69 | 2.55 | 76.80 | 3.10 |
| S2 | 1.71 | 9.13 | 9.76 | 3.56 | 70.21 | 5.63 |
| S3 | 1.54 | 7.30 | 9.55 | 5.30 | 62.90 | 13.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, L.; Gasi, F.; Landers, R.; da Silva Sobrinho, A.; Aragão, E.; Fraga, M.; Petraconi, G.; Chiappim, W.; Pessoa, R. Physicochemical Studies on the Surface of Polyamide 6.6 Fabrics Functionalized by DBD Plasmas Operated at Atmospheric and Sub-Atmospheric Pressures. Polymers 2020, 12, 2128. https://doi.org/10.3390/polym12092128
Nascimento L, Gasi F, Landers R, da Silva Sobrinho A, Aragão E, Fraga M, Petraconi G, Chiappim W, Pessoa R. Physicochemical Studies on the Surface of Polyamide 6.6 Fabrics Functionalized by DBD Plasmas Operated at Atmospheric and Sub-Atmospheric Pressures. Polymers. 2020; 12(9):2128. https://doi.org/10.3390/polym12092128
Chicago/Turabian StyleNascimento, Larissa, Fernando Gasi, Richard Landers, Argemiro da Silva Sobrinho, Eduardo Aragão, Mariana Fraga, Gilberto Petraconi, William Chiappim, and Rodrigo Pessoa. 2020. "Physicochemical Studies on the Surface of Polyamide 6.6 Fabrics Functionalized by DBD Plasmas Operated at Atmospheric and Sub-Atmospheric Pressures" Polymers 12, no. 9: 2128. https://doi.org/10.3390/polym12092128
APA StyleNascimento, L., Gasi, F., Landers, R., da Silva Sobrinho, A., Aragão, E., Fraga, M., Petraconi, G., Chiappim, W., & Pessoa, R. (2020). Physicochemical Studies on the Surface of Polyamide 6.6 Fabrics Functionalized by DBD Plasmas Operated at Atmospheric and Sub-Atmospheric Pressures. Polymers, 12(9), 2128. https://doi.org/10.3390/polym12092128








