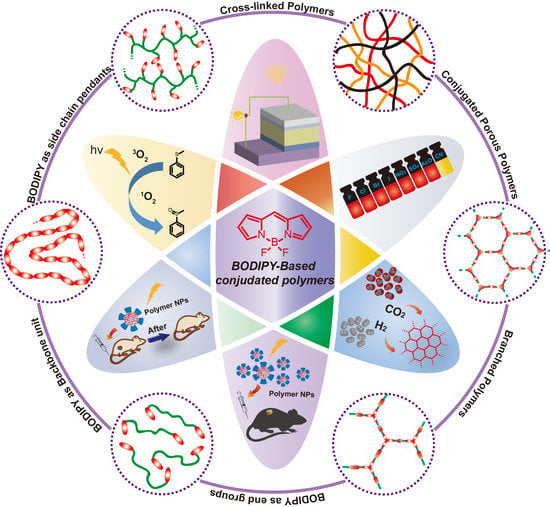
Architectures and Applications of BODIPY-Based Conjugated Polymers
Abstract
1. Introduction
2. Structures of BODIPY-Based Conjugated Polymers
2.1. Linear/Coiled Conjugated Polymers
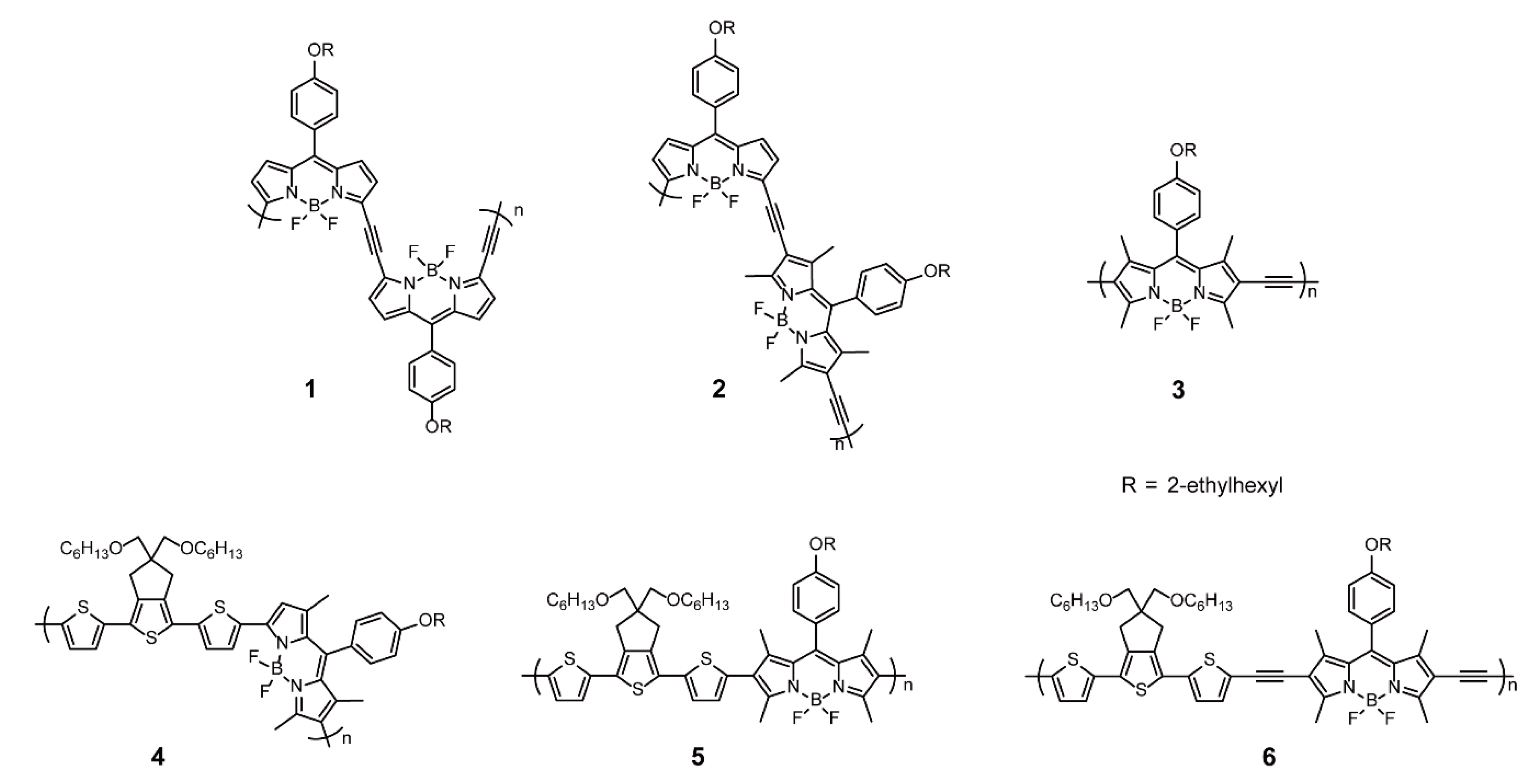
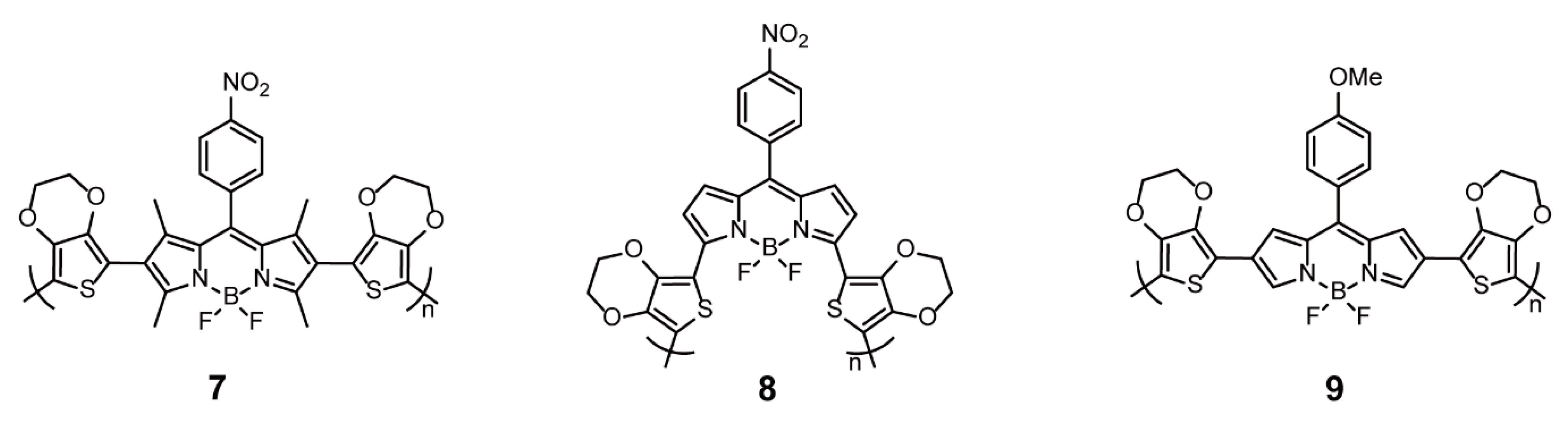
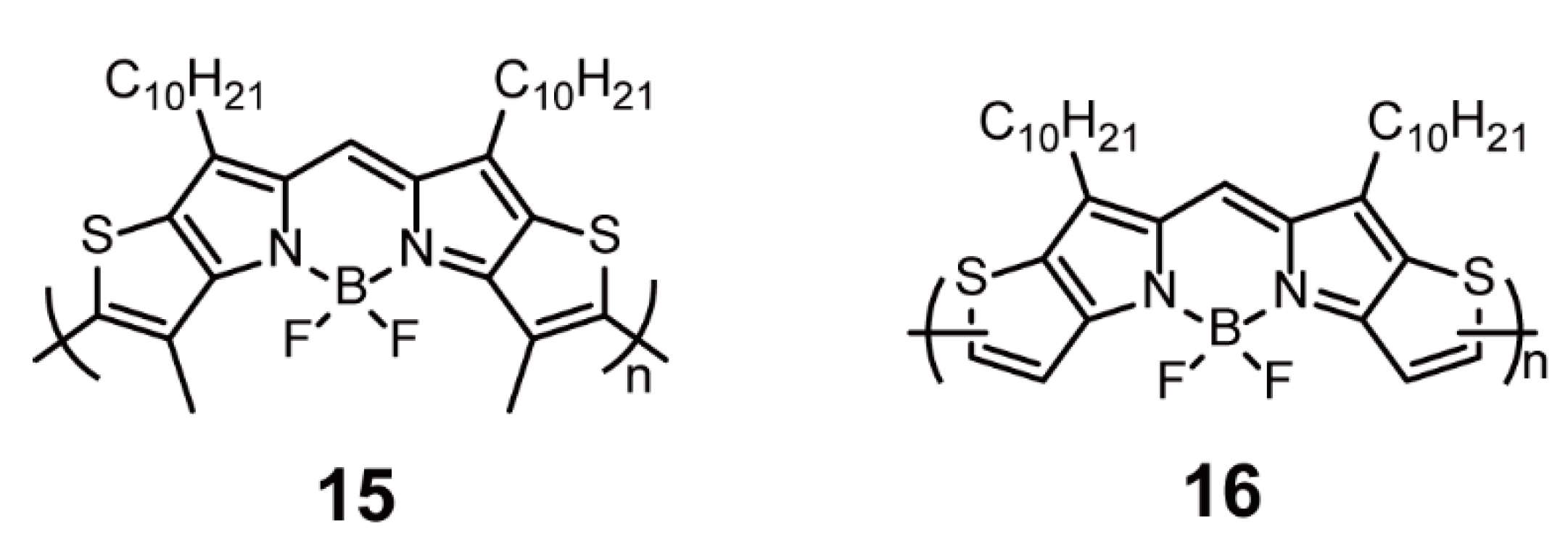
2.1.1. BODIPY as Backbone Units
2.1.2. BODIPY as Pendants Side Chains
2.1.3. BODIPY as End Groups
2.2. Porous Conjugated Polymers
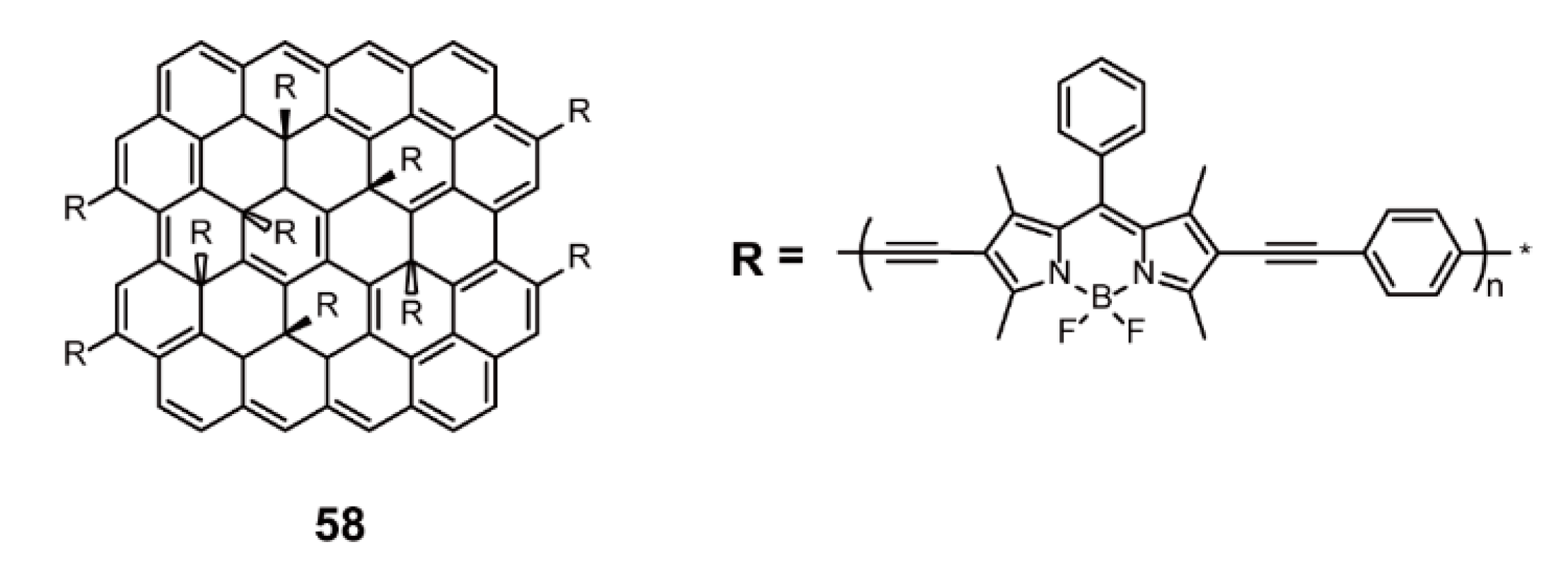
2.3. Other Structures
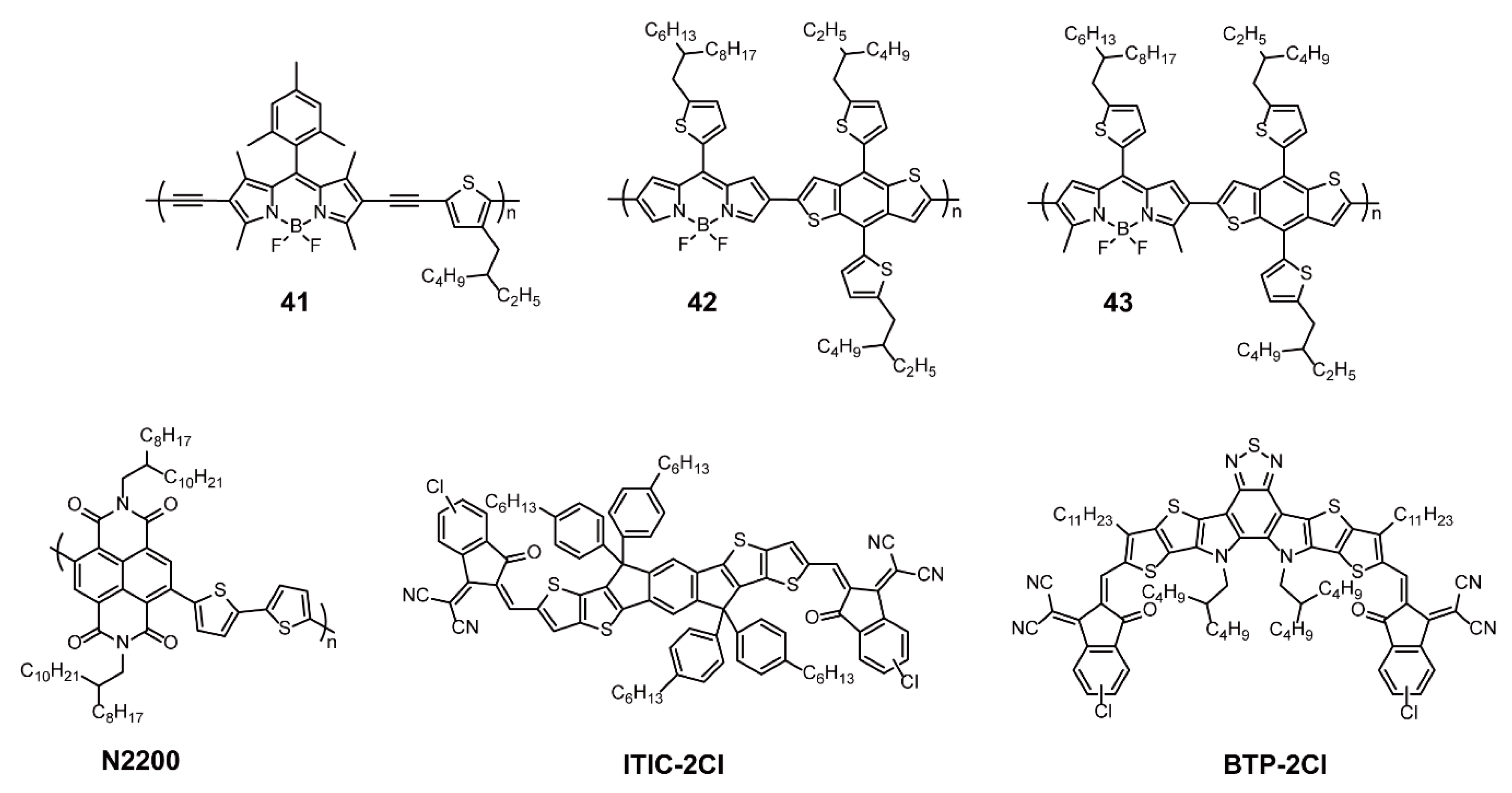
3. Functional Applications of BODIPY-Based Conjugated Polymers
3.1. Optoelectronic Materials
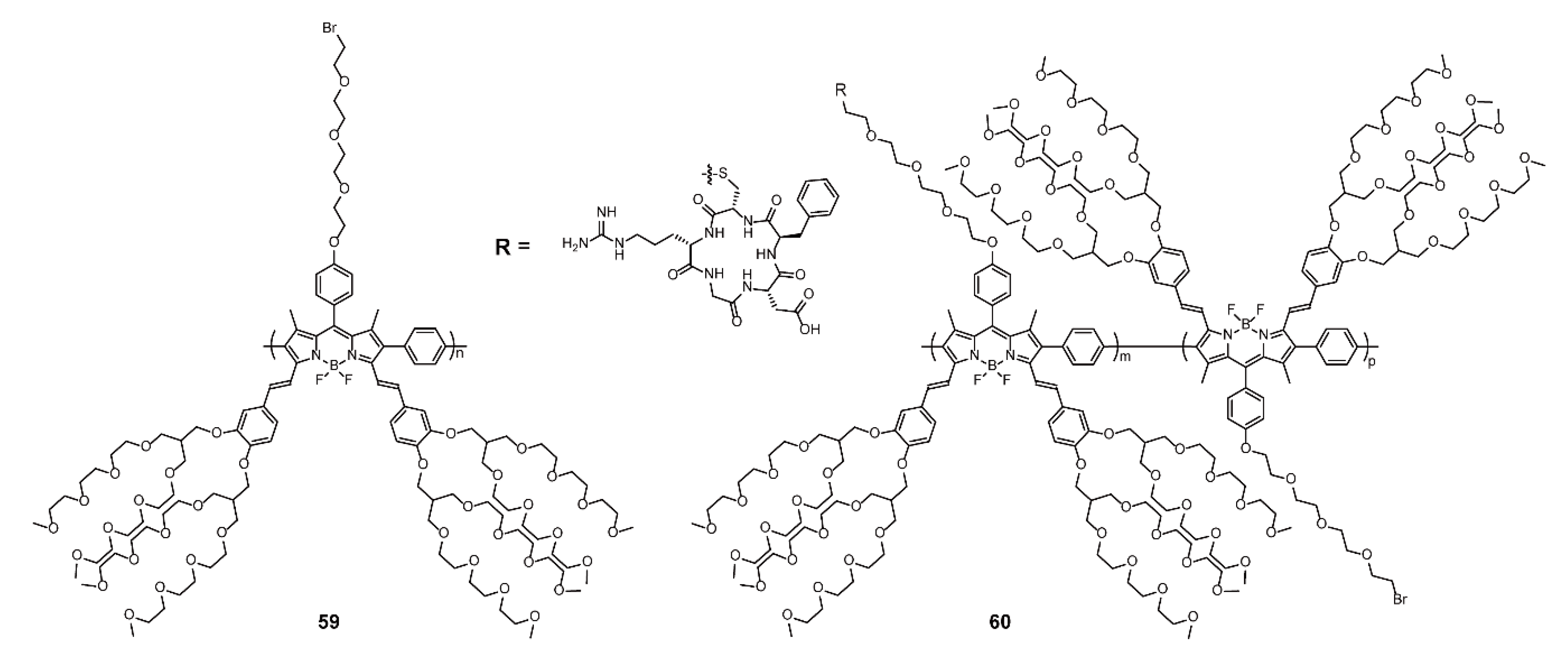
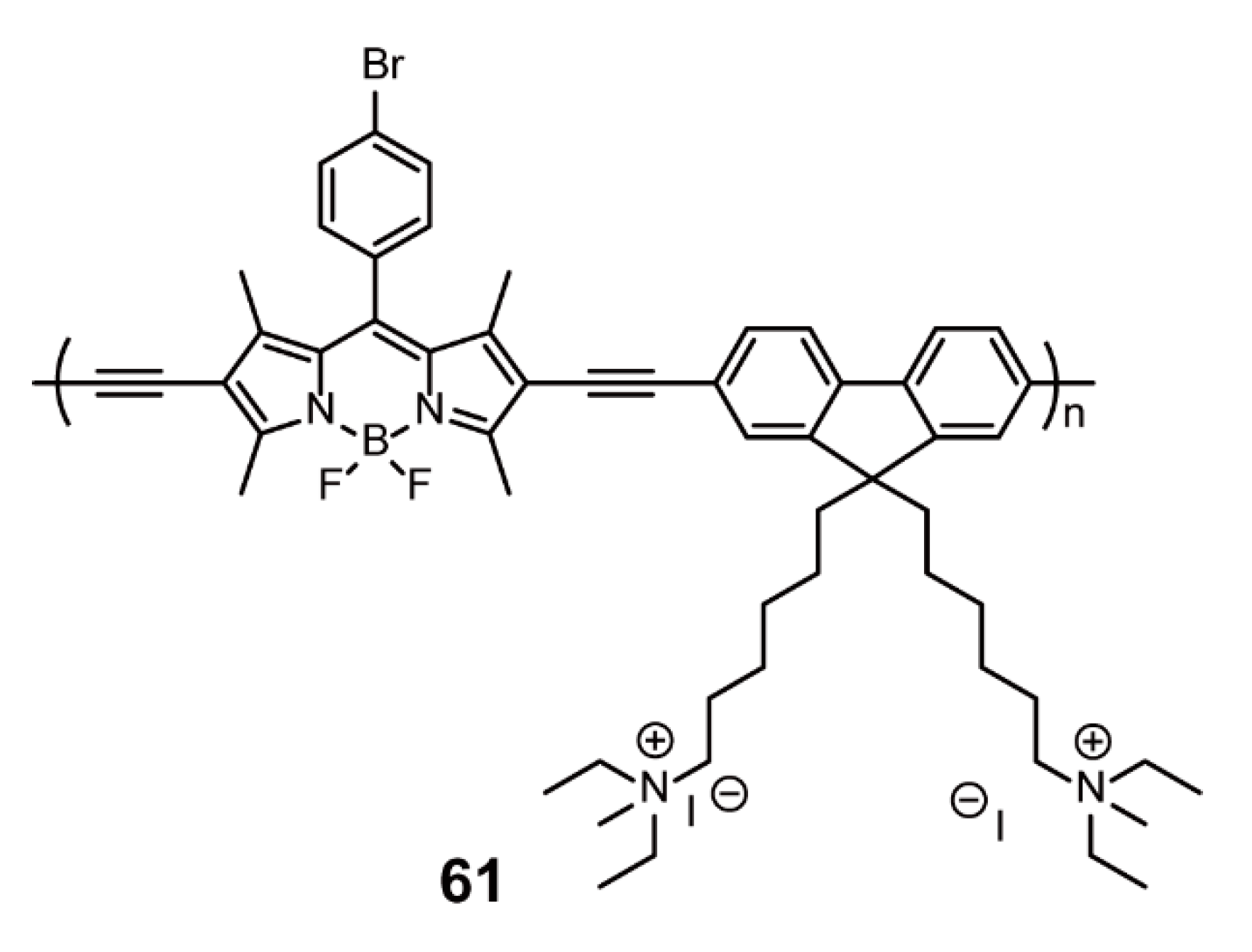
3.2. Bioimaging and Biotherapy
3.3. Sensor
3.4. Gas/Energy Storage
3.5. Other Applications
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiang, C.K.; Fincher, C.R.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical conductivity in doped polyacetylene. Phys. Rev. Lett. 1977, 39, 1098–1101. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Chiang, C.K.; Druy, M.A.; Gau, S.C.; Heeger, A.J.; Louis, E.J.; MacDiarmid, A.G.; Park, Y.W.; Shirakawa, H. Synthesis of highly conducting films of derivatives of polyacetylene, (CH)x. J. Am. Chem. Soc. 1978, 100, 1013–1015. [Google Scholar] [CrossRef]
- Ito, T.; Shirakawa, H.; Ikeda, S. Simultaneous polymerization and formation of polyacetylene film on the surface of concentrated soluble Ziegler-type catalyst solution. J. Polym. Sci. Polym. Chem. Ed. 1974, 12, 11–20. [Google Scholar] [CrossRef]
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Santos, D.A.D.; Brédas, J.L.; Lögdlund, M.; et al. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color control in π-conjugated organic polymers for use in electrochromic devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef]
- Zeglio, E.; Rutz, A.L.; Winkler, T.E.; Malliaras, G.G.; Herland, A. Conjugated polymers for assessing and controlling biological functions. Adv. Mater. 2019, 31, 1806712. [Google Scholar] [CrossRef]
- Inal, S.; Rivnay, J.; Suiu, A.-O.; Malliaras, G.G.; McCulloch, I. Conjugated polymers in bioelectronics. Acc. Chem. Res. 2018, 51, 1368–1376. [Google Scholar] [CrossRef]
- Chen, L.; McBranch, D.W.; Wang, H.-L.; Helgeson, R.; Wudl, F.; Whitten, D.G. Highly sensitive biological and chemical sensors based on reversible fluorescence quenching in a conjugated polymer. Proc. Natl. Acad. Sci. USA 1999, 96, 12287. [Google Scholar] [CrossRef]
- Ho, H.-A.; Leclerc, M. Optical sensors based on hybrid aptamer/conjugated polymer complexes. J. Am. Chem. Soc. 2004, 126, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Zhao, Y.; Zhang, M.; Ma, Y.; Baumgarten, M.; Müllen, K. Detection of TNT explosives with a new fluorescent conjugated polycarbazole polymer. Chem. Commun. 2011, 47, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, S.; Zhang, P.; Lv, F.; Liu, L.; Li, L.; Wang, S. An optical nanoruler based on a conjugated polymer−silver nanoprism pair for label-free protein detection. Adv. Mater. 2015, 27, 6040–6045. [Google Scholar] [CrossRef] [PubMed]
- Squeo, B.M.; Gregoriou, V.G.; Avgeropoulos, A.; Baysec, S.; Allard, S.; Scherf, U.; Chochos, C.L. BODIPY-based polymeric dyes as emerging horizon materials for biological sensing and organic electronic applications. Prog. Polym. Sci. 2017, 71, 26–52. [Google Scholar] [CrossRef]
- Awuah, S.G.; You, Y. Boron dipyrromethene (BODIPY)-based photosensitizers for photodynamic therapy. RSC Adv. 2012, 2, 11169–11183. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Ji, X.; Tao, Y.; Wang, J.; Zhao, W. BODIPY-Based fluorescent probes for biothiols. Chem. Eur. J. 2020, 26, 4172–4192. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Sun, T.; Xie, Z. Unadulterated BODIPY nanoparticles for biomedical applications. Coord. Chem. Rev. 2019, 390, 76–85. [Google Scholar] [CrossRef]
- Poddar, M.; Misra, R. Recent advances of BODIPY based derivatives for optoelectronic applications. Coord. Chem. Rev. 2020, 421, 213462. [Google Scholar] [CrossRef]
- Treibs, A.; Kreuzer, F.-H. Difluorboryl-Komplexe von Di- und Tripyrrylmethenen. Justus Liebigs Ann. Chem. 1968, 718, 208–223. [Google Scholar] [CrossRef]
- Monsma, F.J., Jr.; Barton, A.C.; Chol Kang, H.; Brassard, D.L.; Haugland, R.P.; Sibley, D.R. Characterization of novel fluorescent ligands with high affinity for D1 and D2 dopaminergic receptors. J. Neurochem. 1989, 52, 1641–1644. [Google Scholar] [CrossRef]
- Liu, M.; Ma, S.; She, M.; Chen, J.; Wang, Z.; Liu, P.; Zhang, S.; Li, J. Structural modification of BODIPY: Improve its applicability. Chin. Chem. Lett. 2019, 30, 1815–1824. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef] [PubMed]
- Algı, F.; Cihaner, A. An ambipolar low band gap material based on BODIPY and EDOT. Org. Electron. 2009, 10, 453–458. [Google Scholar] [CrossRef]
- Algi, M.P.; Tirkes, S.; Ertan, S.; Ergun, E.G.C.; Cihaner, A.; Algi, F. Design and synthesis of new 4,4′-difluoro-4-bora-3a,4a-diaza-s-indacene based electrochromic polymers. Electrochim. Acta 2013, 109, 766–774. [Google Scholar] [CrossRef]
- Forgie, J.C.; Skabara, P.J.; Stibor, I.; Vilela, F.; Vobecka, Z. New redox stable low band gap conjugated polymer based on an EDOT−BODIPY−EDOT repeat unit. Chem. Mater. 2009, 21, 1784–1786. [Google Scholar] [CrossRef]
- Nepomnyashchii, A.B.; Bröring, M.; Ahrens, J.; Bard, A.J. Synthesis, photophysical, electrochemical, and electrogenerated chemiluminescence studies. multiple sequential electron transfers in BODIPY monomers, dimers, trimers, and polymer. J. Am. Chem. Soc. 2011, 133, 8633–8645. [Google Scholar]
- Meng, G.; Velayudham, S.; Smith, A.; Luck, R.; Liu, H. Color tuning of polyfluorene emission with BODIPY monomers. Maromolecules 2009, 42, 1995–2001. [Google Scholar] [CrossRef]
- Donuru, V.R.; Vegesna, G.K.; Velayudham, S.; Meng, G.; Liu, H. Deep-red emissive conjugated poly(2,6-BODIPY-ethynylene)s bearing alkyl side chains. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5354–5366. [Google Scholar] [CrossRef]
- Tarafdar, G.; Pandey, U.K.; Sengupta, S.; Ramamurthy, P.C. Effect of structural isomerism in BODIPY based donor-acceptor co-polymers on their photovoltaic performance. Sol. Energy 2019, 186, 215–224. [Google Scholar] [CrossRef]
- Debnath, S.; Singh, S.; Bedi, A.; Krishnamoorthy, K.; Zade, S.S. Site-selective synthesis and characterization of BODIPY–acetylene copolymers and their transistor properties. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1978–1986. [Google Scholar] [CrossRef]
- Debnath, S.; Singh, S.; Bedi, A.; Krishnamoorthy, K.; Zade, S.S. Synthesis, optoelectronic, and transistor properties of BODIPY- and cyclopenta[c]thiophene-containing π-conjugated copolymers. J. Phys. Chem. C 2015, 119, 15859–15867. [Google Scholar] [CrossRef]
- Lin, X.; Tang, D.; He, T.; Xu, Z.; Qiu, H.; Zhang, Q.; Yin, S. A series of novel BODIPY-fluorene copolymers: Synthesis, characterization, optical-electronic and nonlinear optical properties. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 217, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.D.; Aytun, T.; Frasconi, M.; Stupp, S.I.; Stoddart, J.F. Photocurrent generation from a low band-gap and green BODIPY-based electrochromic polymer. Synth. Met. 2014, 197, 52–57. [Google Scholar] [CrossRef]
- Zhu, S.; Dorh, N.; Zhang, J.; Vegesna, G.; Li, H.; Luo, F.-T.; Tiwari, A.; Liu, H. Highly water-soluble neutral near-infrared emissive BODIPY polymeric dyes. J. Mater. Chem. 2012, 22, 2781–2790. [Google Scholar] [CrossRef]
- Donuru, V.R.; Zhu, S.; Green, S.; Liu, H. Near-infrared emissive BODIPY polymeric and copolymeric dyes. Polymer 2010, 51, 5359–5368. [Google Scholar] [CrossRef]
- Bucher, L.; Aly, S.M.; Desbois, N.; Karsenti, P.-L.; Gros, C.P.; Harvey, P.D. Random structural modification of a low-band-gap BODIPY-based polymer. J. Phys. Chem. C 2017, 121, 6478–6491. [Google Scholar] [CrossRef]
- Tanaka, K.; Yanagida, T.; Yamane, H.; Hirose, A.; Yoshii, R.; Chujo, Y. Liquid scintillators with near infrared emission based on organoboron conjugated polymers. Bioorg. Med. Chem. Lett. 2015, 25, 5331–5334. [Google Scholar] [CrossRef]
- Nagai, A.; Chujo, Y. Aromatic ring-fused BODIPY-based conjugated polymers exhibiting narrow near-infrared emission bands. Macromolecules 2010, 43, 193–200. [Google Scholar] [CrossRef]
- Umezawa, K.; Nakamura, Y.; Makino, H.; Citterio, D.; Suzuki, K. Bright, color-tunable fluorescent dyes in the visible−near-infrared region. J. Am. Chem. Soc. 2008, 130, 1550–1551. [Google Scholar] [CrossRef]
- Yoshii, R.; Yamane, H.; Tanaka, K.; Chujo, Y. Synthetic strategy for low-band gap oligomers and homopolymers using characteristics of thiophene-fused boron dipyrromethene. Macromolecules 2014, 47, 3755–3760. [Google Scholar] [CrossRef]
- Wu, Y.; Mao, X.; Ma, X.; Huang, X.; Cheng, Y.; Zhu, C. Synthesis and fluorescence properties of chiral near-infrared emissive polymers incorporating BODIPY derivatives and (S)-binaphthyl. Macromol. Chem. Phys. 2012, 213, 2238–2245. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, X.; Jiao, J.; Cheng, Y.; Zhu, C. Synthesis and characterization of near-infrared emissive BODIPY-based conjugated polymers. Synlett 2012, 23, 778–782. [Google Scholar]
- Donuru, V.R.; Vegesna, G.K.; Velayudham, S.; Green, S.; Liu, H. Synthesis and optical properties of red and deep-red emissive polymeric and copolymeric BODIPY dyes. Chem. Mater. 2009, 21, 2130–2138. [Google Scholar] [CrossRef]
- Alemdaroglu, F.E.; Alexander, S.C.; Ji, D.; Prusty, D.K.; Börsch, M.; Herrmann, A. Poly(BODIPY)s: A new class of tunable polymeric dyes. Macromolecules 2009, 42, 6529–6536. [Google Scholar] [CrossRef]
- Khetubol, A.; Van Snick, S.; Clark, M.L.; Fron, E.; Coutiño-González, E.; Cloet, A.; Kennes, K.; Firdaus, Y.; Vlasselaer, M.; Leen, V.; et al. Improved spectral coverage and fluorescence quenching in donor–acceptor systems involving indolo[3-2-b]carbazole and Boron-dipyrromethene or Diketopyrrolopyrrole. Photochem. Photobiol. 2015, 91, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Morisue, M.; Kawanishi, M.; Nakano, S. An elaborate route of exclusive sonogashira polycondensation to alternating BODIPY–porphyrin ethynylene-conjugated polymer. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2457–2465. [Google Scholar] [CrossRef]
- Thivierge, C.; Loudet, A.; Burgess, K. Brilliant BODIPY−fluorene copolymers with dispersed absorption and emission maxima. Macromolecules 2011, 44, 4012–4015. [Google Scholar] [CrossRef][Green Version]
- Popere, B.C.; Della Pelle, A.M.; Poe, A.; Balaji, G.; Thayumanavan, S. Predictably tuning the frontier molecular orbital energy levels of panchromatic low band gap BODIPY-based conjugated polymers. Chem. Sci. 2012, 3, 3093–3102. [Google Scholar] [CrossRef]
- Cortizo-Lacalle, D.; Howells, C.T.; Gambino, S.; Vilela, F.; Vobecka, Z.; Findlay, N.J.; Inigo, A.R.; Thomson, S.A.J.; Skabara, P.J.; Samuel, I.D.W. BODIPY-based conjugated polymers for broadband light sensing and harvesting applications. J. Mater. Chem. 2012, 22, 14119–14126. [Google Scholar] [CrossRef]
- Li, S.; Liu, K.; Kuang, G.; Masuda, T.; Zhang, A. Thermoresponsive helical poly(phenylacetylene)s. Macromolecules 2014, 47, 3288–3296. [Google Scholar] [CrossRef]
- Algi, F.; Cihaner, A. A novel terthienyl based polymer electrochrome with peripheral BODIPY. Polymer 2012, 53, 3469–3475. [Google Scholar] [CrossRef]
- Cihaner, A.; Algı, F. A new conducting polymer bearing 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) subunit: Synthesis and characterization. Electrochim. Acta 2008, 54, 786–792. [Google Scholar] [CrossRef]
- Yeo, H.; Tanaka, K.; Chujo, Y. Synthesis of dual-emissive polymers based on ineffective energy transfer through cardo fluorene-containing conjugated polymers. Polymer 2015, 60, 228–233. [Google Scholar] [CrossRef]
- Yeo, H.; Tanaka, K.; Chujo, Y. Effective light-harvesting antennae based on BODIPY-tethered cardo polyfluorenes via rapid energy transferring and low concentration quenching. Macromolecules 2013, 46, 2599–2605. [Google Scholar] [CrossRef]
- Liu, B.; Li, L.; Lin, C.; Zhou, J.; Zhu, Z.; Xu, H.; Qiu, H.; Yin, S. Polyacetylenes containing BODIPY pendants with different connectivities: Synthesis, characterization and opto-electronic properties. Polym. Chem. 2014, 5, 372–381. [Google Scholar] [CrossRef]
- Yin, S.; Leen, V.; Jackers, C.; Van der Auweraer, M.; Smet, M.; Boens, N.; Dehaen, W. The synthesis and spectroscopic characterization of poly(p-phenylene ethynylene) with 3-connected BODIPY end groups. Dye. Pigm. 2011, 88, 372–377. [Google Scholar] [CrossRef]
- Yin, S.; Leen, V.; Jackers, C.; Beljonne, D.; Van Averbeke, B.; Van der Auweraer, M.; Boens, N.; Dehaen, W. Oligo(p-phenylene ethynylene)–BODIPY derivatives: Synthesis, energy transfer, and quantum-chemical calculations. Chem. Eur. J. 2011, 17, 13247–13257. [Google Scholar] [CrossRef]
- Dalapati, S.; Gu, C.; Jiang, D. Luminescent porous polymers based on aggregation-induced mechanism: Design, synthesis and functions. Small 2016, 12, 6513–6527. [Google Scholar] [CrossRef]
- Huang, N.; Day, G.; Yang, X.; Drake, H.; Zhou, H.-C. Engineering porous organic polymers for carbon dioxide capture. Sci. China Chem. 2017, 60, 1007–1014. [Google Scholar] [CrossRef]
- Bildirir, H.; Gregoriou, V.G.; Avgeropoulos, A.; Scherf, U.; Chochos, C.L. Porous organic polymers as emerging new materials for organic photovoltaic applications: Current status and future challenges. Mater. Horiz. 2017, 4, 546–556. [Google Scholar] [CrossRef]
- Zhang, Y.; Riduan, S.N. Functional porous organic polymers for heterogeneous catalysis. Chem. Soc. Rev. 2012, 41, 2083–2094. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, S.; Xu, H.; Nagai, A.; Jiang, D. Conjugated microporous polymers: Design, synthesis and application. Chem. Soc. Rev. 2013, 42, 8012–8031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, S.; Ma, S.; Xiao, F.-S.; Sun, Q. Exploration of advanced porous organic polymers as a platform for biomimetic catalysis and molecular recognition. Chem. Commun. 2020, 56, 10631–10641. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zhang, H.; Wang, D.-G.; Chen, T.; Yang, S.; Kuang, G.-C. Imine-linked porous organic polymers showing mesoporous microspheres architectures with tunable surface roughness. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 319–327. [Google Scholar] [CrossRef]
- Wang, D.-G.; Li, Q.; Zhu, Y.; Tang, H.; Song, M.; Kuang, G.-C. BODIPY-based porous organic polymers: How the monomeric methyl substituents and isomerization affect the porosity and singlet oxygen generation. Macromol. Chem. Phys. 2017, 218, 1700101. [Google Scholar] [CrossRef]
- Wang, D.-G.; Song, F.; Tang, H.; Jia, X.-R.; Song, M.; Kuang, G.-C. A facile route to prepare dimeric BODIPY-based porous organic polymers using FeCl3. New J. Chem. 2017, 41, 5263–5266. [Google Scholar] [CrossRef]
- Baran, D.; Tuladhar, S.; Economopoulos, S.P.; Neophytou, M.; Savva, A.; Itskos, G.; Othonos, A.; Bradley, D.D.C.; Brabec, C.J.; Nelson, J.; et al. Photovoltaic limitations of BODIPY:fullerene based bulk heterojunction solar cells. Synth. Met. 2017, 226, 25–30. [Google Scholar] [CrossRef]
- Economopoulos, S.P.; Chochos, C.L.; Ioannidou, H.A.; Neophytou, M.; Charilaou, C.; Zissimou, G.A.; Frost, J.M.; Sachetan, T.; Shahid, M.; Nelson, J.; et al. Novel BODIPY-based conjugated polymers donors for organic photovoltaic applications. RSC Adv. 2013, 3, 10221–10229. [Google Scholar] [CrossRef]
- Yoshii, R.; Yamane, H.; Nagai, A.; Tanaka, K.; Taka, H.; Kita, H.; Chujo, Y. π-conjugated polymers composed of BODIPY or Aza-BODIPY derivatives exhibiting high electron mobility and low threshold voltage in electron-only devices. Macromolecules 2014, 47, 2316–2323. [Google Scholar] [CrossRef]
- Squeo, B.M.; Gregoriou, V.G.; Han, Y.; Palma-Cando, A.; Allard, S.; Serpetzoglou, E.; Konidakis, I.; Stratakis, E.; Avgeropoulos, A.; Anthopoulos, T.D.; et al. α,β-unsubstituted meso-positioning thienyl BODIPY: A promising electron deficient building block for the development of near infrared (NIR) p-type donor–acceptor (D–A) conjugated polymers. J. Mater. Chem. C 2018, 6, 4030–4040. [Google Scholar] [CrossRef]
- He, A.; Qin, Y.; Dai, W.; Luo, X. Novel D-A type dyes based on BODIPY for solution processed organic polymer solar cells. Dye. Pigm. 2019, 162, 671–679. [Google Scholar] [CrossRef]
- Kim, B.; Ma, B.; Donuru, V.R.; Liu, H.; Fréchet, J.M.J. Bodipy-backboned polymers as electron donor in bulk heterojunction solar cells. Chem. Commun. 2010, 46, 4148–4150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Miao, J.; Dou, C.; Liu, J.; Wang, L. BODIPY bearing alkylthienyl side chains: A new building block to design conjugated polymers with near infrared absorption for organic photovoltaics. Polym. Chem. 2020, 11, 5750–5756. [Google Scholar] [CrossRef]
- Bucher, L.; Desbois, N.; Harvey, P.D.; Gros, C.P.; Sharma, G.D. Porphyrin antenna-enriched BODIPY–thiophene copolymer for efficient solar cells. ACS Appl. Mater. Interfaces 2018, 10, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Squeo, B.M.; Gasparini, N.; Ameri, T.; Palma-Cando, A.; Allard, S.; Gregoriou, V.G.; Brabec, C.J.; Scherf, U.; Chochos, C.L. Ultra low band gap α,β-unsubstituted BODIPY-based copolymer synthesized by palladium catalyzed cross-coupling polymerization for near infrared organic photovoltaics. J. Mater. Chem. A 2015, 3, 16279–16286. [Google Scholar] [CrossRef]
- Bucher, L.; Desbois, N.; Harvey, P.D.; Gros, C.P.; Misra, R.; Sharma, G.D. Nonfullerene polymer solar cells reaching a 9.29% efficiency using a BODIPY-thiophene backboned donor material. ACS Appl. Energy Mater. 2018, 1, 3359–3368. [Google Scholar]
- Guo, Y.; Xia, D.; Liu, B.; Wu, H.; Li, C.; Tang, Z.; Xiao, C.; Li, W. Small band gap boron dipyrromethene-based conjugated polymers for all-polymer solar cells: The effect of methyl units. Macromolecules 2019, 52, 8367–8373. [Google Scholar] [CrossRef]
- Liu, B.; Ma, Z.; Xu, Y.; Guo, Y.; Yang, F.; Xia, D.; Li, C.; Tang, Z.; Li, W. Non-fullerene organic solar cells based on a BODIPY-polymer as electron donor with high photocurrent. J. Mater. Chem. C 2020, 8, 2232–2237. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Grätzel, M. The light and shade of perovskite solar cells. Nat. Mater. 2014, 13, 838–842. [Google Scholar] [CrossRef]
- Kyeong, M.; Lee, J.; Lee, K.; Hong, S. BODIPY-based conjugated polymers for use as dopant-free hole transporting materials for durable perovskite solar cells: Selective tuning of HOMO/LUMO levels. ACS Appl. Mater. Interfaces 2018, 10, 23254–23262. [Google Scholar] [CrossRef]
- Usta, H.; Yilmaz, M.D.; Avestro, A.-J.; Boudinet, D.; Denti, M.; Zhao, W.; Stoddart, J.F.; Facchetti, A. BODIPY–thiophene copolymers as p-channel semiconductors for organic thin-film transistors. Adv. Mater. 2013, 25, 4327–4334. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, D.; Liu, X.; Kim, M.-J.; Nashchadin, A.; Sharapov, V.; Yu, L. BODIPY-containing polymers with ultralow band gaps and ambipolar charge mobilities. Macromolecules 2020, 53, 2014–2020. [Google Scholar] [CrossRef]
- Popere, B.C.; Della Pelle, A.M.; Thayumanavan, S. BODIPY-based donor–acceptor π-conjugated alternating copolymers. Maromolecules 2011, 44, 4767–4776. [Google Scholar] [CrossRef]
- Singh, S.; Chithiravel, S.; Krishnamoorthy, K. Copolymers comprising monomers with various dipoles and quadrupole as active material in organic field effect transistors. J. Phys. Chem. C 2016, 120, 26199–26205. [Google Scholar] [CrossRef]
- Ozdemir, M.; Kim, S.W.; Kim, H.; Kim, M.-G.; Kim, B.J.; Kim, C.; Usta, H. Semiconducting copolymers based on meso-substituted BODIPY for inverted organic solar cells and field-effect transistors. Adv. Electron. Mater. 2018, 4, 1700354. [Google Scholar] [CrossRef]
- Sun, S.; Zhuang, X.; Wang, L.; Liu, B.; Zhang, B.; Chen, Y. BODIPY-based conjugated polymer covalently grafted reduced graphene oxide for flexible nonvolatile memory devices. Carbon 2017, 116, 713–721. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, J.; Rong, Y.; Ye, F.; Chiu, D.T.; Uvdal, K. High-intensity near-IR fluorescence in semiconducting polymer dots achieved by cascade FRET strategy. Chem. Sci. 2013, 4, 2143–2151. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, W.; Li, C.; Liu, S.; Hu, X.; Xie, Z. Rational design of BODIPY-diketopyrrolopyrrole conjugated polymers for photothermal tumor ablation. ACS Appl. Mater. Interfaces 2019, 11, 32720–32728. [Google Scholar] [CrossRef]
- Zhang, W.; Lin, W.; Wang, X.; Li, C.; Liu, S.; Xie, Z. Hybrid nanomaterials of conjugated polymers and albumin for precise photothermal therapy. ACS Appl. Mater. Interfaces 2019, 11, 278–287. [Google Scholar] [CrossRef]
- Rong, Y.; Yu, J.; Zhang, X.; Sun, W.; Ye, F.; Wu, I.C.; Zhang, Y.; Hayden, S.; Zhang, Y.; Wu, C.; et al. Yellow fluorescent semiconducting polymer dots with high brightness, small size, and narrow emission for biological applications. ACS Macro Lett. 2014, 3, 1051–1054. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, J.; Janjanam, J.; Bi, J.; Vegesna, G.; Tiwari, A.; Luo, F.-T.; Wei, J.; Liu, H. Highly water-soluble, near-infrared emissive BODIPY polymeric dye bearing RGD peptide residues for cancer imaging. Anal. Chim. Acta 2013, 758, 138–144. [Google Scholar] [CrossRef]
- Du, R.; Cui, S.; Sun, Z.; Liu, M.; Zhang, Y.; Wu, Q.; Wu, C.; Guo, F.; Zhao, L. Highly fluorescent hyperbranched BODIPY-based conjugated polymer dots for cellular imaging. Chem. Commun. 2017, 53, 8612–8615. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Qian, Y.; Xu, Z.; Lv, Z.; Tao, P.; Xie, M.; Liu, S.; Huang, W.; Zhao, Q. Enhancing singlet oxygen generation in semiconducting polymer nanoparticles through fluorescence resonance energy transfer for tumor treatment. Chem. Sci. 2019, 10, 5085–5094. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fang, G.; Cao, D. Synthesis of a cationic BODIPY-containing conjugated polymer for detection of DNA and cellular imaging. J. Fluoresc. 2016, 26, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fang, G.; Cao, D. Highly selective and sensitive detection of F− and CN− ions simultaneously by a reaction-based BODIPY-containing conjugated polymer. Sens. Actuators B Chem. 2015, 221, 63–74. [Google Scholar] [CrossRef]
- He, T.; Tang, D.; Lin, C.; Shen, X.; Lu, C.; Xu, L.; Gu, Z.; Xu, Z.; Qiu, H.; Zhang, Q.; et al. Conjugated polymers containing BODIPY and fluorene units for sensitive detection of CN− Ions: Site-selective synthesis, photo-physical and electrochemical properties. Polymers 2017, 9, 512. [Google Scholar] [CrossRef]
- Sen, C.P.; Devendar Goud, V.; Shrestha, R.G.; Shrestha, L.K.; Ariga, K.; Valiyaveettil, S. BODIPY based hyperbranched conjugated polymers for detecting organic vapors. Polym. Chem. 2016, 7, 4213–4225. [Google Scholar] [CrossRef]
- Sen, C.P.; Shrestha, R.G.; Shrestha, L.K.; Ariga, K.; Valiyaveettil, S. Low-band-gap BODIPY conjugated copolymers for sensing volatile organic compounds. Chem. Eur. J. 2015, 21, 17344–17354. [Google Scholar] [CrossRef]
- Xu, Y.; Chang, D.; Feng, S.; Zhang, C.; Jiang, J.-X. BODIPY-containing porous organic polymers for gas adsorption. New J. Chem. 2016, 40, 9415–9423. [Google Scholar] [CrossRef]
- Lin, Y.; Yin, J.; Li, X.; Pan, C.; Kuang, G. Luminescent BODIPY-based porous organic polymer for CO2 adsorption. J. Wunan Univ. Technol. 2019, 34, 440–445. [Google Scholar] [CrossRef]
- Li, G.; Yin, J.-F.; Guo, H.; Wang, Z.; Zhang, Y.; Li, X.; Wang, J.; Yin, Z.; Kuang, G.-C. BODIPY-based conjugated porous polymer and its derived porous carbon for lithium-ion storage. ACS Omega 2018, 3, 7727–7735. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Anil, A.G.; James, A.; Patra, A. Multifunctional porous organic polymers: Tuning of porosity, CO2, and H2 storage and visible-light-driven photocatalysis. ACS Appl. Mater. Interfaces 2016, 8, 27669–27678. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, Z.; Li, Y.; Yang, W. Synthesis and properties of BODIPY polymers and their photocatalytic performance for aerobic oxidation of benzylamine. Catal. Commun. 2015, 64, 96–100. [Google Scholar] [CrossRef]
- Liras, M.; Iglesias, M.; Sánchez, F. Conjugated microporous polymers incorporating BODIPY moieties as light-emitting materials and recyclable visible-light photocatalysts. Macromolecules 2016, 49, 1666–1673. [Google Scholar] [CrossRef]
- Tobin, J.M.; Liu, J.; Hayes, H.; Demleitner, M.; Ellis, D.; Arrighi, V.; Xu, Z.; Vilela, F. BODIPY-based conjugated microporous polymers as reusable heterogeneous photosensitisers in a photochemical flow reactor. Polym. Chem. 2016, 7, 6662–6670. [Google Scholar] [CrossRef]
- Zhu, Y.; Ji, Y.-J.; Wang, D.-G.; Zhang, Y.; Tang, H.; Jia, X.-R.; Song, M.; Yu, G.; Kuang, G.-C. BODIPY-based conjugated porous polymers for highly efficient volatile iodine capture. J. Mater. Chem. A 2017, 5, 6622–6629. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Zhang, Z.; Sun, Z.; Shao, X.; Guo, J.; Xi, L.; Yuan, Z.; Zhang, X.; Chiu, D.T.; et al. Narrow-band polymer dots with pronounced fluorescence fluctuations for dual-color super-resolution imaging. Nanoscale 2020, 12, 7522–7526. [Google Scholar] [CrossRef]









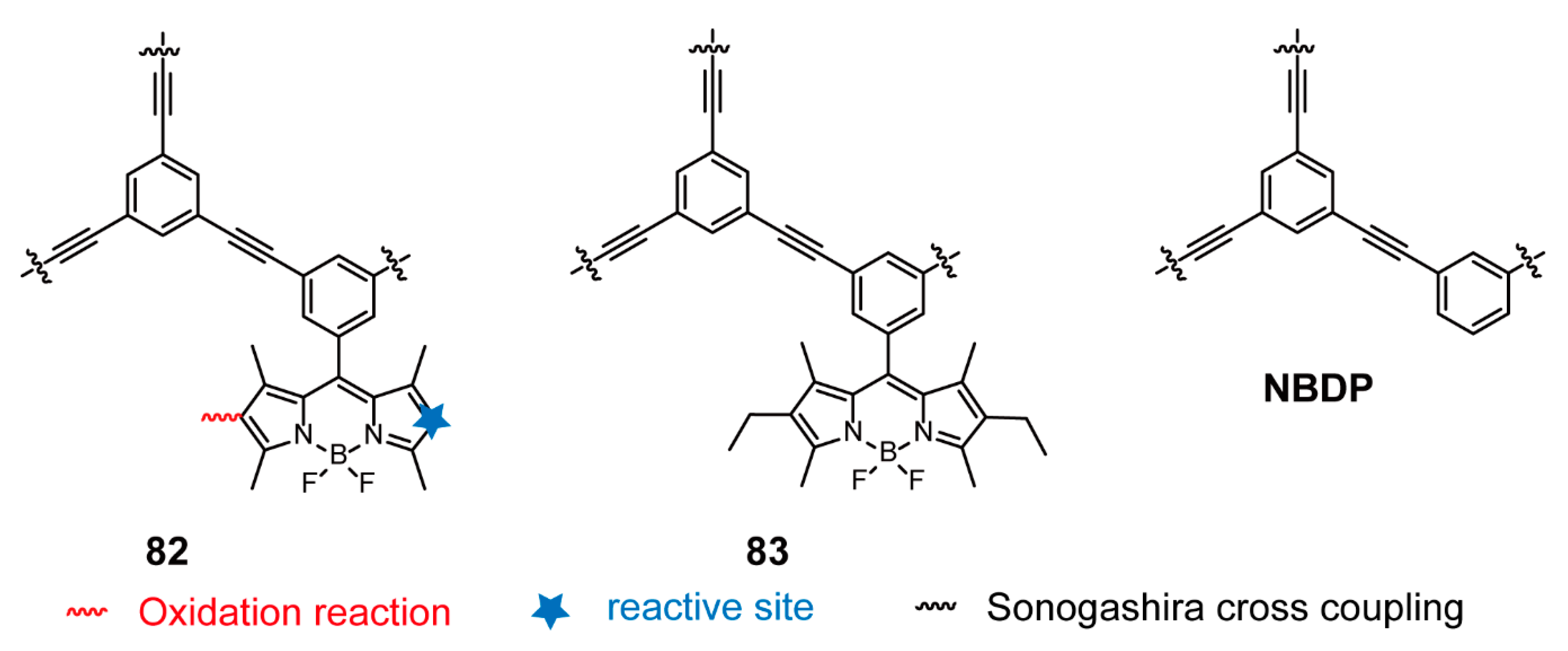
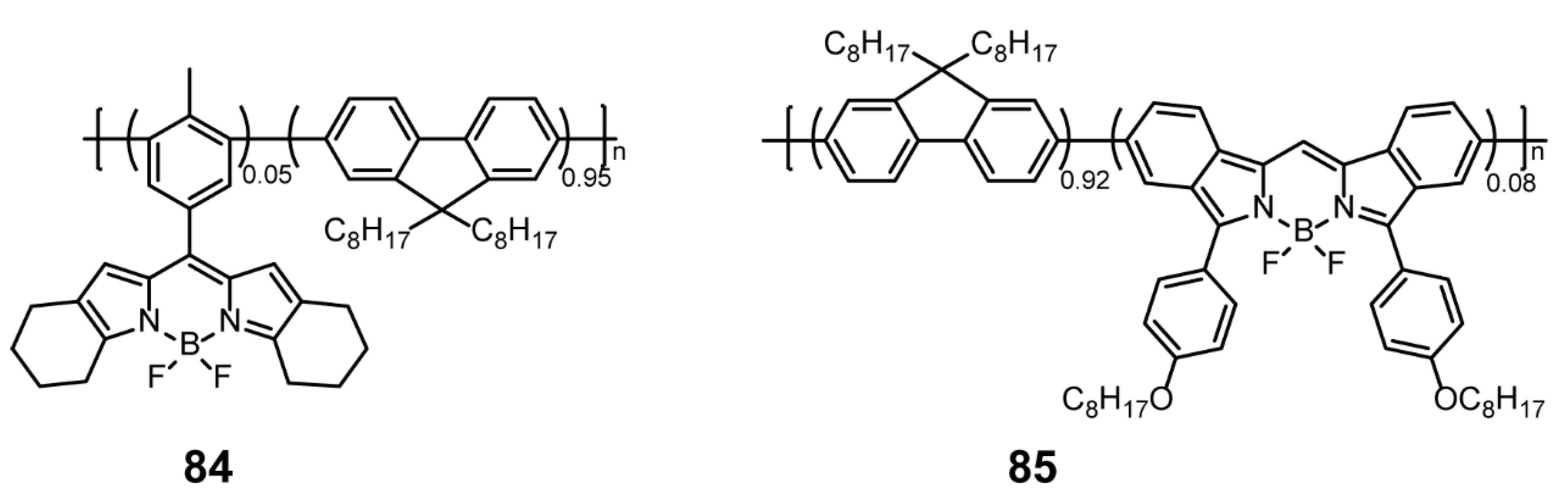
| Structures of BODIPY-Based Conjugated Polymers | Synthetic Methods | Compounds | References | |
|---|---|---|---|---|
| Linear/Coiled Conjugated Polymers | BODIPY as Backbone Units | Stille Cross-coupling Reaction | 4, 5, 22, 23 | [31,49] |
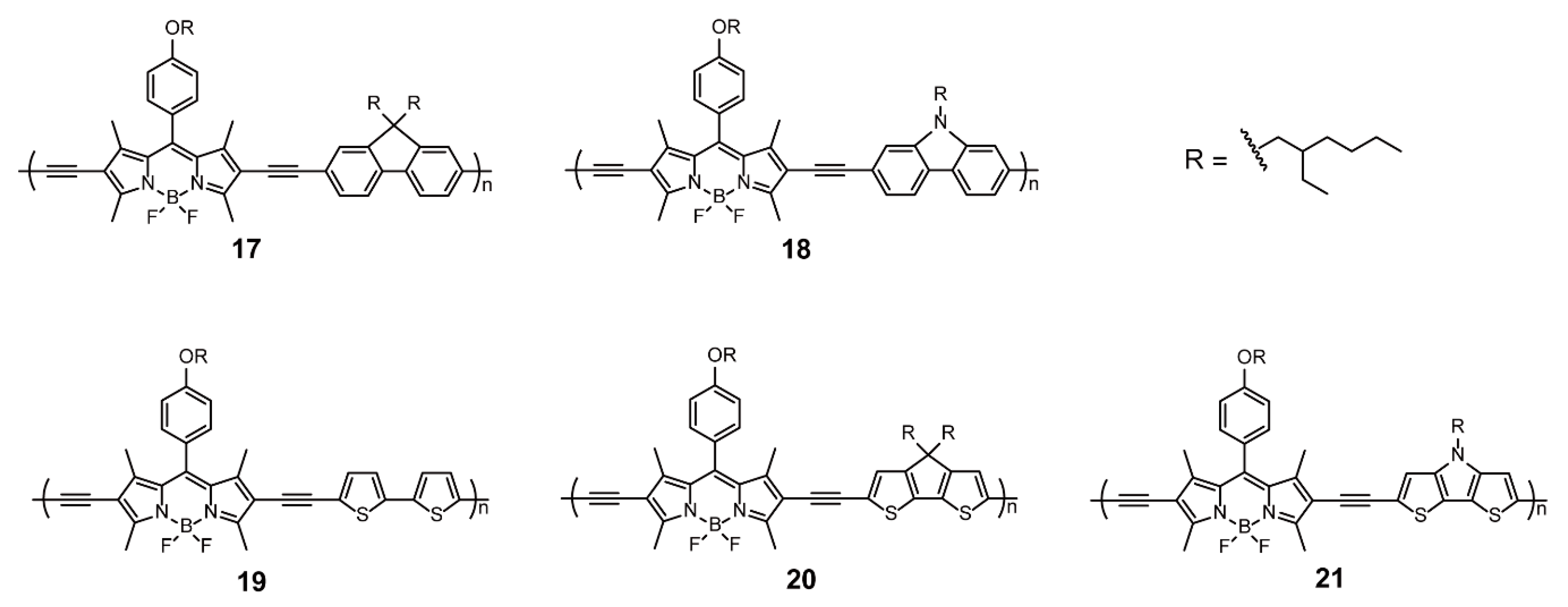
| Sonogashira Cross-coupling Reaction | 1–3, 6, 10–14, 17–21 | [30,31,34] | ||
| Oxidation Coupling | 15, 16 | [40] | ||
| Electrochemical Polymerization | 7–9 | [23,25,33] | ||
| BODIPY as Pendants Side Chains | Suzuki Cross-coupling Reaction | 24–26 | [54] | |
| Coordination Polymerization | 27–29 | [55] | ||
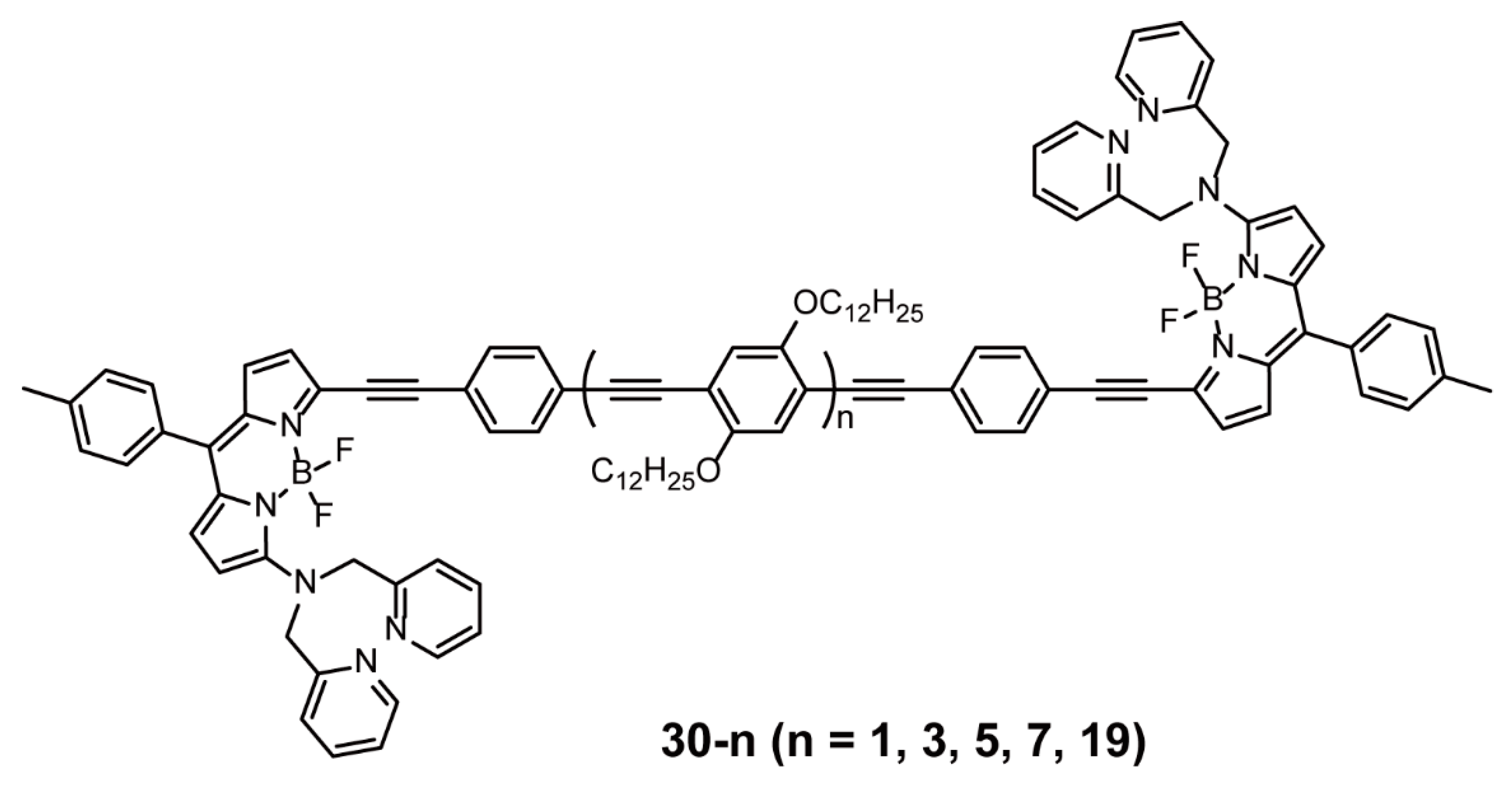
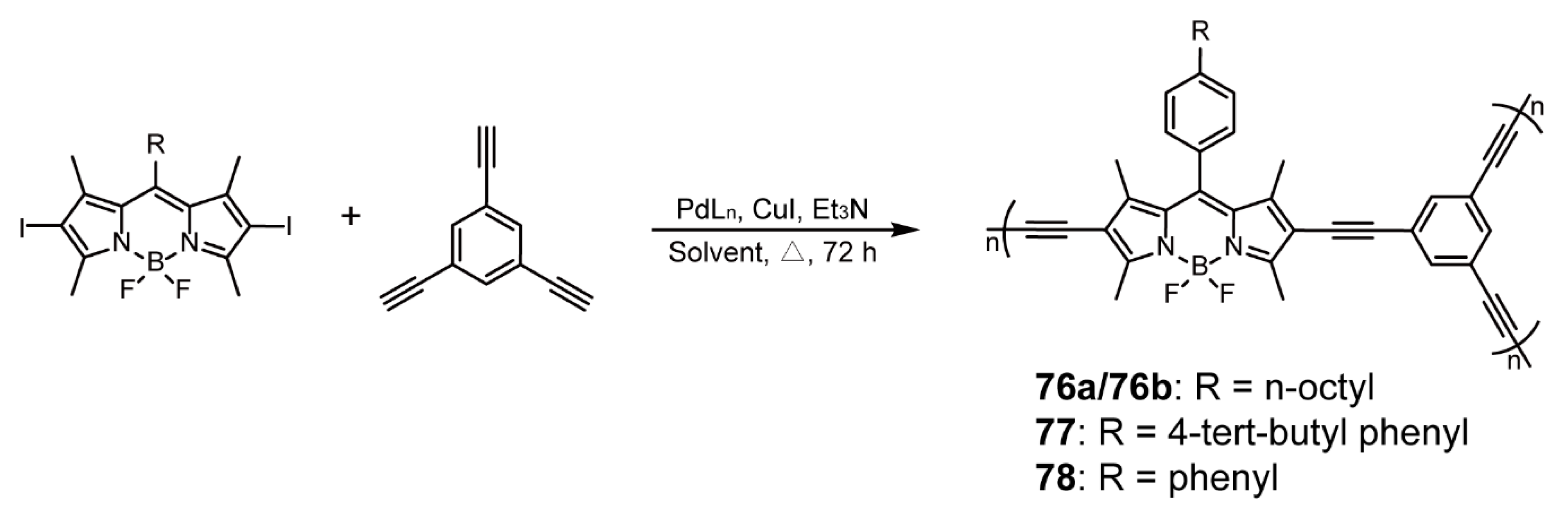
| BODIPY as End Groups | Sonogashira Cross-coupling Reaction | 30-n | [56,57] | |
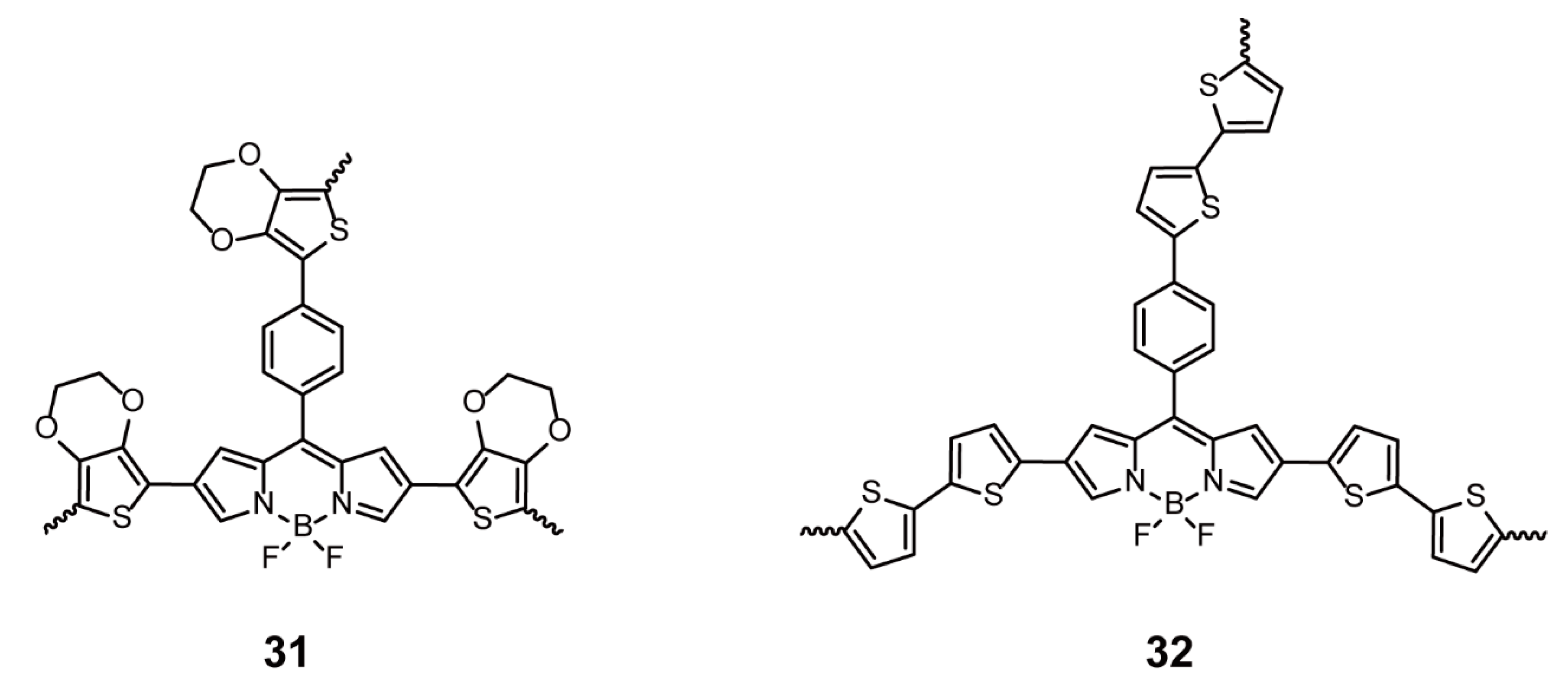
| Porous Conjugated Polymers | Electrochemical Polymerization | 31, 32 | [24] | |
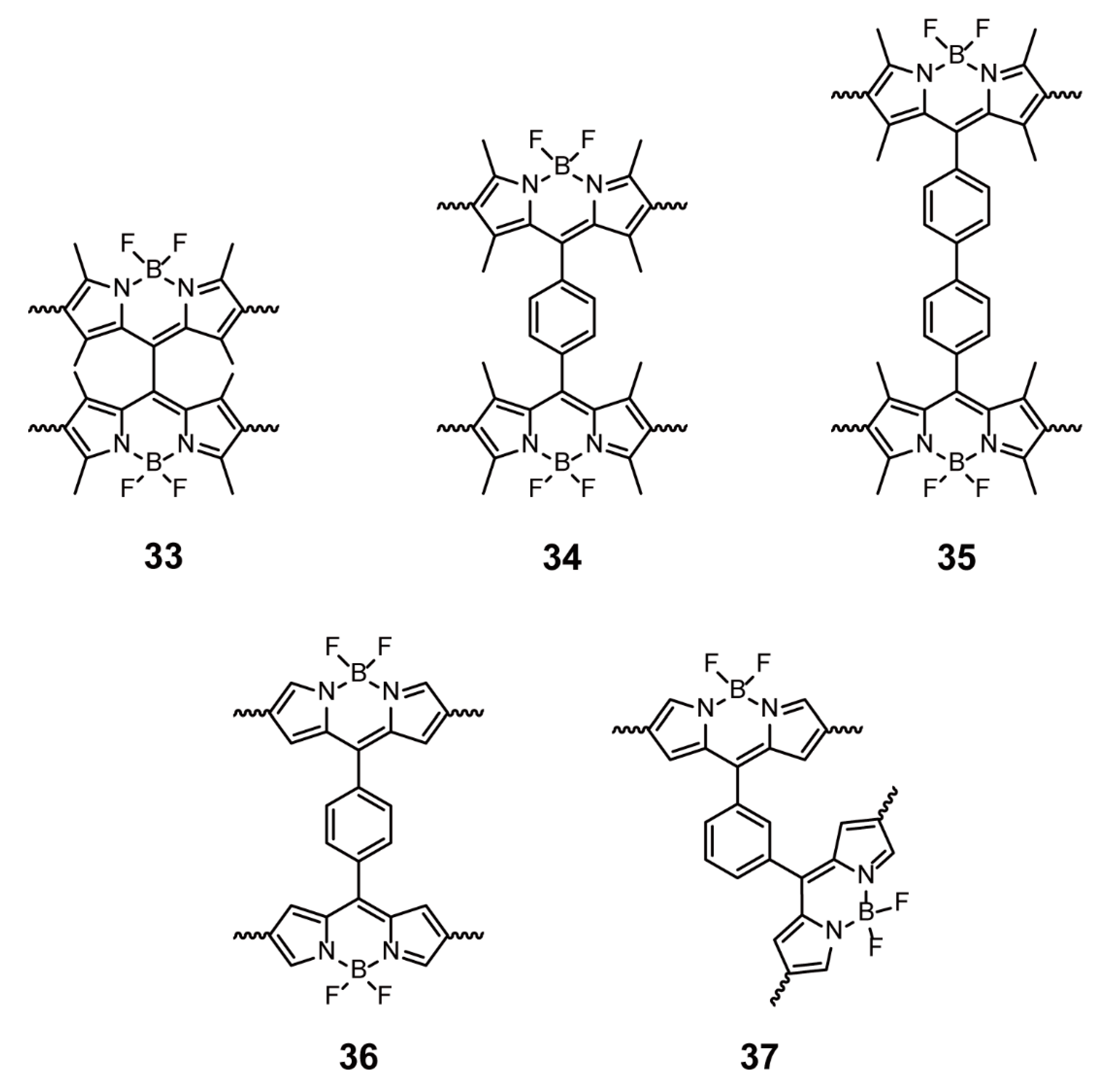
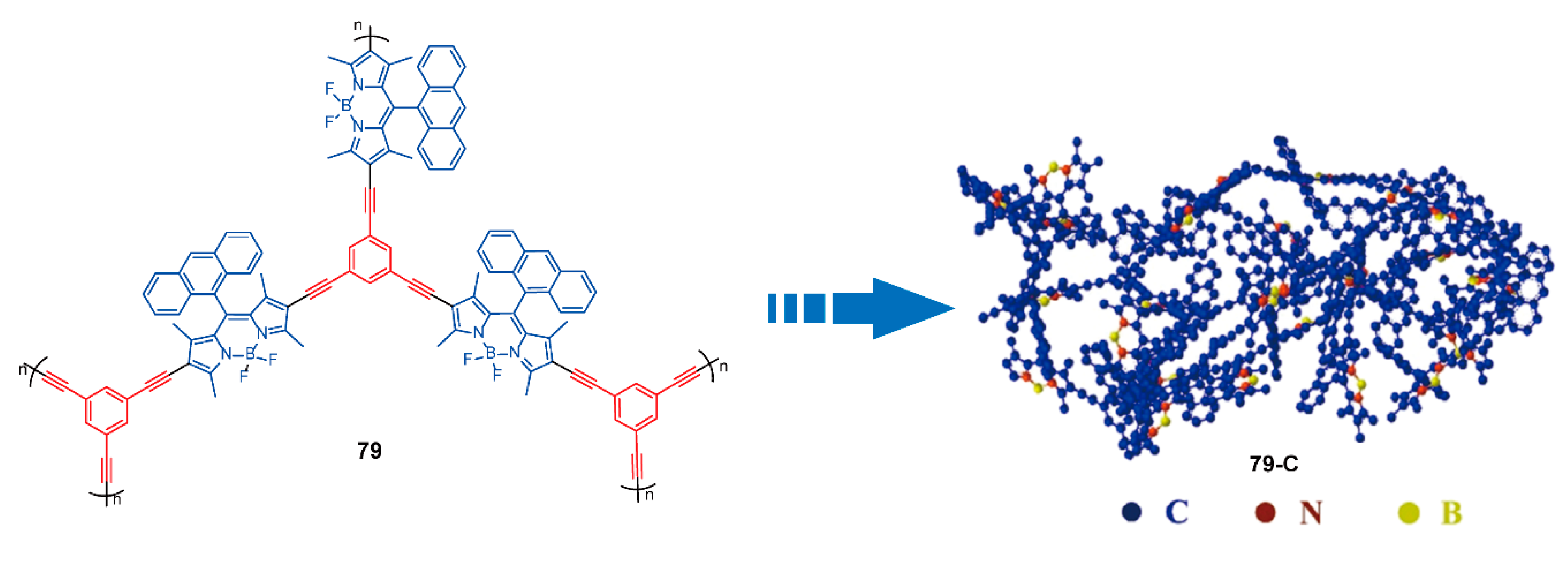
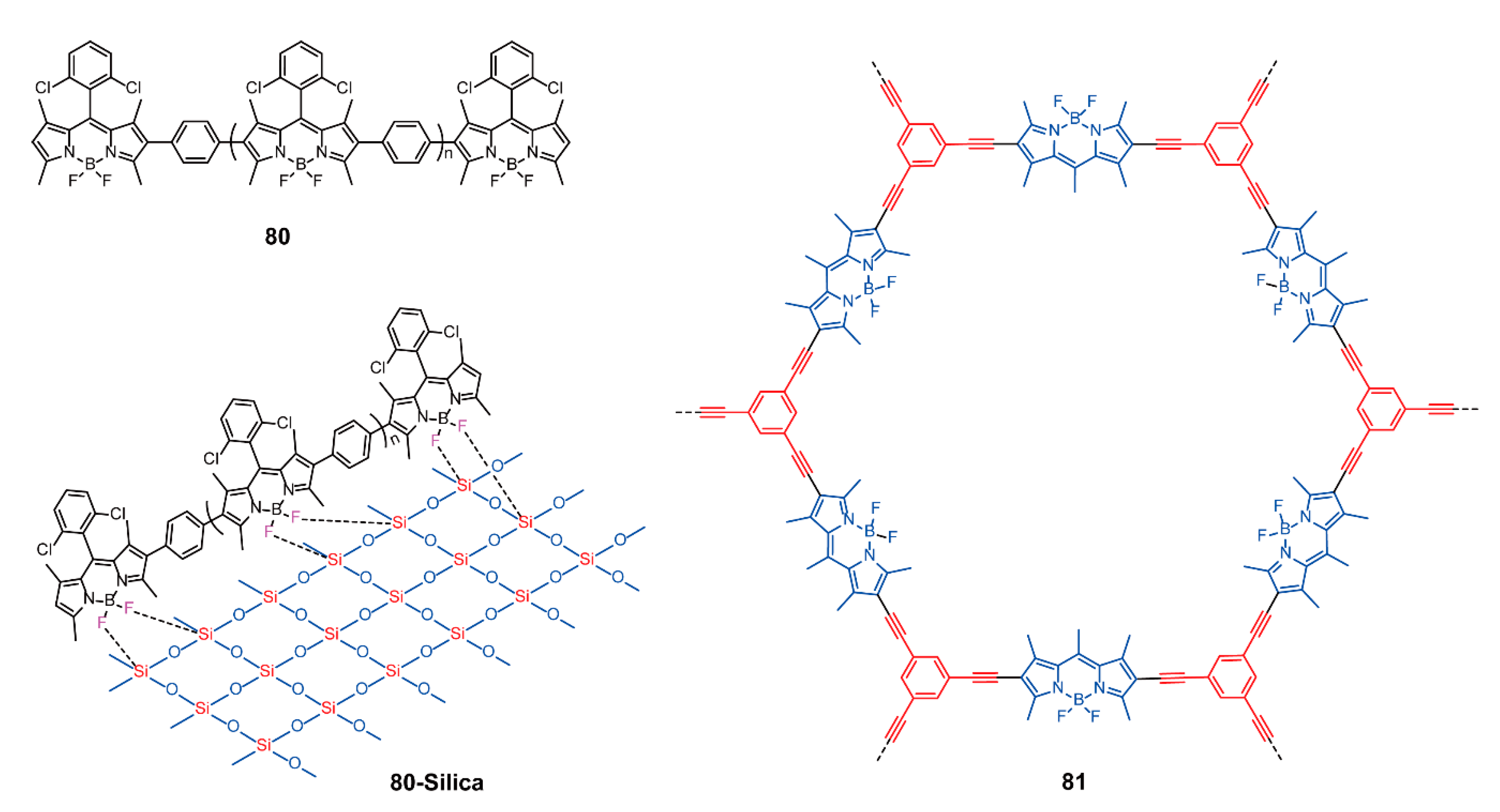
| Friedel-Crafts cross-coupling reactions | 33–37 | [65,66] | ||
| Other Structures | Crosslinked Polymers | Yamamoto Cross-coupling Reaction | 62 | [91] |
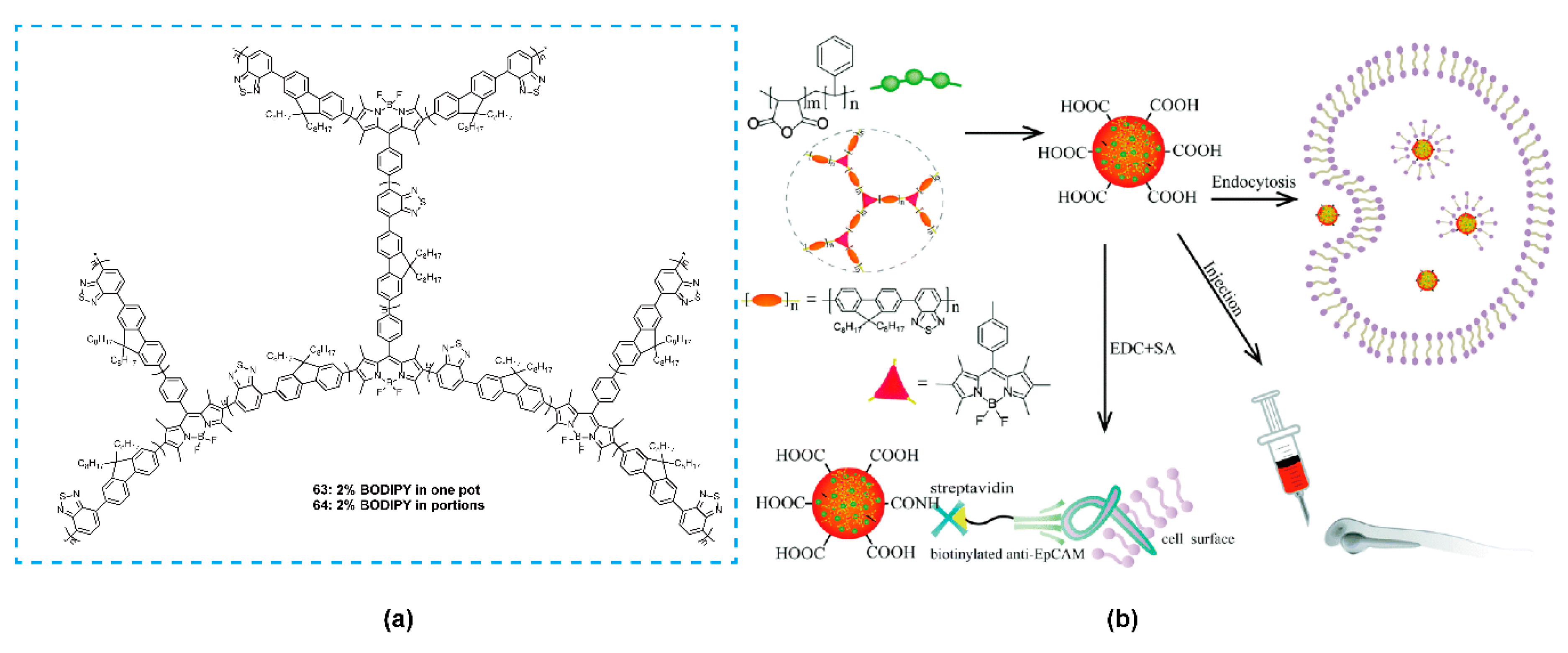
| Branched Polymers | Suzuki Cross-coupling Reaction | 63, 64 | [93] | |
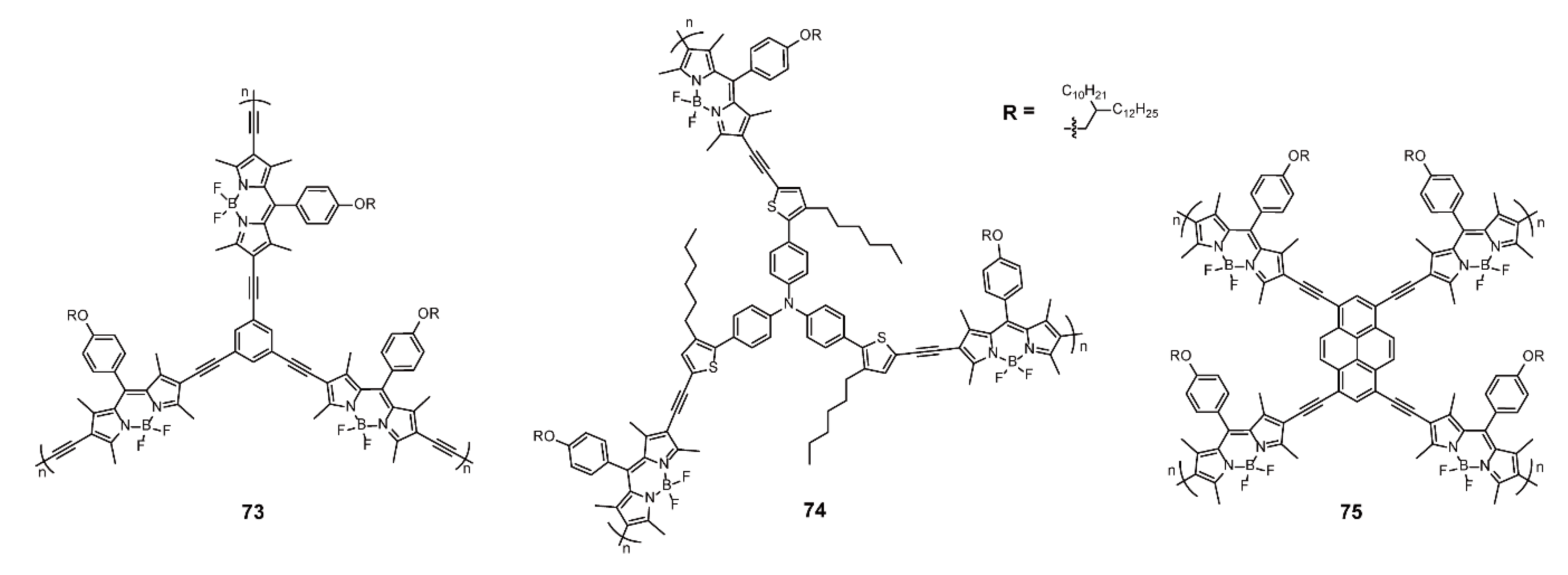
| Sonogashira Cross-coupling Reaction | 73–75 | [98] | ||
| Functional Applications of BODIPY-Based Conjugated Polymers | Synthetic Methods | Compounds | References | |
|---|---|---|---|---|
| Optoelectronic Materials | Solar Cells | Stille Cross-coupling Reaction | 38, 42–49 | [75,77,78,81] |
| Sonogashira Cross-coupling Reaction | 39–41 | [74,76] | ||
| Organic Thin-film Transistors | Stille Cross-coupling Reaction | 50–56 | [82,83] | |
| Sonogashira Cross-coupling Reaction | 57 | [84] | ||
| Memory Devices | Sonogashira Cross-coupling Reaction | 58 | [87] | |
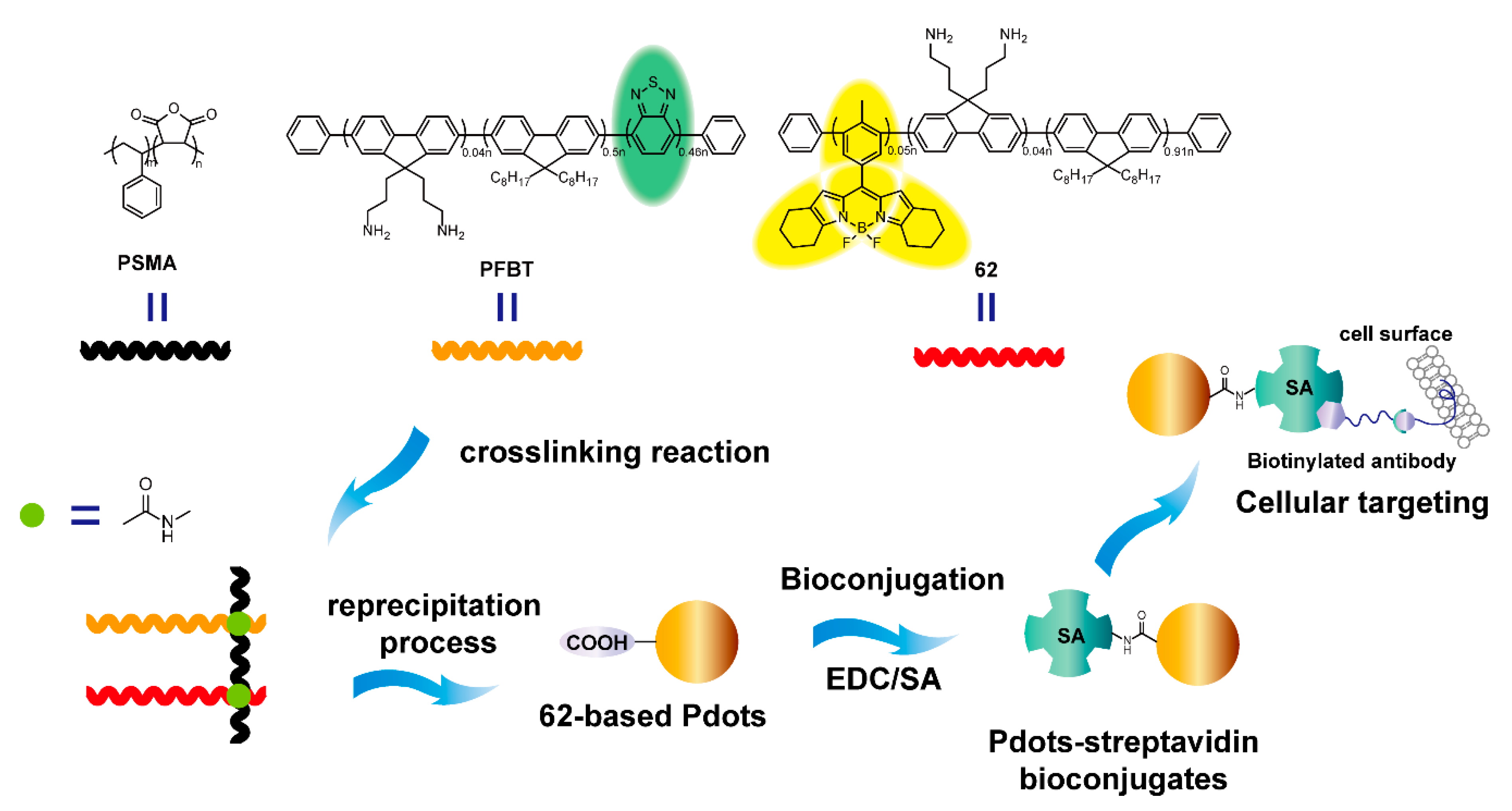
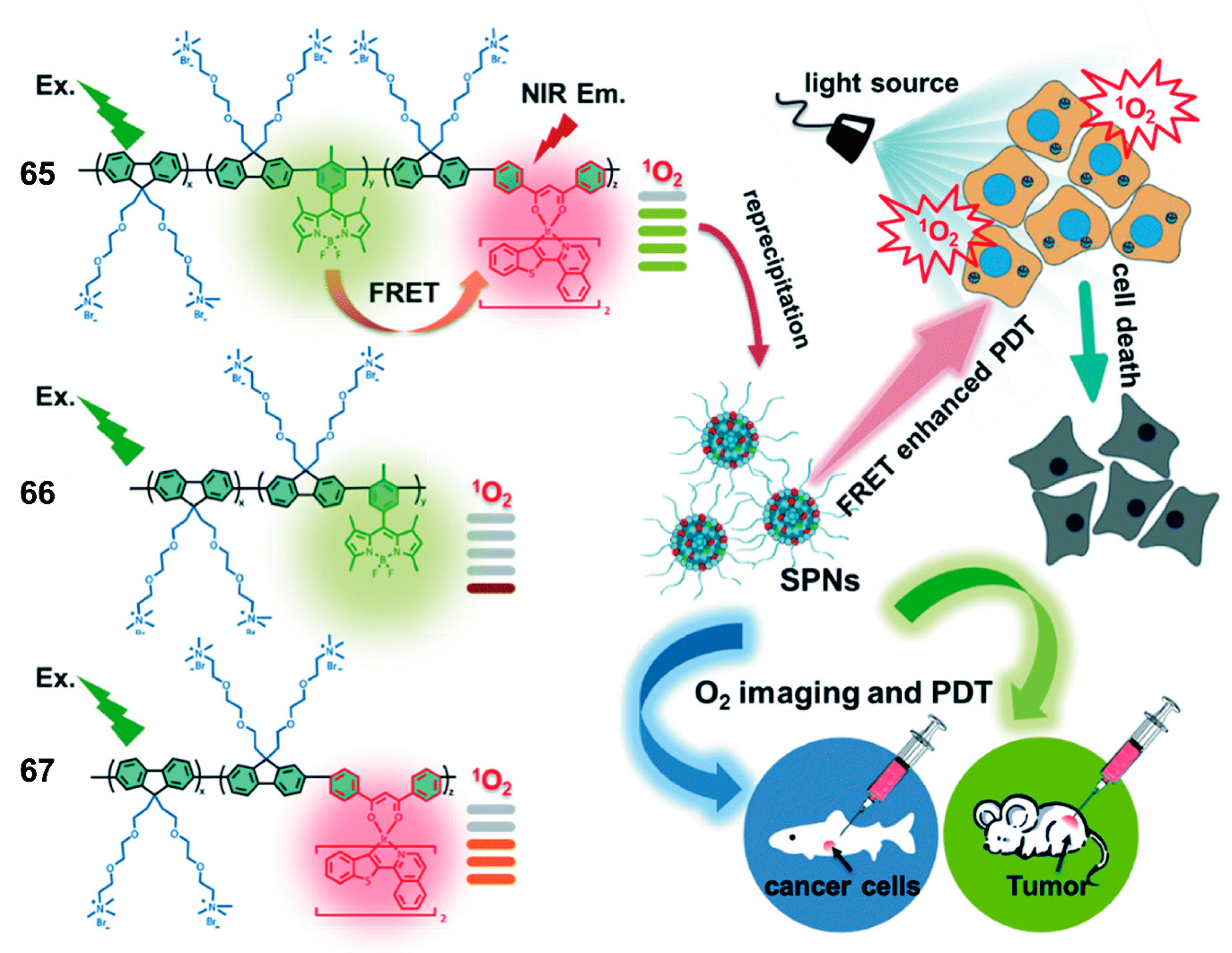
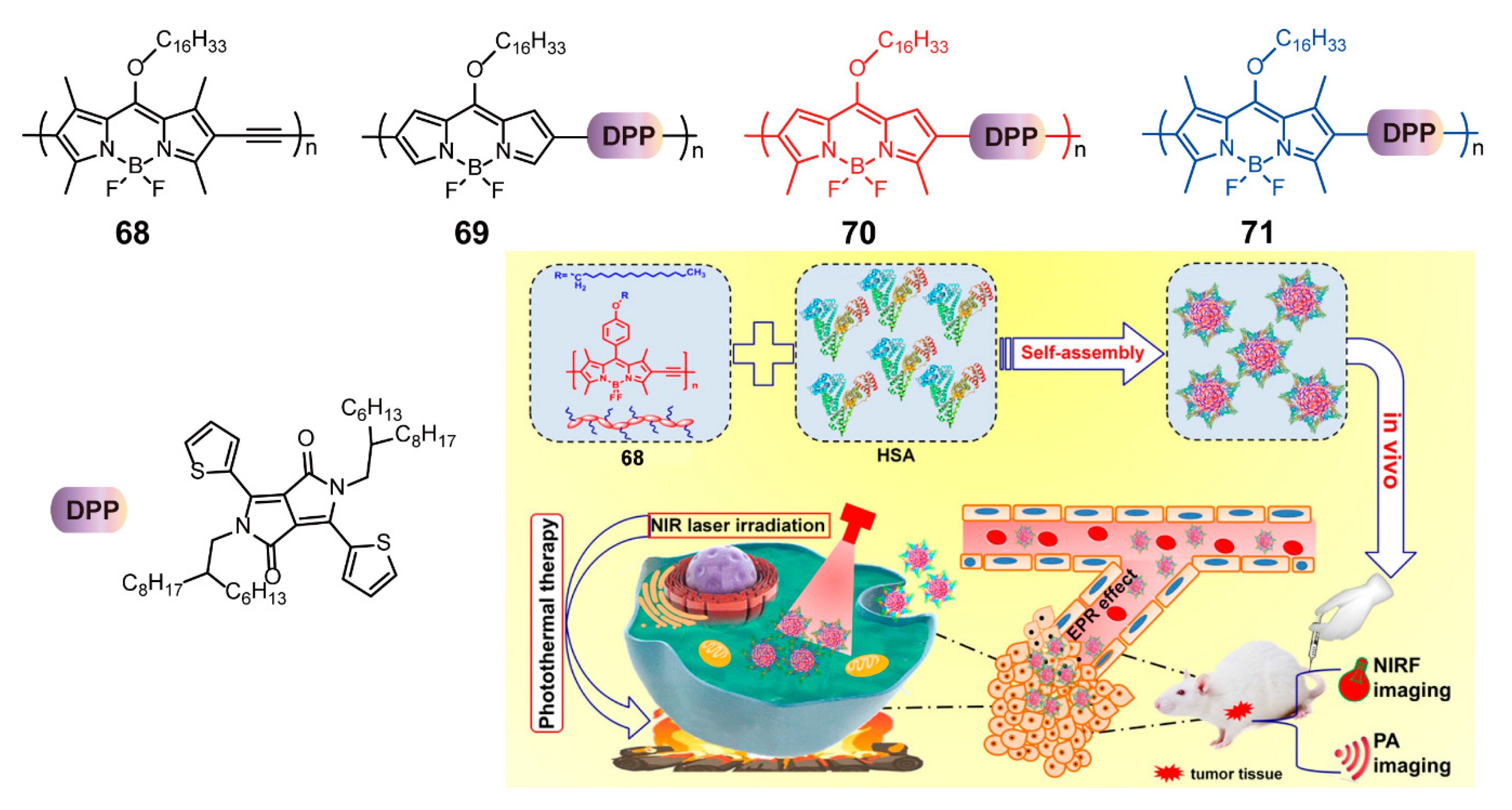
| Bioimaging and Biotherapy | Suzuki Cross-coupling Reaction | 59, 60, 63–66, 69–91 | [90,92,93,94] | |
| Sonogashira Cross-coupling Reaction | 61, 68 | [90,95] | ||
| Yamamoto Cross-coupling Reaction | 62 | [91] | ||
| Sensor | Sonogashira Cross-coupling Reaction | 72–75 | [96,98] | |
| Gas/Energy Storage | Sonogashira Cross-coupling Reaction | 76–79 | [102,103] | |
| Other Applications | Suzuki Cross-coupling Reaction | 80 | [104] | |
| Sonogashira Cross-coupling Reaction | 81–83 | [105,107] | ||
| Yamamoto Cross-coupling Reaction | 84, 85 | [108] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Zhang, J.; Hong, Z.; Qiu, H.; Li, Y.; Yin, S. Architectures and Applications of BODIPY-Based Conjugated Polymers. Polymers 2021, 13, 75. https://doi.org/10.3390/polym13010075
Fan Y, Zhang J, Hong Z, Qiu H, Li Y, Yin S. Architectures and Applications of BODIPY-Based Conjugated Polymers. Polymers. 2021; 13(1):75. https://doi.org/10.3390/polym13010075
Chicago/Turabian StyleFan, Yiqi, Jinjin Zhang, Zhouyi Hong, Huayu Qiu, Yang Li, and Shouchun Yin. 2021. "Architectures and Applications of BODIPY-Based Conjugated Polymers" Polymers 13, no. 1: 75. https://doi.org/10.3390/polym13010075
APA StyleFan, Y., Zhang, J., Hong, Z., Qiu, H., Li, Y., & Yin, S. (2021). Architectures and Applications of BODIPY-Based Conjugated Polymers. Polymers, 13(1), 75. https://doi.org/10.3390/polym13010075