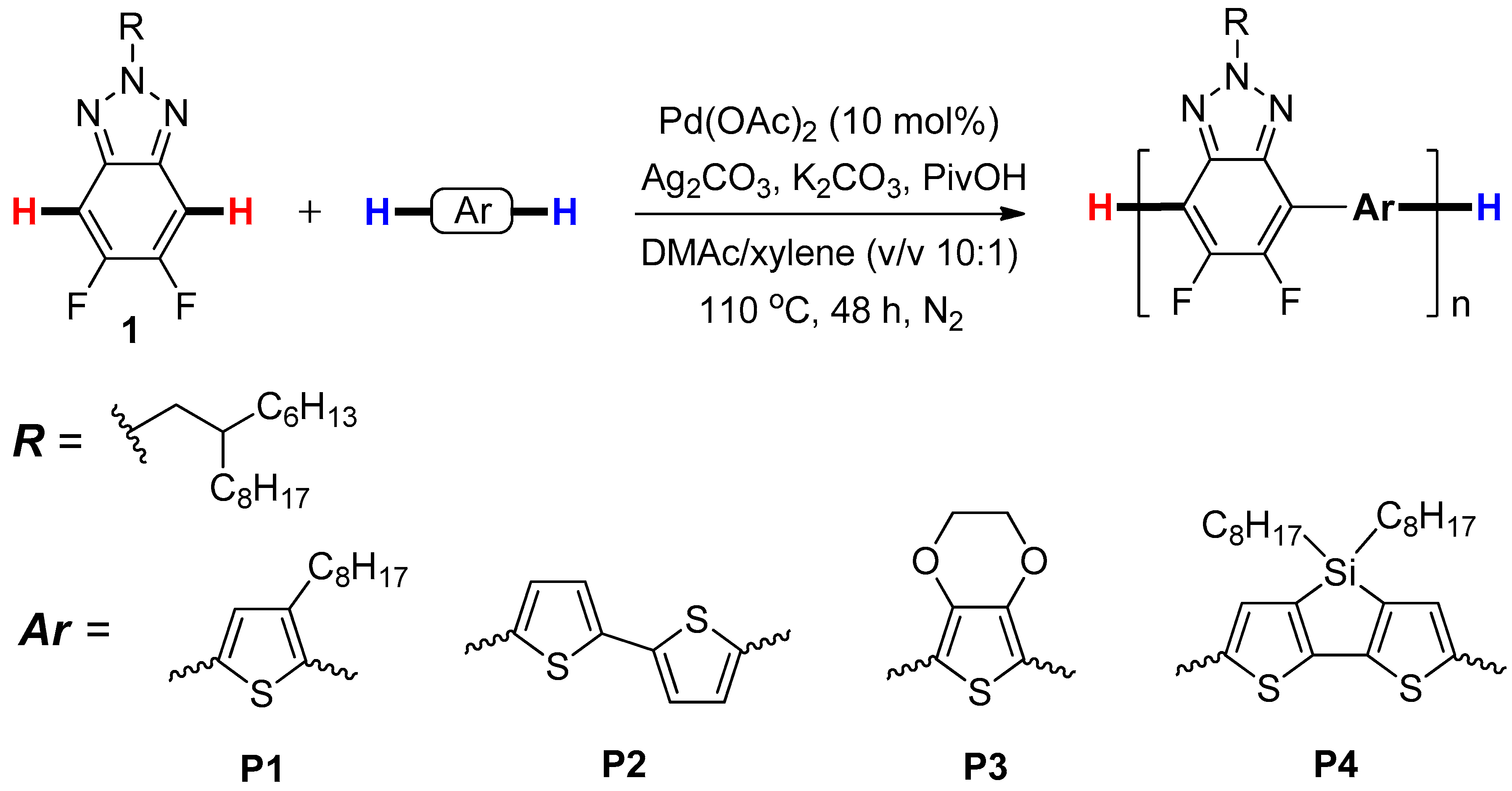
Abstract
Four D-π-A conjugated polymers, namely P1–P4, which contain benzotriazole building blocks in their backbone as acceptor, are synthesized via palladium-catalyzed direct C-H cross-coupling polycondensation of 5,6-difluorobenzotriazole with different thiophene derivatives, including 3-octylthiophene, 2,2’-bithiophene, thieno[3,4-b][1,4]dioxine, and 4,4-dioctyl-4H-silolo-[3,2-b:4,5-b’]dithiophene as donor units, respectively. Taking the polymer P1 as an example, the chemical structure of the polymer is demonstrated by 1H and 19F NMR spectra. The optical, electrochemical, and thermal properties of these polymers are assessed by UV–vis absorption and fluorescence spectroscopy, cyclic voltammetry (CV), and thermal gravimetric analysis (TGA), respectively. DFT simulations of all polymers are also performed to understand their physicochemical properties. Furthermore, P1 and P2, which have relatively higher molecular weights and better fluorescent quantum efficiency than those of P3 and P4, are utilized as lighting emitters for organic light-emitting diodes (OLEDs), affording promising green and red luminescence with 0.07% and 0.14% of maximum external quantum efficiency, respectively, based on a device with an architecture of ITO/PEDOT:PSS/PTAA/the polymer emitting layer/TPBi/LiF/Al.
1. Introduction
Organic light-emitting diodes (OLEDs) have received much attention both in academia and industry owing to their wide applications in fullcolor displays and solid-state lighting. π-Conjugated polymers have many unique photophysical properties that are inherited from the delocalized electronic structures of their backbones and, thus, they exhibit promising advantages, such as solution-processing, light-weight, low-cost, and facile preparation of flexible devices in various functional applications, such as OLEDs, solar cells, field-effect transistors (FETs), photodetectors, etc. [1,2,3,4,5,6].
A variety of π-conjugated polymers have been synthesized based on reliable coupling methods including Suzuki–Miyaura [7], Stille [8], and Kumada–Corriu couplings [9], etc. For example, Todd and coworkers used brominated 5,6-difluorobenzotriazole and 4-hexylthiophene as starting materials and iPrMgCl·LiCl as Grignard reagents to synthesize the alternating polymer poly(5,6-difluorobenzotriazole-alt-4-hexylthiophene) via Kumada catalyst-transfer polycondensation [10]. In 2014, several benzotriazole-containing π-conjugated polymers were also achieved through Stille coupling reaction of 4,7-dibromo-5,6-difluorobenzotriazole with 2,5-bis(trimethylstannyl)thiophene derivatives for polymer field-effect transistors (FETs) applications [11]. These synthetic methods above for benzotriazole-based π-conjugated polymers generally require halogenations and/or organometallic pre-functionalization steps of the corresponding monomers prior to polymerization. It is often difficult to synthesize and purify the metalated monomers due to their instability. In addition, in some cases, these polymerization processes can produce a toxic byproduct, for example, trialkyltin bromide. To circumvent these problems, direct arylation polymerization (DArP) has been developed, exhibiting several advantages, including fewer synthetic steps, easier purification of monomers, and reduced environmental pollution. For instance, Luscombe et al. successfully synthesized the alternating copolymer of 5,6-difluorobenzotriazole and 2,5-dithienylsilole with Mn = 10,000 via DArP [12]. Most recently, transition-metal catalyzed direct C-H coupling strategy was proposed and has been applied for preparing various π-conjugated polymers, including our work [13,14,15,16,17]. This synthetic protocol fully avoids the steps of pre-functionalized starting monomers, and allows straightforward access to π-conjugated polymers [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. However, this method is mainly limited to the homo-coupling of some aromatic monomers such as thienopyrroledione (TPD) [13], bithiazole [14], benzodiimidazole [25,26,27], and ester-functionalized thiophene [16,17,18,19] at present. Obviously, it is more challenging to achieve the highly selective cross-coupling of two different aromatic monomers as there are many possible multiple coupling patterns.
In this work, a series of D-π-A type conjugated polymers containing benzotriazole as acceptor units were successfully synthesized via Pd-catalyzed direct C-H cross-coupling polycondensation reaction of 5,6-difluorobenzotriazole with thiophene derivatives. Furthermore, the as-prepared polymers’ physicochemical properties and light-emitting performance were also assessed.
2. Experimental
2.1. Materials
5,6-Difluoro-2-(2-hexyldecyl)-2H-benzo[d][1,2,3]triazole, 3-octylthiophene, 2,2’-bithiophene, thieno[3,4-b][1,4]dioxine, 4,4-dioctyl-4H-silolo[3,2-b:4,5-b]dithiophene, palladium acetate (Pd(OAc)2), pivalic acid (PivOH), potassium carbonate (K2CO3), and silver carbonate (Ag2CO3) were purchased from Zhengzhou HQ Material Co., Ltd. (Zhengzhou, China) for direct use. N,N-Dimethylacetamide (DMAc) and xylene were obtained from J&K Chemical (Beijing, China) and used without further purification. Poly(sodium-p-styrenesulfonate) (PSS), poly(2,3-dihydrothieno-1,4-dioxin) (PEDOT), poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] (PTAA), and 1,3,5-tris(1-phenyl-1H-benzimidazol-2-yl)benzene (TPBi) were provided by Xi’an Polymer Light Technology Co. (Xi’an, China).
2.2. Instruments
1H and 13C NMR spectra of polymers were measured on a Bruker AV400 at 25 °C utilizing the residual solvent peak as reference. Molecular weights of polymers were determined by gel permeation chromatography (GPC) on Waters 1525 equipped with Waters Styragel HT gel columns using tetrahydrofuran (THF) as eluent at 35 °C and monodisperse polystyrene as standard. Thermogravimetric analyses (TGA) were carried out on a Frontier Mid-IR FTIR/STA6000-TL9000-Clarus SQ8 instrument with a 10 °C·min−1 heating rate under a purified nitrogen gas flow. Optical properties of polymers including UV–vis absorption and photoluminescence (PL) spectra were obtained on a Shimadzu UV-2550 and Hitachi F-4600, respectively. Solid-state samples for the test of photophysical property were prepared by spin-casting of polymers chloroform solutions on quartz plates. Optical bandgaps were calculated based on the onset of the absorption spectra of the thin film. Cyclic voltammetry (CV) measurements are carried out on a LK98B II electrochemical analyzer at room temperature with the scan rate was 100 mV s−1. In this experiment, a conventional three-electrode configuration was adopted, i.e., a glassy carbon electrode as the working electrode, a saturated calomel electrode (SCE) as the reference electrode, and a Pt wire as the counter electrode. Acetonitrile, freshly prepared (CH3CN) by distilling from calcium hydride (CaH) under dry N2, was used as solvent. Tetrabutylammonium phosphorus hexafluoride (Bu4NPF6) with a concentration of 0.1 M in CH3CN was applied as the support electrolyte.
2.3. Synthesis of Polymer
The polymers were prepared according to a similar procedure as reported in our recent work [27]. In detail, 5,6-difluoro-2-(2-hexyldecyl)-2H-benzo[d][1,2,3]triazole (1) (113.7 mg, 0.3 mmol), 3-octylthiophene (58.8 mg, 0.3 mmol) were dissolved in 3 mL of DMAc/xylene (v/v, 10/1). Silver carbonate (248.2 mg, 0.9 mmol) and potassium carbonate (124.4 mg, 0.9 mmol), pivalic acid (61.3 mg, 0.6 mmol), Pd(OAc)2 (6.7 mg, 10 mol%) were added into the above solution. The mixture was stirred for 48 h at 110 °C under N2. After the mixture was cooled to room temperature, it was poured into cold methanol (100 mL). The precipitate was collected and subsequently subjected to successive Soxhlet extractions with anhydrous methanol and hexanes for 24 h to remove possible catalytic residues and oligomers. The residue was then extracted with chloroform for another 12 h. The CHCl3 solution was concentrated to about 2 mL using a rotary evaporator and was then poured into methanol (60 mL). The precipitate was collected and dried under vacuum at 30 °C for 12 h to provide P1 as a black solid (159 mg, 93 % yield). Mn = 22.3 kDa, PDI = 1.82 (GPC). 1H NMR (400 MHz, CDCl3) δ 8.38 (m, J = 10.5 Hz, 1H), 4.86-4.37 (m, 2H), 2.70 (s, 2H), 2.28 (s, 1H), 1.68 (s, 2H), 1.45-1.04 (m, 34H), 0.84 (s, 9H).
P2, P3, and P4 were synthesized based on a similar procedure as that for P1, except for the use of different monomers. P2: 131 mg, 82% yield, Mn = 28.1 kDa, PDI = 1.22 (GPC). 1H NMR (400 MHz, CDCl3) δ 8.21 (m, 1H), 6.98 (br, 3H), 4.71 (d, 2H), 2.25 (s, 1H), 1.32 (d, 32H), 0.86 (s, 8H). P3: 15.6 mg, 10% yield, Mn = 15.0 kDa, PDI = 1.59 (GPC). 1H NMR (400 MHz, CDCl3) δ 4.75-3.96 (m, 6H), 2.25 (s, 1H), 1.41 (d, 24H), 0.87 (d, J = 7.2 Hz, 6H). P4: 47.8 mg, 20% yield, Mn = 3.5 kDa, PDI = 1.34 (GPC). 1H NMR (400 MHz, CDCl3) δ 6.98 (br, J = 15.4 Hz, 2H), 4.60 (d, 2H), 2.25 (s, 1H), 1.73-0.97 (m, 52H), 0.86 (s, 12H).
2.4. Device Fabrication and Characterization
The OLED devices with P1 and P2 as a light-emitting layer were fabricated with the following configuration: ITO/PSS:PEDOT/PTAA/polymer light-emitting layer/TPBi/LiF/Al.
In this experiment, indium tin oxide (ITO) glass with a conductivity of 10 Ω/square was first pre-cleaned with acetone and ethanol under ultrasound and was subsequently treated in an ultraviolet–ozone chamber. A thin 40 nm-thick PSS-PEDOT layer was spin-coated onto the treated ITO glass which was placed on a hot plate at 3000 rpm for 10 min at 110 °C. The coated ITO glass was then transferred to a glovebox filled with N2 and re-dried for 10 min at 110 °C. The solution of PTAA (5 mg) in 1 mL of DMF was then spin-coated onto the top of PSS:PEDOT layer on ITO to form an electron blocking layer with a thickness of 30 nm. After the above substrate was baked for 20 min at 100 °C, the toluene solution of P1 or P2 (10 mg/mL) was spin-coated onto PTAA layer to form a uniform 40 nm-thick light-emitting layer, which was followed by an annealing treatment for 10 min at 90 °C. Finally, using conventional thermal evaporation under vacuum, TPBi, LiF, and Al were deposited onto the surface of the active layer in sequence with the thicknesses of 30, 1, and 120 nm, respectively. The devices provided an active area of 5 × 3 mm2. Based on the above devices, the electroluminescence (EL) spectra, current density–voltage (J–V) and luminance–voltage (L–V) were obtained on a Keithley 2400 Source Meter system. The luminance of OLEDs was measured with a PhotoResearch SpectraScan PR-650 Colorimeter.
3. Results and Discussion
3.1. Synthesis and Characterization of Polymers
As depicted in Scheme 1, P1–P4 were prepared by Pd-catalyzed direct C-H cross-coupling reaction of 5,6-difluoro-2-(2-hexyldecyl)-2H-benzotriazole (1) with four different thiophene derivatives, including 3-octylthiophene, 2,2’-bithiophene, thieno[3,4-b][1,4]dioxine, and 4,4-dioctyl-4H-silolo-[3,2-b:4,5-b’]dithiophene under the optimized conditions as reported in our recent work [29], namely, Pd(OAc)2 (10 mol%) as catalyst, Ag2CO3 (3.0 equiv) as oxidant, K2CO3 (3.0 equiv) as base, PivOH (2.0 equiv) as additive, and DMAc/xylene (v/v, 10/1) as solvents at 110 °C for 48 h. A branched side chain (2-hexyldecyl) was attached to benzotriazole units, aiming to improve the solubility of compound 1 and the resultant copolymers in the common organic solvents, such as DMAc, DMF, CHCl3, etc. [11]. The results of the polycondensation reactions and the thermophysical properties of P1–P4 were shown in Table 1.
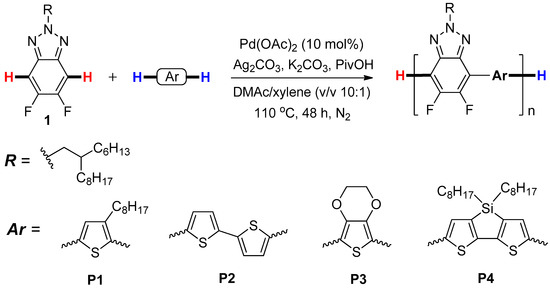
Scheme 1.
Synthetic routes of the polymers P1–P4.

Table 1.
Results of the polycondensation and the thermostability of P1–P4.
The polycondensation of 5,6-difluoro-2-(2-hexyldecyl)-2H-benzotriazole (1) with 3-octylthiophene provides the polymer P1 with an average molecular weight of Mn = 22,300 in an excellent yield (93%). In the case of 2,2’-bithiophene as donor units, P2 with the highest molecular weight of Mn = 28,100 was obtained in a good yield (82%). By contrast, the polymerization reaction of 1 with thieno[3,4-b][1,4]dioxine mainly yielded an insoluble solid in common organic solvents, and only a small number of products can be isolated by Soxhlet extractions with CHCl3 to provide a total yield of about 10%. This result may be due to the cross-linking reaction in multiple C-H systems [30,31]. Dithienosilole cores are common building blocks of various active materials used in photovoltaics, FETs, and OLEDs, etc. [32,33,34,35,36] because of their strong donating electron ability, photostability, and chemical stability. Under the same reaction conditions as that for P1–P3, the polycondensation of 1 with 4,4-dioctyl-4H-silolo-[3,2-b:4,5-b’]dithiophene yields a soluble polymer P4 in CHCl3 with low yield (about 20%) (Table 1, entry 4), which is similar to the case of P3. Most of the as-prepared polymers exhibit good thermal stability with the degradation temperatures at 5% weight loss are 385, 317, and 392 °C for P1, P2, and P4, respectively (Table 1), which is suitable for the applications in the semiconductor devices.
3.2. Structural Characterization
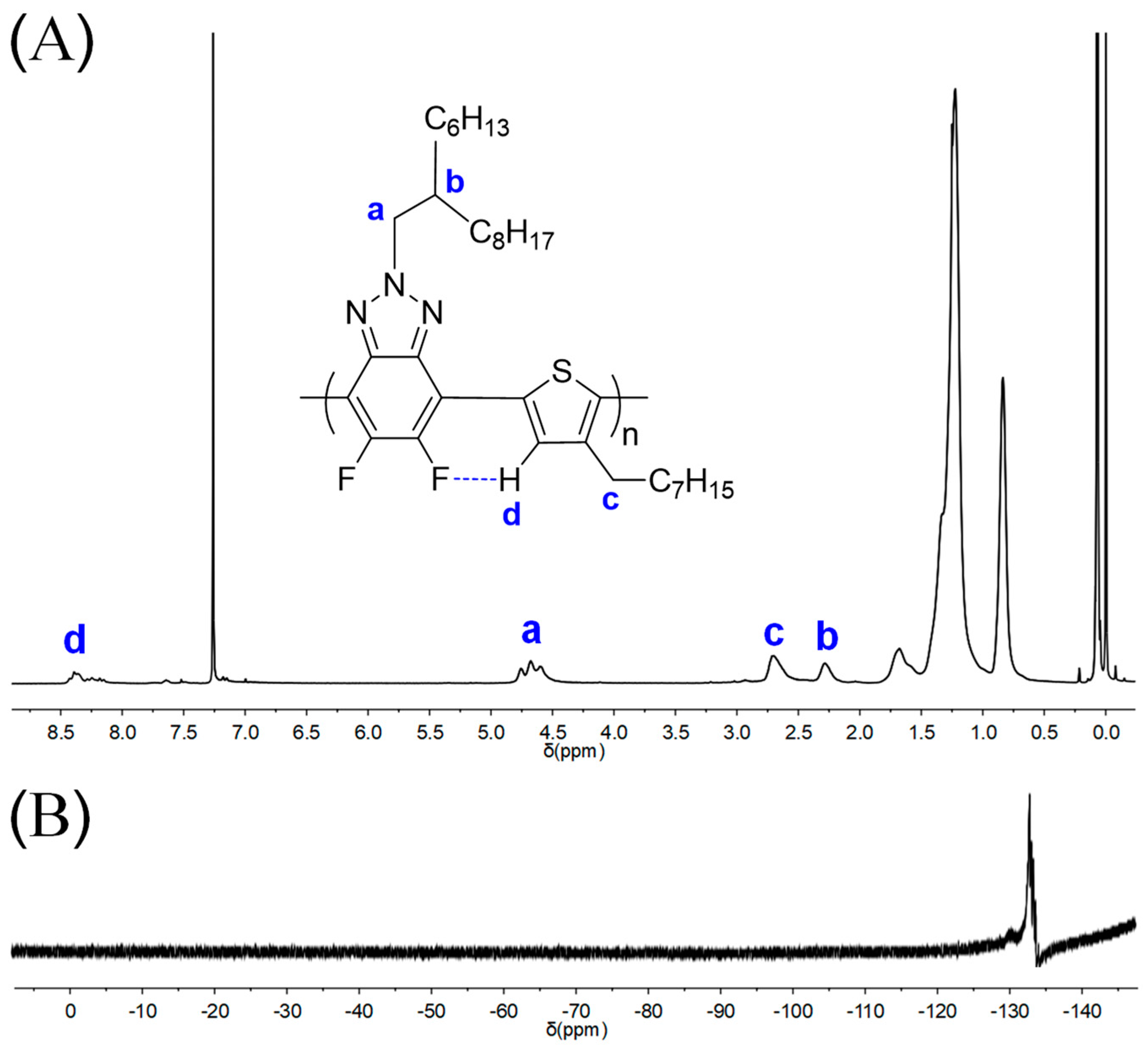
The chemical structure of polymers was determined by 1H and 19F NMR spectra (Figure 1 and Figures S1–S8). Taking P1 as an example, all characteristic proton peaks corresponding to the benzotriazole and thiophene units can be observed as shown in Figure 1A, which is similar to the analogous polymers obtained via the Kumada method except for different alkyl side chains as reported by Bielawski and co-workers [10]. As seen in Figure 1A, the multiple peaks at 4.60–4.76 ppm (a in Figure 1A) and the broad peak at 2.28 ppm (b in Figure 1A) were assigned to the -CH2 groups close to the triazole ring and -CH signal of the branched groups, respectively. The peak at 2.70 nm (c in Figure 1A) corresponds to -CH2 groups of thiophene side chains. It was noted that the signal of aromatic hydrogen of thiophene units (d in Figure 1A) significantly shifted to low field compared with the corresponding thiophene monomer, which was possibly caused by F⋯H noncovalent interaction between the fluorine atoms on benzotriazole unit and the hydrogen atoms on thiophene units as demonstrated by our recent work [29] and the literature [11].
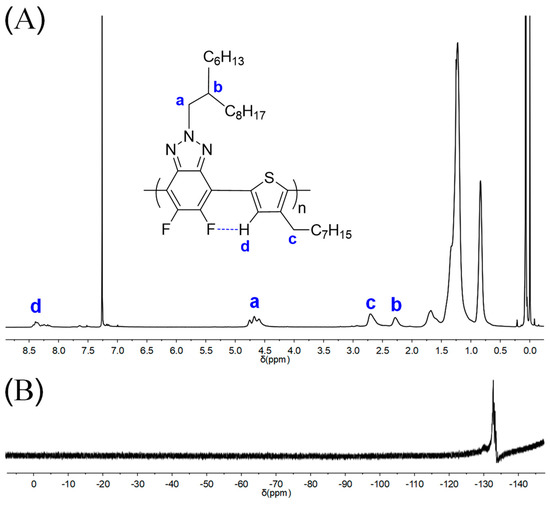
Figure 1.
1H NMR (A) and 19F NMR (B) of P1 in CDCl3.
Besides, 19F NMR was also applied to confirm the polymers’ chemical structure since it has many merits, including high sensitivity, little interference, a large range of chemical shifts, and similar structures that are not easy to overlap, etc. As depicted in Figure 1B, the 19F NMR of P1 displayed an obvious main peak at −133.0 ppm that corresponds to the fluorine atoms on the polymeric chains. The remaining weak signals could be assigned to the end groups, where the fluorine atoms are not chemically equivalent to those along the backbone [37]. All the NMR results of P1 suggest an alternating structure along the polymer main chain.
3.3. DFT Simulation
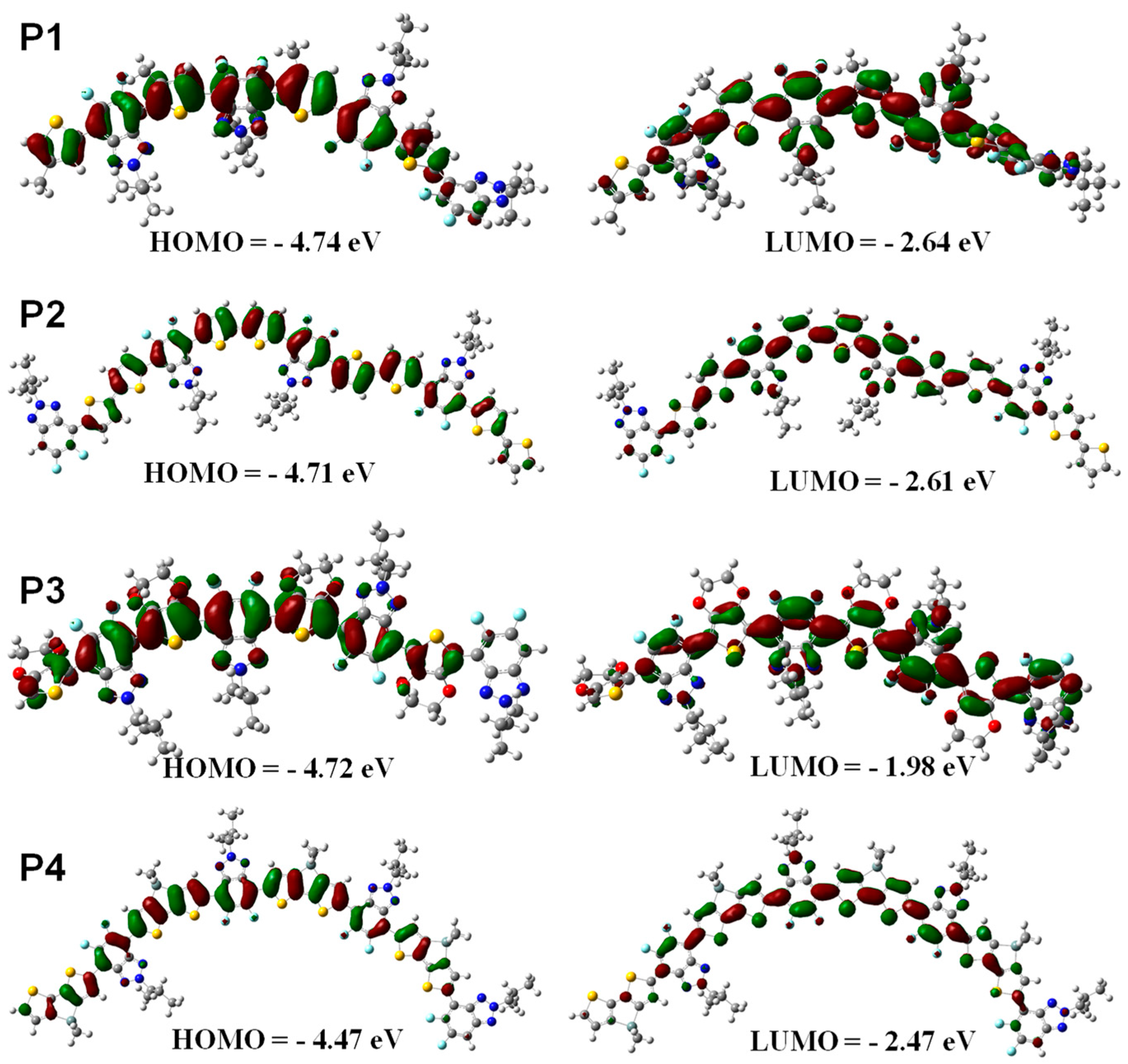
Density functional theory (DFT) simulation was performed at the B3LYP/6-31G* level using Gaussian 09 programs to check the effect of the structure of thiophene donor units on the polymer backbone on the HOMO and LUMO energy levels of the resultant polymers. In this study, four repeated D-A units were used and all side chains were replaced with methyl groups to simplify the calculation. The HOMO and LUMO level values were obtained by MO topologies on the basis of the optimized geometry. As shown in Figure 2, the contour plots indicated the effective separation of HOMO and LUMO levels for all polymers P1–P4, which is beneficial to charge-carrier transport. Thus, these polymers were suitable for active materials of OLED devices. Additionally, it can be clearly seen from the molecular orbital diagrams that the HOMO energy levels of the four polymers all remain delocalized along the main chain of polymers, and the HOMO energy levels of P1-P4 are −4.74, −4.71, −4.72, and −4.47 eV, respectively. While the electron density is successively distributed on the whole D-A units, the LUMO values of P1–P4 are calculated to be −2.61, −2.64, −1.98, and −2.47 eV, respectively. It is important to note that the thiophene segments tend to be trans-coplanar with benzotriazole units in the optimized geometries of P1 (Figure 2), where the sulfur atom of the thiophene unit is in the opposite position to the fluorine atom of the thiazole unit. This conformation is undoubtedly conducive to the formation of F⋯H non-covalent interactions as demonstrated by 1H NMR spectra shown in Figure 1A.
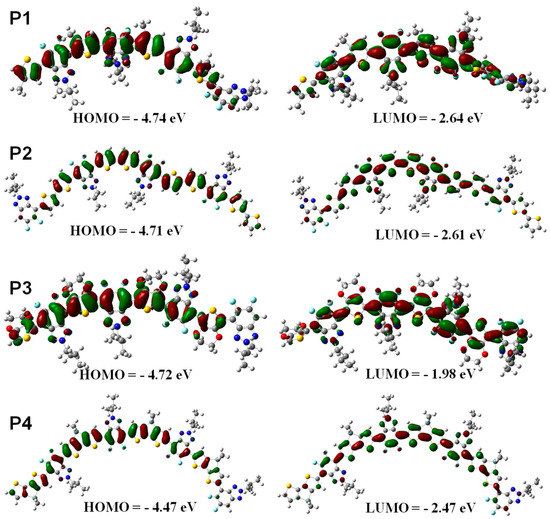
Figure 2.
Optimized geometries and molecular orbital surfaces for the HOMO and LUMO values of P1–P4 obtained using a DFT/B3LYP/6-31G* method.
3.4. UV–vis Absorption Spectra
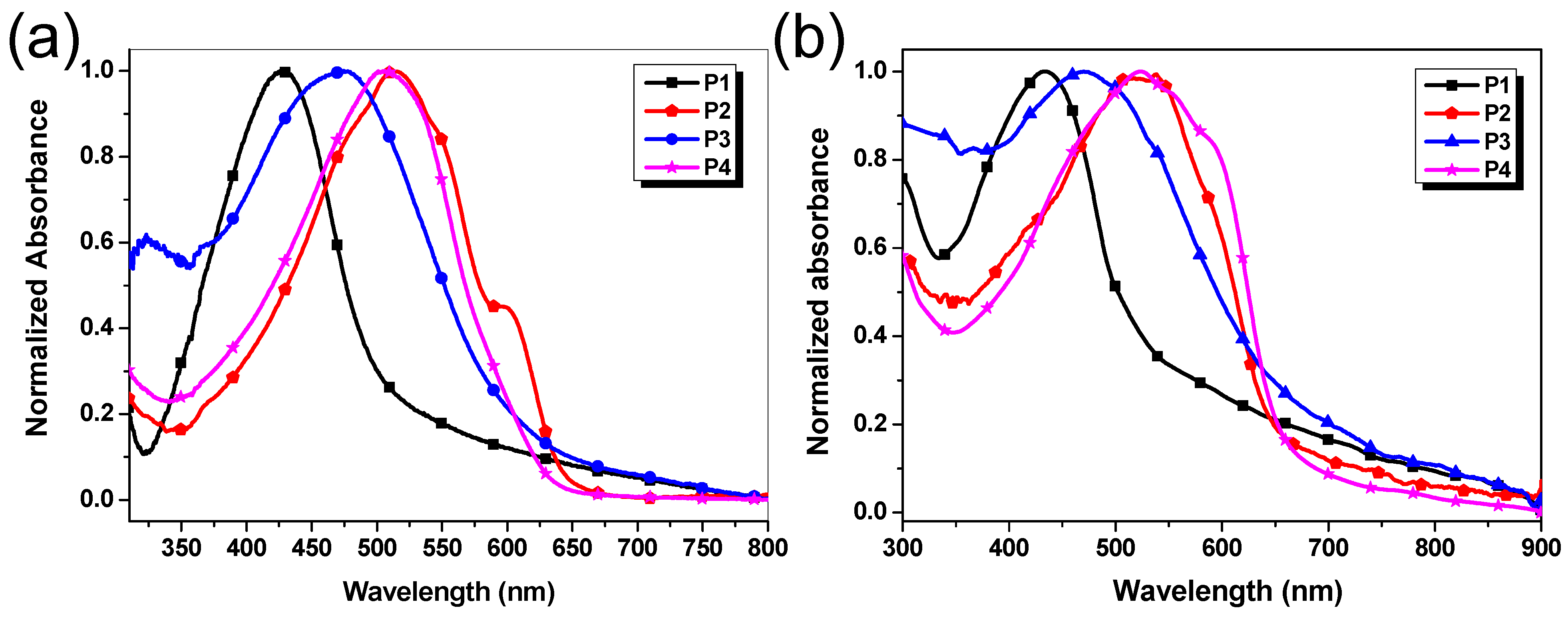
The UV–vis absorption spectra of P1–P4 in dilute chloroform (CHCl3) solutions and in film states were measured on a Shimadzu UV-2550 spectrometer. The concentration of the polymer in CHCl3 solution was 10−5 mol/L based on repeated units. The film samples were prepared by casting the chloroform solutions (3 mg/mL) on the surface of quartz and subsequently annealing for 10 min at 100 °C. Their UV–vis absorption spectra of polymer solutions and thin films are presented in Figure 3, and the absorption maxima and the optical band gaps estimated from the absorption edge of the thin film are listed in Table 2.
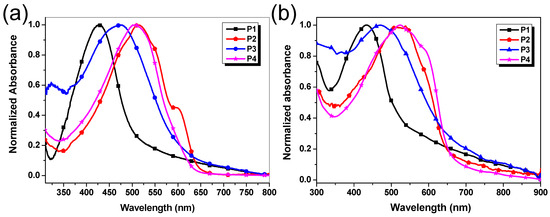
Figure 3.
Normalized absorption spectra of P1–P4 (a) in dilute chloroform solution (1 × 10−5 mol/L) and (b) in the thin film state (spin-cast from chloroform solution).

Table 2.
Photophysical and electrochemical properties of P1–P4.
As shown in Figure 3a and Table 2, the λmax of P1–P4 in CHCl3 solutions are 427, 515, 471, and 506 nm, respectively. Compared with P1, a remarkable redshift by 88 nm was observed for P2 in CHCl3 solutions, possibly due to P2 having longer conjugated chains and a more efficient conjugated effect caused by smaller steric hindrance between benzotriazole units and dithiophene units. In addition, the λmax of all polymers in the thin film states exhibit negligible changes in comparison with that in solutions beside P4. The results should be attributed to only P4 tending to form a more coplanar configuration between benzotriazole segments and silole ring in the solid-state due to the completely symmetrical and rigidly planar structure of silole units as demonstrated by DFT calculation (Figure 2). The optical band gaps (Egopt) of P1–P4 were determined to be 2.16, 1.81, 1.85, and 1.88 eV, respectively, from the onsets of the spectra in the films. The results are close to the corresponding Egopt values of the other benzotriazole-based D-π-A conjugated polymers reported in the literature, including ours [9,27].
3.5. Photoluminescence Property
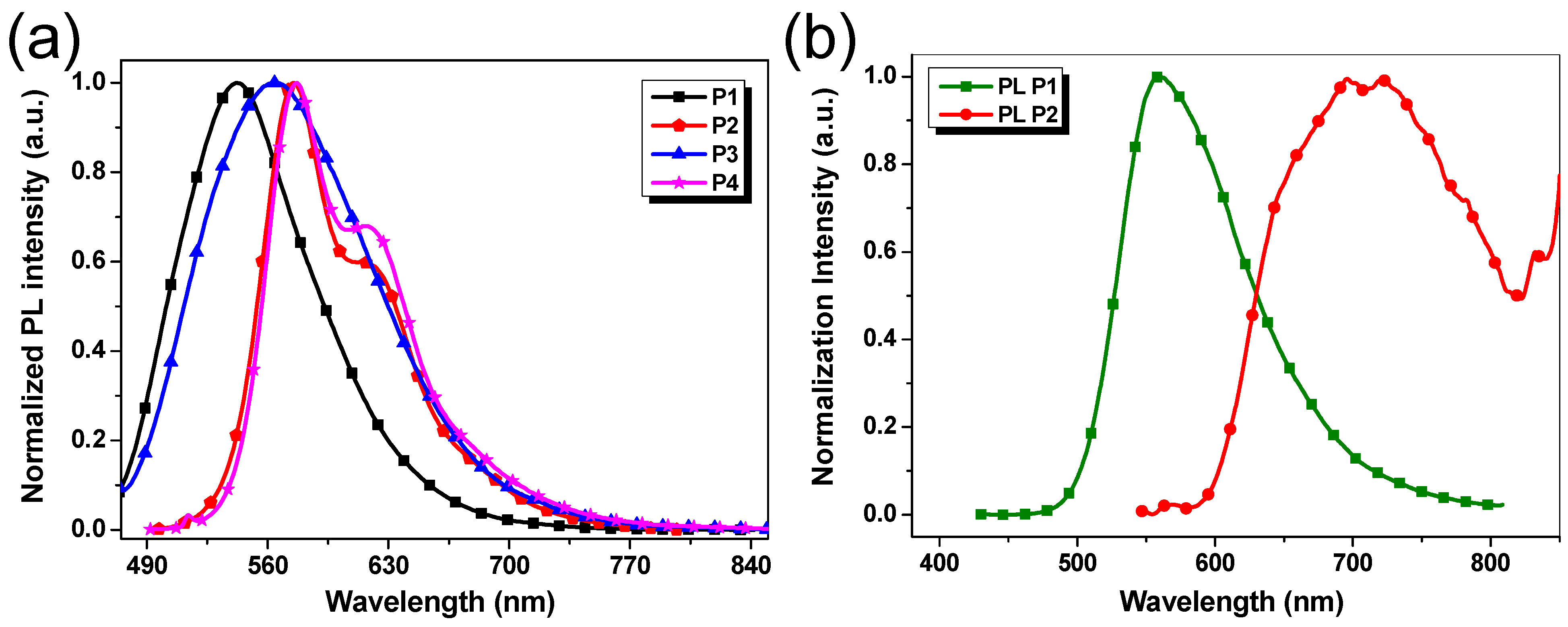
Figure 4a shows the photoluminescence (PL) spectra in CHCl3 solutions. P1–P4 exhibit broad emission peaks in the range of 480–770 nm upon excitation at 420 nm. In the case of P2 and P4, the obvious shoulder bands at about 630 nm could be attributed to the intramolecular charge transfer (ICT) from thiophene units to benzotriazole units (Figure 4a) [38,39]. Additionally, it was noted that emission peaks of polymers vary with the different donor units, exhibiting a bathochromic displacement observed in the following order: P1 < P3 < P2 ≈ P4 (Figure 4a). Thus, the polymers P2 and P4 with the more extended conjugated backbones show the maximum emission peaks at the highest wavelengths between 550 and 650 nm (Figure 4a).
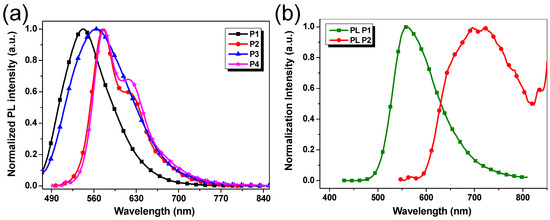
Figure 4.
(a) Normalized PL spectra of P1–P4 in dilute chloroform solutions (1 × 10−5 mol/L); (b) Normalized PL spectra in the thin film states of P1 and P2.
Different emission behavior of the polymers was noticed in solid-state. P1 maintained a similar emission profile to that in solution but consisted of a larger spectral domain, extended to most of the visible light region from 500 to 700 nm. The solid-state P2 displayed a very wide emission extended into the near-infrared domain, with an emission peak located at near 700 nm (Figure 4b). Compared with the emission peak of P2 in solution, its significant red-shift in thin-film state was noted. This should be attributed to more effective conjugation of polymer chains due to the intramolecular rotation that was restricted in solid-state. For the films of P3 and P4, only very weak emissions were observed (not provided), which agrees with the results of similar polymers reported in the literature [40].
3.6. Electrochemical Property
The electrochemical properties of all polymers P1–P4 were assessed by standard cyclic voltammetry (CV) using a saturated calomel electrode as reference electrode and the energy level of ferrocene/ferrocenium (−4.80 eV) as the internal standard (Figures S10 and S11). The energy levels of P1–P4, calculated on the basis of CV curves, are presented in Table 2. The HOMO levels of P1–P4 are −5.57, −5.69, −5.45, and −5.67 eV, respectively versus Fc/Fc+, and obviously lower than that of classical poly(3-hexylthiophene) (P3HT) materials (−5.10 eV) [41]. The results also indicate that the HOMO levels of benzotriazole-based conjugated polymers can be finely tuned by the introduction of different donor units. After magnification of the CV plots, the onsets of reduction potentials (Eonsetred) could be determined to be −0.73, −0.81, −0.82, and −0.83 V for P1–P4, respectively. Hence, the LUMO energy levels of polymers P1–P4 can be obtained to be −3.60, −3.68, −3.59, and −3.58 eV, respectively, versus Fc/Fc+ according to the corresponding Eonsetred. Since the conjugated polymers containing fluorinated benzotriazole segments can display excellent oxidational stability due to the stabilized HOMO and LUMO levels by the strong inductive effect of fluorine atoms [11,42], such polymer materials should have a huge potential for application in various photoelectric devices.
3.7. Electroluminescence Characteristics
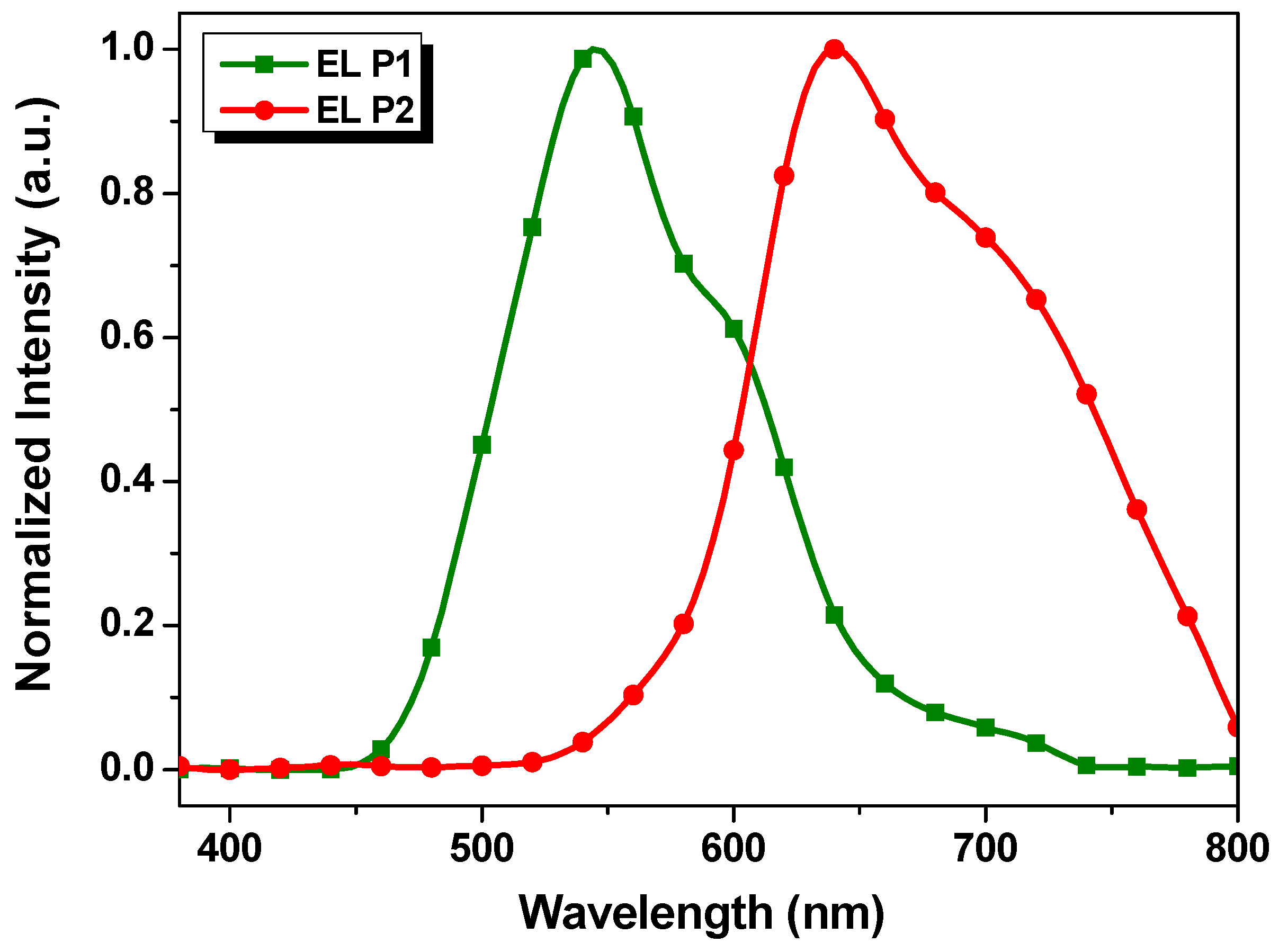
Considering that P1 and P2 have relatively high Mn and good fluorescent quantum efficiency in solutions (η = 0.3837 and 0.3805 for P1 and P2, respectively, measured on a FLS1000 fluorescence spectrometer, Edinburgh, UK), the solution-processed OLED devices with the P1 and P2 as emissive layers were fabricated based on the following configuration: ITO/PEDOT:PSS/PTAA/emissive layer/TPBi/LiF/Al. Figure 5 shows the electroluminescent (EL) spectra of the devices with the P1 and P2 as the emissive layer, respectively. The emission maxima of P1 and P2-based EL devices were 544 and 640 nm, respectively, which were slightly blue-shifted in comparison with the corresponding photoluminescence ones in the solid films as shown in Figure 4b. These results suggest that the color tuning on the EL device of benzotriazole-based polymers through the incorporation of various donor units on their backbone is feasible.
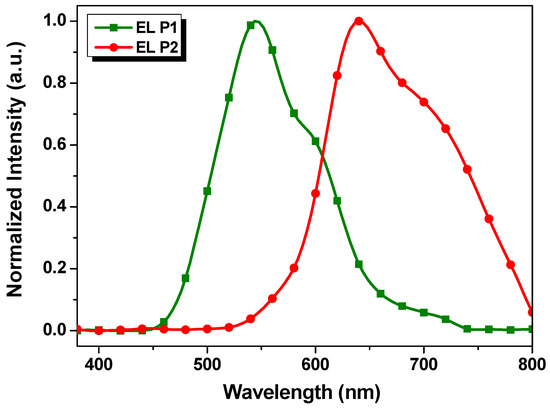
Figure 5.
EL spectra P1 and P2-based devices.
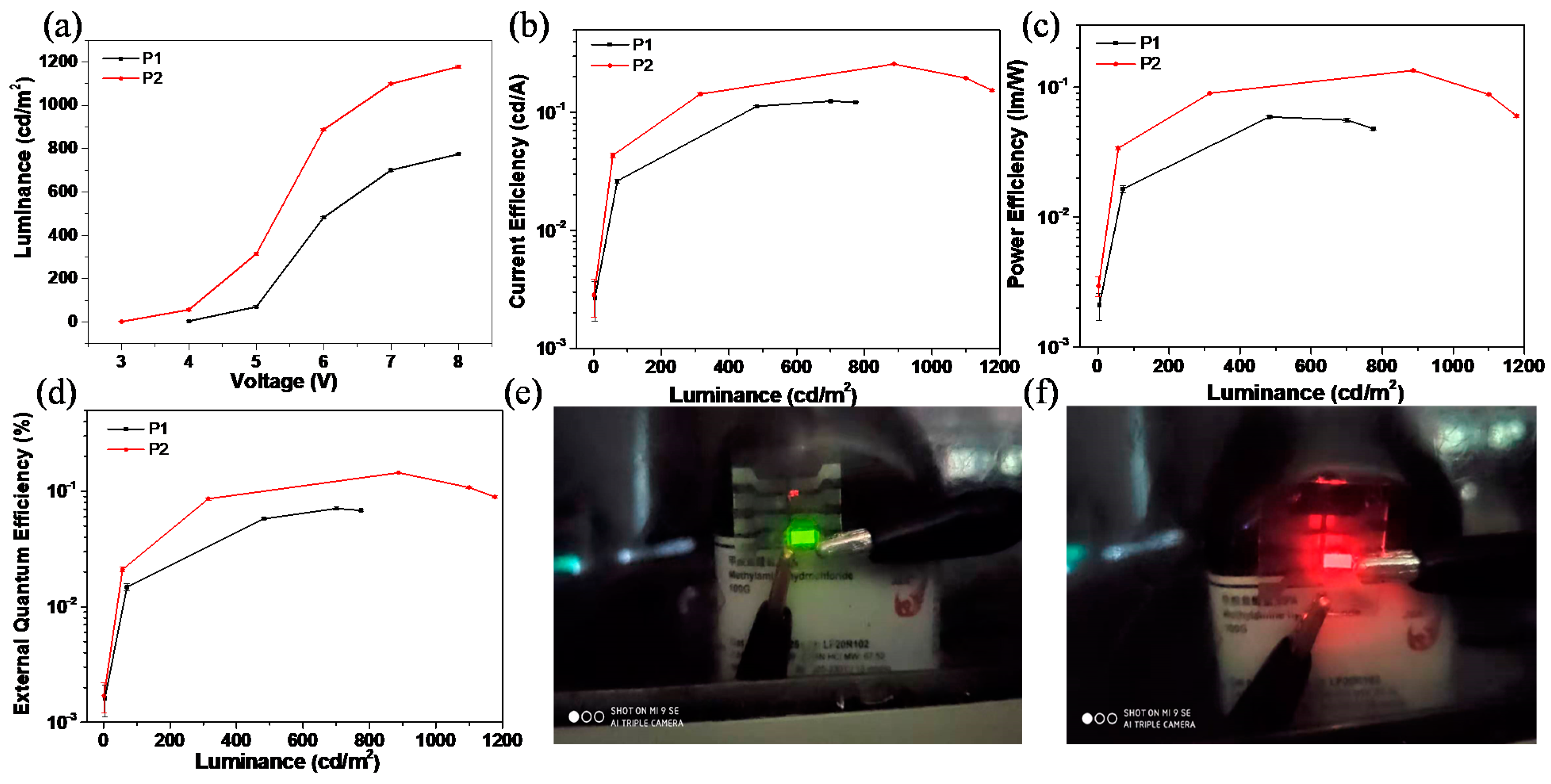
Figure 6 shows the typical EL performances of P1 and P2-based devices and their EL characteristics that were also listed in Table 3. The rectification behavior of P1 and P2-based devices can be observed from their J–V curves (Figure S12) originating from the intrinsic properties of the diodes. The turn-on voltages (Von) of P1- and P2-based devices were 4.0 and 3.0 V, respectively, as shown in Figure 6a. The P2-based device provided a higher luminance and current efficiency than that of P1 at a certain bias, as shown in Figure 6a,b. As a result, the maximum power efficiency (ηp = 0.13 lm/W) and the maximum external quantum efficiency (EQEmax = 0.14%) for P2-based OLED devices, which is almost twice as much as that for P1-based devices (Figure 6c,d). The P1-based device exhibited a bright green emission with CIE coordinates of (0.40, 0.52) (Figure 6e), while a red emission with CIE coordinates of (0.66, 0.33) at 1200 cd/m2 was observed for P2-based devices (Figure 6f). This result indicates P2 is a promising red emissive material for OLEDs.
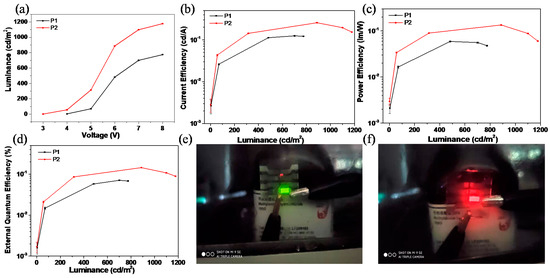
Figure 6.
(a) The luminance–voltage (L–V) characteristics; (b) Current efficiency–luminance (J–L) curves; (c) Power efficiency versus luminance plots; (d) External quantum efficiency–luminance characteristics of P1 and P2. (e) The photograph of P1-based device with green emission at 8 V; (f) The photograph of P2-based device with red emission at 8 V.

Table 3.
Luminescence characteristics of P1 and P2-based OLED devices.
4. Conclusions
Four benzotriazole-based π-conjugated polymers with donor-acceptor alternating structure were successfully prepared via Pd-catalyzed direct C-H cross-coupling polycondensation. The protocol offers a straightforward and effective assessment of such polymers without any pre-functionalized steps. This work extends the range of aromatic monomers for direct C-H cross-coupling polycondensation and achieves the effective copolymerization of electron-deficient benzotriazole with electron-rich thiophene derivatives in a structurally controllable mode. Although the as-synthesized polymer materials only displayed moderate OLED performances, the present method could have enormous potential for the synthesis of various semiconducting materials by tuning donor and acceptor units for different organic optoelectronic device applications.
Supplementary Materials
The supplementary materials are available online at https://www.mdpi.com/2073-4360/13/2/254/s1. Figures S1–S8: 1H NMR and 19F NMR Spectra of P1-P4; Figure S9: TGA curves of P1-P4; Figure S10: Cyclic voltammograms of P1-P4; Figure S11: Schematic energy-level diagrams of P1-P4; Figure S12: The current density-voltage curve of P1 and P2-based devices.
Author Contributions
Investigation, D.H., J.L., Q.Z., Z.H., Z.W. and J.C.; Project administration, Q.Z. and Y.L.; Supervision, Y.L.; Validation, D.H., J.L. and Q.Z.; Writing—orginal draft, D.H. and Q.Z.; Writing—review & editing, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the financial assistance of the NSFC (No. 21604063), the Natural Science Foundation of Tianjin (No. 18JCZDJC34600), the Program for Prominent Young College Teachers and Youth Reserve Talent of Tianjin Educational Committee, the Scientific Developing Foundation of Tianjin Education Commission (2017ZD14) and Undergraduate Innovation and Entrepreneurship Training Program (201910060093) for financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hildner, R.; Köhler, A.; Müller-Buschbaum, P.; Panzer, F.; Thelakkat, M. π-Conjugated Donor Polymers: Structure Formation and Morphology in Solution, Bulk and Photovoltaic Blends. Adv. Energy Mater. 2017, 7, 1700314. [Google Scholar] [CrossRef]
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting π-Conjugated Systems in Field-Effect Transistors: A Material Odyssey of Organic Electronics. Chem. Rev. 2012, 112, 2208–2267. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Abdalla, H.; Kemerink, M. Conjugated Polymer Blends for Organic Thermoelectrics. Adv. Electron. Mater. 2019, 5, 1800821. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting Polymers: The Third Generation. Chem. Soc. Rev. 2010, 39, 2354–2371. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.; Brasiunas, B.; Damaskaite, A.; Plikusiene, I.; Ramanavicius, A.; Ramanaviciene, A. Electrodeposited Gold Nanostructures for the Enhancement of Electrochromic Properties of PANI-PEDOT Film Deposited on Transparent Electrode. Polymers 2020, 12, 2778. [Google Scholar] [CrossRef]
- Popov, A.; Brasiunas, B.; Mikoliunaite, L.; Bagdziunas, G.; Ramanavicius, A.; Ramanaviciene, A. Comparative Study of Polyaniline (PANI), Poly(3,4-ethylenedioxythiophene) (PEDOT) and PANI-PEDOT Films Electrochemically Deposited on Transparent Indium Thin Oxide Based Electrodes. Polymer 2019, 172, 133–141. [Google Scholar] [CrossRef]
- Sakamoto, J.; Rehahn, M.; Wegner, G.; Schlüter, A.D. Suzuki Polycondensation: Polyarylenes à la Carte. Macromol. Rapid Commun. 2009, 30, 653–687. [Google Scholar] [CrossRef]
- Carsten, B.; He, F.; Son, H.J.; Xu, T.; Yu, L. Stille Polycondensation for Synthesis of Functional Materials. Chem. Rev. 2011, 111, 1493–1528. [Google Scholar] [CrossRef]
- Yokozawa, T.; Yokoyama, A. Chain-Growth Condensation Polymerization for the Synthesis of Well-Defined Condensation Polymers and π-Conjugated Polymers. Chem. Rev. 2009, 109, 5595–5619. [Google Scholar] [CrossRef]
- Todd, A.D.; Bielawski, C.W. Controlled Synthesis of an Alternating Donor-Acceptor Conjugated Polymer via Kumada Catalyst-Transfer Polycondensation. ACS Macro Lett. 2015, 4, 1254–1258. [Google Scholar] [CrossRef]
- Yum, S.; An, T.K.; Wang, X.; Lee, W.; Uddin, M.A.; Kim, Y.J.; Nguyen, T.L.; Xu, S.; Hwang, S.; Park, C.E.; et al. Benzotriazole-Containing Planar Conjugated Polymers with Noncovalent Conformational Locks for Thermally Stable and Efficient Polymer Field-Effect Transistors. Chem. Mater. 2014, 26, 2147–2154. [Google Scholar] [CrossRef]
- Scott, C.N.; Bisen, M.D.; Stemer, D.M.; McKinnon, S.; Luscombe, C.K. Direct Arylation Polycondensation of 2,5-Dithienylsilole with a Series of Difluorobenzodiimine-Based Electron Acceptors. Macromolecules 2017, 50, 4623–4628. [Google Scholar] [CrossRef]
- Zhang, Q.; Wan, X.; Lu, Y.; Li, Y.; Li, Y.; Li, C.; Wu, H.; Chen, Y. The Synthesis of 5-Alkyl[3,4-c]thienopyrrole-4,6-dione-Based Polymers Using a Pd-catalyzed Oxidative C-H/C-H Homopolymerization Reaction. Chem. Commun. 2014, 50, 12497–12499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, Y.; Lu, Y.; Zhang, H.; Li, M.; Yang, Y.; Wang, J.; Chen, Y.; Li, C. Pd-catalysed Oxidative C-H/C-H Coupling Polymerization for Polythiazole-Based Derivatives. Polymer 2015, 68, 227–233. [Google Scholar] [CrossRef]
- Zhang, Q.; Chang, M.; Lu, Y.; Sun, Y.; Li, C.; Yang, X.; Zhang, M.; Chen, Y. A Direct C-H Coupling Method for Preparing π-Conjugated Functional Polymers with High Regioregularity. Macromolecules 2018, 51, 379–388. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Deng, L.; Zhao, L.; Li, C.; Lu, Y. Synthesis of Highly Regioregular, Head-to-Tail Coupled Poly(3-octylesterthiophene) via C-H/C-H Coupling Polycondensation. Chin. J. Polym. Sci. 2018, 36, 1019–1026. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, Q.; Zhao, L.; Lu, Y. Direct C-H Coupling Polymerization of Asymmetric Monomer: Synthesis and Properties of Regioregular Ppoly(alkyl thiophene-3-carboxylates). Eur. Polym. J. 2018, 109, 72–81. [Google Scholar] [CrossRef]
- Gobalasingham, N.S.; Noh, S.; Thompson, B.C. Palladium-Catalyzed Oxidative Direct Arylation Polymerization (Oxi-DArP) of an Ester-Functionalized Thiophene. Polym. Chem. 2016, 7, 1623–1631. [Google Scholar] [CrossRef]
- Gobalasingham, N.S.; Pankow, R.M.; Thompson, B.C. Synthesis of Random Poly(hexyl thiophene-3-carboxylate) Copolymers via Oxidative Direct Arylation Polymerization (oxi-DArP). Polym. Chem. 2017, 8, 1963–1971. [Google Scholar] [CrossRef]
- Gobalasingham, N.S.; Thompson, B.C. Direct Arylation Polymerization: A Guide to Optimal Conditions for Effective Conjugated Polymers. Prog. Polym. Sci. 2018, 83, 135–201. [Google Scholar] [CrossRef]
- Yang, Y.; Lan, J.; You, J. Oxidative C-H/C-H Coupling Reactions between Two (Hetero)arenes. Chem. Rev. 2017, 117, 8787–8863. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.J.; Xing, L.; Luscombe, C.K. Exploration and Development of Gold- and Silver-Catalyzed Cross Dehydrogenative Coupling toward Donor-Acceptor π-Conjugated Polymer Synthesis. Polym. Chem. 2019, 10, 486–493. [Google Scholar] [CrossRef]
- Collier, G.S.; Reynolds, J.R. Exploring the Utility of Buchwald Ligands for C–H Oxidative Direct Arylation Polymerizations. ACS Macro Lett. 2019, 8, 931–936. [Google Scholar] [CrossRef]
- Aoki, H.; Saito, H.; Shimoyama, Y.; Kuwabara, J.; Yasuda, T.; Kanbara, T. Synthesis of Conjugated Polymers Containing Octafluorobiphenylene Unit via Pd-Catalyzed Cross-Dehydrogenative-Coupling Reaction. ACS Macro Lett. 2018, 7, 90–94. [Google Scholar] [CrossRef]
- Guo, Q.; Wu, D.; You, J. Oxidative Direct Arylation Polymerization Using Oxygen as the Sole Oxidant: Facile, Green Access to Bithiazole-Based Polymers. ChemSusChem 2016, 9, 2765–2768. [Google Scholar] [CrossRef]
- Guo, Q.; Jiang, R.; Wu, D.; You, J. Rapid Access to 2,2′-Bithiazole-Based Copolymers via Sequential Palladium-Catalyzed C-H/C-X and C-H/C-H Coupling Reactions. Macromol. Rapid Commun. 2016, 37, 794–798. [Google Scholar] [CrossRef]
- Huang, Q.; Qin, X.; Li, B.; Lan, J.; Guo, Q.; You, J. Cu-catalysed Oxidative C-H/C-H Coupling Polymerisation of Benzodiimidazoles: An Efficient Approach to Regioregular Polybenzodiimidazoles for Blue-Emitting Materials. Chem. Commun. 2014, 50, 13739–13741. [Google Scholar] [CrossRef]
- Yang, Y.; Nishiura, M.; Wang, H.; Hou, Z. Metal-Catalyzed C-H Activation for Polymer Synthesis and Functionalization. Coord. Chem. Rev. 2018, 376, 506–532. [Google Scholar] [CrossRef]
- Li, J.; Han, D.; Zhang, Q.; HE, Z.; Lu, Y. Synthesis and Properties of Fluorinated Benzotriazole-Based Donor-Acceptor-Type Conjugated Polymers via Pd-Catalyzed Direct C-H/C-H Coupling Polymerization. J. Polym. Sci. 2021. [Google Scholar] [CrossRef]
- Pankow, R.M.; Ye, L.; Thompson, B.C. Influence of An Ester Directing-Group on Defect Formation in the Synthesis of Conjugated Polymers via Direct Arylation Polymerization (DArP) Using Sustainable Solvents. Polym. Chem. 2019, 10, 4561–4572. [Google Scholar] [CrossRef]
- Lombeck, F.; Marx, F.; Strassel, K.; Kunz, S.; Lienert, C.; Komber, H.; Friend, R.; Sommer, M. To Branch or not to Branch: C-H Selectivity of Thiophene-Based Donor-Acceptor-Donor Monomers in Direct Arylation Polycondensation Exemplified by PCDTBT. Polym. Chem. 2017, 8, 4738–4745. [Google Scholar] [CrossRef]
- Adachi, Y.; Ooyama, Y.; Ren, Y.; Yin, X.; Jäkle, F.; Ohshita, J. Hybrid Conjugated Polymers with Alternating Dithienosilole or Dithienogermole and Tricoordinate Boron Units. Polym. Chem. 2018, 9, 291–299. [Google Scholar] [CrossRef]
- Fei, Z.; Kim, Y.; Smith, J.; Domingo, E.B.; Stingelin, N.; McLachlan, M.A.; Song, K.; Anthopoulos, T.D.; Heeney, M. Comparative Optoelectronic Study between Copolymers of Peripherally Alkylated Dithienosilole and Dithienogermole. Macromolecules 2012, 45, 735–742. [Google Scholar] [CrossRef]
- Zhong, L.; Bin, H.; Angunawela, I.; Jia, Z.; Qiu, B.; Sun, C.; Li, X.; Zhang, Z.; Ade, H.; Li, Y. Effect of Replacing Thiophene by Selenophene on the Photovoltaic Performance of Wide Bandgap Copolymer Donors. Macromolecules 2019, 52, 4776–4784. [Google Scholar] [CrossRef]
- Hu, H.; Ye, L.; Ghasemi, M.; Balar, N.; Rech, J.J.; Stuard, S.J.; You, W.; O’Connor, B.T.; Ade, H. Highly Efficient, Stable, and Ductile Ternary Nonfullerene Organic Solar Cells from a Two-Donor Polymer Blend. Adv. Mater. 2019, 31, 1808279. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Song, W.; Xiao, B.; Guo, J.; Min, J.; Ge, Z.; Zhang, J.; Wei, Z.; Zhou, E. Benzotriazole-Based Acceptor and Donors, Coupled with Chlorination, Achieve a High Voc of 1.24 V and an Efficiency of 10.5% in Fullerene-Free Organic Solar Cells. Chem. Mater. 2019, 31, 3941–3947. [Google Scholar] [CrossRef]
- Livi, F.; Gobalasingham, N.S.; Bundgaard, E.; Thompson, B.C. Influence of Functionality on Direct Arylation of Model Systems as a Route Toward Fluorinated Copolymers via Direct Arylation Polymerization (DArP). J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2598–2605. [Google Scholar] [CrossRef]
- Wu, W.-C.; Liu, C.-L.; Chen, W.-C. Synthesis and Characterization of New Fluorene-Acceptor Alternating and Random Copolymers for Light-Emitting Applications. Polymer 2006, 47, 527–538. [Google Scholar] [CrossRef]
- Torres-Moya, I.; Vázquez-Guilló, R.; Fernández-Palacios, S.; Carrillo, J.R.; Díaz-Ortiz, Á.; López Navarrete, J.T.; Ponce Ortiz, R.; Ruiz Delgado, M.C.; Mallavia, R.; Prieto, P. Fluorene-Based Donor-Acceptor Copolymers Containing Functionalized Benzotriazole Units: Tunable Emission and their Electrical Properties. Polymers 2020, 12, 256. [Google Scholar] [CrossRef]
- Tutuncu, E.; Cihaner, A.; Icli Ozkut, M. Synthesis and Electropolymerization of a Donor-Acceptor-Donor Trimeric Monomer Containing 3,4-Propylenedioxythiophene and Dithienosilole Units. Eur. Polym. J. 2019, 118, 239–243. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, J.B.; Kim, H.U.; Kang, I.-N.; Hwang, D.-H. High Open-Circuit Voltage Organic Photovoltaic Cells Fabricated Using Semiconducting Copolymers Consisting of Bithiophene and Fluorinated Quinoxaline or Triazole Derivatives. Synth. Met. 2014, 194, 88–96. [Google Scholar] [CrossRef]
- Chen, J.; Yan, Z.; Tang, L.; Uddin, M.A.; Yu, J.; Zhou, X.; Yang, K.; Tang, Y.; Shin, T.J.; Woo, H.Y.; et al. 1,4-Di(3-alkoxy-2-thienyl)-2,5-Difluorophenylene: A Building Block Enabling High-Performance Polymer Semiconductors with Increased Open-Circuit Voltages. Macromolecules 2018, 51, 5352–5363. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).