Cationic UV-Curing of Epoxidized Biobased Resins
Abstract
:1. Introduction
2. Cationic UV-Curing
- It does not require an inert atmosphere during the curing process since it is not affected by oxygen inhibition.
- It can continue after the light source has been removed. This phenomenon is noted as a “dark reaction”, which can lead to enhanced monomers conversion either in ambient temperature or with a thermal treatment.
- The cationic photocurable monomers are generally not toxic and irritating.
2.1. Cationic Photoinitiators
- Shifting the activation region by introduction of chromophoric groups in the aromatic rings [34].
2.2. Cationic UV-Curable Monomers
3. Cationic Photocurable Bio-Based Epoxy Monomers
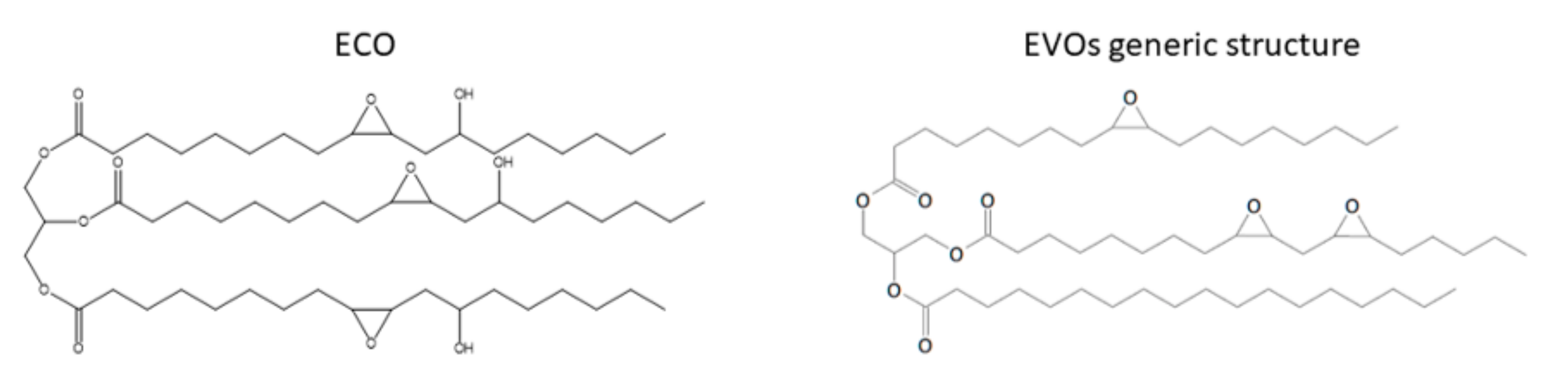
3.1. Epoxidized Vegetable Oils (EVOs)
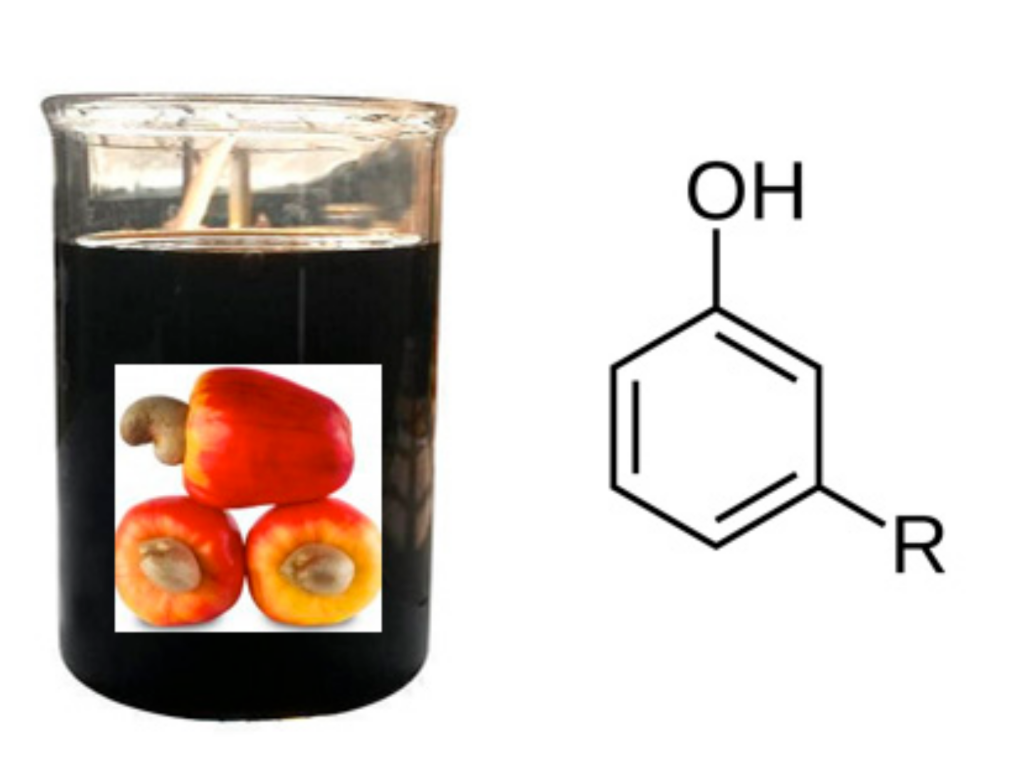
3.2. Epoxidized Cardanols
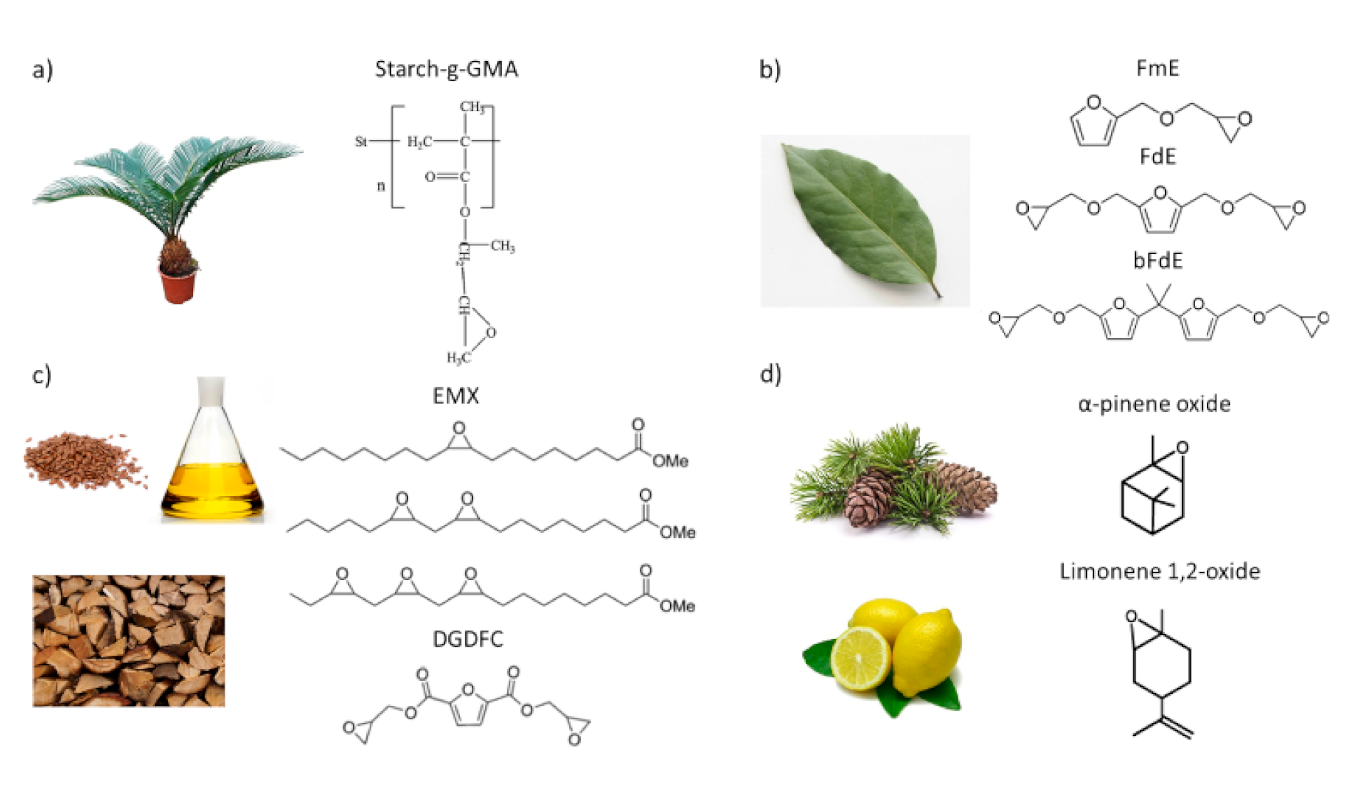
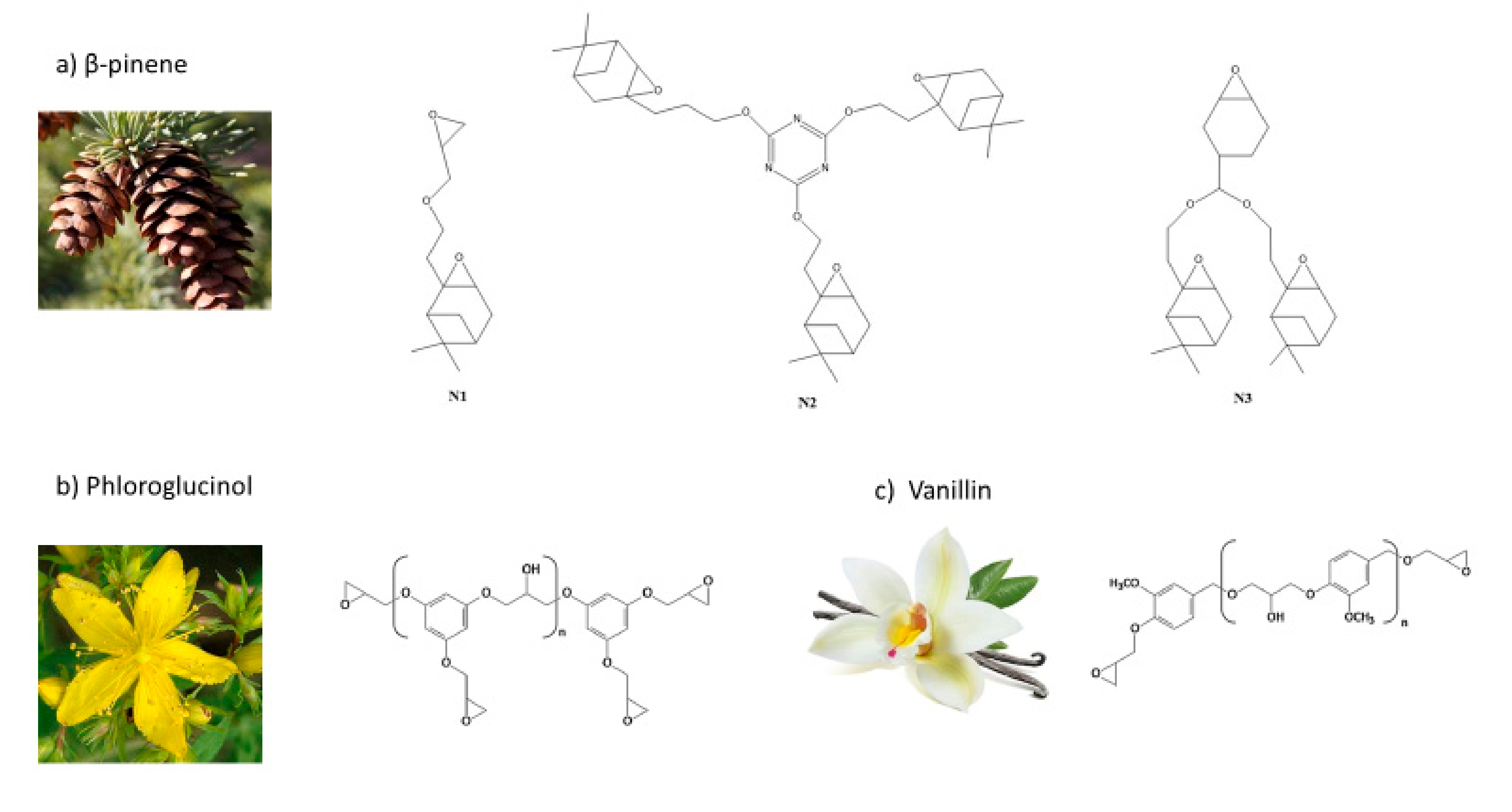
3.3. Others
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Epoxy resin market to reach $11 22 bn by 2021. Focus Powder Coat. 2017, 2017, 7. [CrossRef]
- Aggarwal, L.; Thapliyal, P.; Karade, S.R. Anticorrosive properties of the epoxy–cardanol resin based paints. Prog. Org. Coat. 2007, 59, 76–80. [Google Scholar] [CrossRef]
- Dodds, E.C.; Lawson, W. Synthetic strogenic Agents without the Phenanthrene Nucleus. Nat. Cell Biol. 1936, 137, 996. [Google Scholar] [CrossRef]
- Ma, S.; Li, T.; Liu, X.; Zhu, J. Research progress on bio-based thermosetting resins. Polym. Int. 2016, 65, 164–173. [Google Scholar] [CrossRef]
- Flint, S.; Markle, T.; Thompson, S.; Wallace, E. Bisphenol A exposure, effects, and policy: A wildlife perspective. J. Environ. Manag. 2012, 104, 19–34. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.-P. Biobased Thermosetting Epoxy: Present and Future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef]
- Gerbase, A.E.; Petzhold, C.L.; Costa, A.P.O. Dynamic mechanical and thermal behavior of epoxy resins based on soybean oil. J. Am. Oil Chem. Soc. 2002, 79, 797–802. [Google Scholar] [CrossRef]
- Tan, S.G.; Chow, W.S. Biobased Epoxidized Vegetable Oils and Its Greener Epoxy Blends: A Review. Polym. Technol. Eng. 2010, 49, 1581–1590. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Biocomposites Composed of Epoxidized Soybean Oil Cured with Terpene-Based Acid Anhydride and Cellulose Fibers. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar]
- Miyagawa, H.; Misra, M.; Drzal, L.T.; Mohanty, A.K. Fracture toughness and impact strength of anhydride-cured biobased epoxy. Polym. Eng. Sci. 2005, 45, 487–495. [Google Scholar] [CrossRef]
- Park, S.-J.; Jin, F.-L.; Lee, J.-R. Thermal and mechanical properties of tetrafunctional epoxy resin toughened with epoxidized soybean oil. Mater. Sci. Eng. A 2004, 374, 109–114. [Google Scholar] [CrossRef]
- Cook, W.; Chausson, S.; Chen, F.; Le Pluart, L.; Bowman, C.N.; Scott, T.F. Thermomechanical Behavior of Epoxy Resins Modified with Epoxidized Vegetable Oils. Polym. Int. 2008, 57, 469–478. [Google Scholar] [CrossRef]
- Uyama, H.; Kuwabara, M.; Tsujimoto, T.; Kobayashi, S. Enzymatic Synthesis and Curing of Biodegradable Epoxide-Containing Polyesters from Renewable Resources. Biomacromolecules 2003, 4, 211–215. [Google Scholar] [CrossRef]
- Caillol, S. Cardanol: A promising building block for biobased polymers and additives. Curr. Opin. Green Sustain. Chem. 2018, 14, 26–32. [Google Scholar] [CrossRef]
- Devi, A.; Srivastava, D. Studies on the blends of cardanol-based epoxidized novolac type phenolic resin and carboxyl-terminated polybutadiene (CTPB), I. Mater. Sci. Eng. A 2007, 458, 336–347. [Google Scholar] [CrossRef]
- Yadav, R.; Srivastava, D. Blends of cardanol-based epoxidized novolac resin and CTBN for application in surface coating: A study on thermal, mechanical, chemical, and morphological characteristics. J. Coat. Technol. Res. 2010, 7, 557–568. [Google Scholar] [CrossRef]
- Tan, S.G.; Chow, W.S. Curing Characteristics and Thermal Properties of Epoxidized Soybean Oil Based Thermosetting Resin. J. Am. Oil Chem. Soc. 2011, 88, 915–923. [Google Scholar] [CrossRef]
- Ng, F.; Couture, G.; Philippe, C.; Boutevin, B.; Caillol, S. Bio-Based Aromatic Epoxy Monomers for Thermoset Materials. Molecules 2017, 22, 149. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Q.; Huang, W.; Jiang, Y.H.; Zhu, J.; Zhang, C.Z. Preparation of a bio-based epoxy with comparable properties to those of petroleum-based counterparts. Express Polym. Lett. 2012, 6, 293–298. [Google Scholar] [CrossRef]
- Ferdosian, F.; Zhang, Y.; Yuan, Z.; Anderson, M.; Xu, C.C. Curing kinetics and mechanical properties of bio-based epoxy composites comprising lignin-based epoxy resins. Eur. Polym. J. 2016, 82, 153–165. [Google Scholar] [CrossRef]
- Baroncini, E.; Yadav, S.K.; Palmese, G.R.; Stanzione, J.F. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 44103. [Google Scholar] [CrossRef] [Green Version]
- Ramon, E.; Sguazzo, C.; Moreira, P. A Review of Recent Research on Bio-Based Epoxy Systems for Engineering Applications and Potentialities in the Aviation Sector. Aerospace 2018, 5, 110. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Akram, D.; Sharmin, E.; Zafar, F.; Ahmad, S. Vegetable oil based eco-friendly coating materials: A review article. Arab. J. Chem. 2014, 7, 469–479. [Google Scholar] [CrossRef]
- Bobade, S.K.; Paluvai, N.R.; Mohanty, S.; Nayak, S.K. Bio-Based Thermosetting Resins for Future Generation: A Review. Polym. Technol. Eng. 2016, 55, 1863–1896. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef]
- Sharmin, E.; Zafar, F.; Akram, D.; Alam, M.; Ahmad, S. Recent advances in vegetable oils based environment friendly coatings: A review. Ind. Crop. Prod. 2015, 76, 215–229. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Diaryliodonium Salts. A New Class of Photoinitiators for Cationic Polymerization. Macromolecules 1977, 10, 1307–1315. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-Curing: Technology and Applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Sangermano, M.; Roppolo, I.; Chiappone, A. New Horizons in Cationic Photopolymerization. Polymers 2018, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Gomurashvili, Z.; Crivello, J.V. Phenothiazine photosensitizers for onium salt photoinitiated cationic polymerization. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 1187–1197. [Google Scholar] [CrossRef]
- DeVoe, R.J.; Sahyun, M.R.V.; Serpone, N.; Sharma, D.K. Transient intermediates in the photolysis of iodonium cations. Can. J. Chem. 1987, 65, 2342–2349. [Google Scholar] [CrossRef]
- Chen, Y.; Yamamura, T.; Igarashi, K. Photosensitization of carbazole derivatives in cationic polymerization with a novel sensitivity to near-UV light. J. Polym. Sci. Polym. Chem. Ed. 2000, 38, 90–100. [Google Scholar] [CrossRef]
- Bulut, U.; Crivello, J.V. Investigation of the Reactivity of Epoxide Monomers in Photoinitiated Cationic Polymerization. Macromolecules 2005, 38, 3584–3595. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Burr, D.; Crivello, J.V. Photochemistry and Photopolymerization Activity Of Diaryliodonium Salts. J. Macromol. Sci. Part A 1994, 31, 677–701. [Google Scholar] [CrossRef]
- Yaǧci, Y.; Ledwith, A. Mechanistic and kinetic studies on the photoinitiated polymerization of tetrahydrofuran. J. Polym. Sci. Part A Polym. Chem. 1988, 26, 1911–1918. [Google Scholar] [CrossRef]
- Yagci, Y.; Schnabel, W. New aspects on the photoinitiated free radical promoted cationic polymerization. Makromol. Chem. Macromol. Symp. 1992, 60, 133–143. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lee, J.L. Photosensitized cationic polymerizations using dialkylphenacylsulfonium and dialkyl(4-hydroxyphenyl)sulfonium salt photoinitiators. Macromolecules 1981, 14, 1141–1147. [Google Scholar] [CrossRef]
- Nelson, E.W.; Carter, T.P.; Scranton, A.B. The role of the triplet state in the photosensitization of cationic polymerizations by anthracene. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 247–256. [Google Scholar] [CrossRef]
- Yagci, Y.; Schnabel, W.; Wilpert, A.; Bendig, J. Electron transfer from aromatic compounds to phenyliodinium and diphenylsulfinium radical cations. J. Chem. Soc. Faraday Trans. 1994, 90, 287–291. [Google Scholar] [CrossRef]
- Hizal, G.; Yaḡci, Y.; Schnabel, W. Charge-transfer complexes of pyridinium ions and methyl- and methoxy-substituted benzenes as photoinitiators for the cationic polymerization of cyclohexene oxide and related compounds. Polymers 1994, 35, 2428–2431. [Google Scholar] [CrossRef]
- Hizal, G.; Emiroglu, S.E.; Yagci, Y. Photoinitiated radical polymerization using charge transfer complex ofN-ethoxy-p-cyanopyridinium salt and 1,2,4-trimethoxybenzene. Polym. Int. 1998, 47, 391–392. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Photoinitiated cationic polymerization by dialkylphenacylsulfonium salts. J. Polym. Sci. Polym. Chem. Ed. 1979, 17, 2877–2892. [Google Scholar] [CrossRef]
- Yagci, Y.; Durmaz, Y.Y.; Aydogan, B. Phenacyl onium salt photoinitiators: Synthesis, photolysis, and applications. Chem. Rec. 2007, 7, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Yaḡci, Y.; Kornowski, A.; Schnabel, W. N-alkoxy-pyridinium and N-alkoxy-quinolinium salts as initiators for cationic photopolymerizations. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 1987–1991. [Google Scholar] [CrossRef]
- Shi, S.; Croutxé-Barghorn, C.; Allonas, X. Photoinitiating systems for cationic photopolymerization: Ongoing push toward long wavelengths and low light intensities. Prog. Polym. Sci. 2017, 65, 1–41. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, P.; Dietlin, C.; Campolo, D.; Dumur, F.; Gigmes, D.; Morlet-Savary, F.; Fouassier, J.P.; Lalevée, J. Cationic Photoinitiators for Near UV and Visible LEDs: A Particular Insight into One-Component Systems. Macromol. Chem. Phys. 2016, 217, 1214–1227. [Google Scholar] [CrossRef]
- Sangermano, M. Recent Advances in Cationic Photopolymerization. J. Photopolym. Sci. Technol. 2019, 32, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, M.; Magaldi, D.; Hijazi, A.; Graff, B.; Dumur, F.; Fouassier, J.; Bui, T.-T.; Goubard, F.; Lalevée, J. Development of new high-performance visible light photoinitiators based on carbazole scaffold and their applications in 3d printing and photocomposite synthesis. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2081–2092. [Google Scholar] [CrossRef]
- Hola, E.; Topa, M.; Chachaj-Brekiesz, A.; Pilch, M.; Fiedor, P.; Galek, M.; Ortyl, J. New, highly versatile bimolecular photoinitiating systems for free-radical, cationic and thiol–ene photopolymerization processes under low light intensity UV and visible LEDs for 3D printing application. RSC Adv. 2020, 10, 7509–7522. [Google Scholar] [CrossRef] [Green Version]
- Breloy, L.; Brezová, V.; Blacha-Grzechnik, A.; Presset, M.; Yildirim, M.S.; Yilmaz, I.; Yagci, Y.; Versace, D.-L. Visible Light Anthraquinone Functional Phthalocyanine Photoinitiator for Free-Radical and Cationic Polymerizations. Macromolecules 2020, 53, 112–124. [Google Scholar] [CrossRef]
- Sangermano, M.; Malucelli, G.; Morel, F.; Decker, C.; Priola, A. Cationic photopolymerization of vinyl ether systems: Influence of the presence of hydrogen donor additives. Eur. Polym. J. 1999, 35, 639–645. [Google Scholar] [CrossRef]
- Crivello, J.V.; Liu, S. Photoinitiated cationic polymerization of epoxy alcohol monomers. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 389–401. [Google Scholar] [CrossRef]
- Crivello, J.V.; Bi, D.; Lu, Y. Cationic photopolymerization of ambifunctional monomers. Macromol. Symp. 1995, 95, 79–89. [Google Scholar] [CrossRef]
- Sangermano, M. UV-Cured Nanostructured Epoxy Coatings. In Epoxy Polymers; Pascault, J.P., Williams, R.J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 235–251. ISBN 9783527324804. [Google Scholar]
- Sangermano, M.; Pegel, S.; Pötschke, P.; Voit, B. Antistatic Epoxy Coatings with Carbon Nanotubes Obtained by Cationic Photopolymerization. Macromol. Rapid Commun. 2008, 29, 396–400. [Google Scholar] [CrossRef]
- Sangermano, M.; Voit, B.; Sordo, F.; Eichhorn, K.-J.; Rizza, G. High refractive index transparent coatings obtained via UV/thermal dual-cure process. Polymers 2008, 49, 2018–2022. [Google Scholar] [CrossRef]
- Sangermano, M.; Messori, M. Scratch Resistance Enhancement of Polymer Coatings. Macromol. Mater. Eng. 2010, 295, 603–612. [Google Scholar] [CrossRef]
- Sangermano, M.; Yagci, Y.; Rizza§, G. In Situ Synthesis of Silver−Epoxy Nanocomposites by Photoinduced Electron Transfer and Cationic Polymerization Processes. Macromolecules 2007, 40, 8827–8829. [Google Scholar] [CrossRef]
- Yagci, Y.; Sangermano, M.; Rizza, G. Synthesis and Characterization of Gold−Epoxy Nanocomposites by Visible Light Photoinduced Electron Transfer and Cationic Polymerization Processes. Macromolecules 2008, 41, 7268–7270. [Google Scholar] [CrossRef]
- Yagci, Y.; Sangermano, M.; Rizza, G. A visible light photochemical route to silver–epoxy nanocomposites by simultaneous polymerization–reduction approach. Polymers 2008, 49, 5195–5198. [Google Scholar] [CrossRef]
- Sangermano, M.; Borella, E.; Priola, A.; Messori, M.; Taurino, R.; Pötschke, P. Use of Single-Walled Carbon Nanotubes as Reinforcing Fillers in UV-Curable Epoxy Systems. Macromol. Mater. Eng. 2008, 293, 708–713. [Google Scholar] [CrossRef]
- Sangermano, M.; Messori, M.; Galleco, M.M.; Rizza, G.; Voit, B. Scratch resistant tough nanocomposite epoxy coatings based on hyperbranched polyesters. Polymers 2009, 50, 5647–5652. [Google Scholar] [CrossRef]
- Sangermano, M.; Tonin, M.; Yagci, Y. Degradable epoxy coatings by photoinitiated cationic copolymerization of bisepoxide with ε-caprolactone. Eur. Polym. J. 2010, 46, 254–259. [Google Scholar] [CrossRef]
- Crivello, J.V.; Narayan, R. Epoxidized triglycerides as renewable monomers in photoinitiated cationic polymerization. Chem. Mater. 1992, 4, 692–699. [Google Scholar] [CrossRef]
- Malburet, S.; Di Mauro, C.; Noè, C.; Mija, A.; Sangermano, M.; Graillot, A. Sustainable access to fully biobased epoxidized vegetable oil thermoset materials prepared by thermal or UV-cationic processes. RSC Adv. 2020, 10, 41954–41966. [Google Scholar] [CrossRef]
- Chakrapani, S.; Crivello, J.V. Synthesis and Photoinitiated Cationic Polymerization of Epoxidized Castor Oil and Its Derivatives. J. Macromol. Sci. Part A 1998, 35, 1–20. [Google Scholar] [CrossRef]
- Noè, C.; Malburet, S.; Bouvet-Marchand, A.; Graillot, A.; Loubat, C.; Sangermano, M. Cationic photopolymerization of bio-renewable epoxidized monomers. Prog. Org. Coat. 2019, 133, 131–138. [Google Scholar] [CrossRef]
- Ortiz, R.A.; López, D.P.; Cisneros, M.D.L.G.; Valverde, J.C.R.; Crivello, J.V. A kinetic study of the acceleration effect of substituted benzyl alcohols on the cationic photopolymerization rate of epoxidized natural oils. Polymers 2005, 46, 1535–1541. [Google Scholar] [CrossRef]
- Venturello, C.; D’Aloisio, R. Quaternary ammonium tetrakis(diperoxotungsto)phosphates(3-) as a new class of catalysts for efficient alkene epoxidation with hydrogen peroxide. J. Org. Chem. 1988, 53, 1553–1557. [Google Scholar] [CrossRef]
- Yang, Z.; Narayanan, J.; Ravalli, M.; Rupp, B.T.; Ryu, C.Y. Structure-Property Relationships of Epoxy Thermoset Networks from Photoinitiated Cationic Polymerization of Epoxidized Vegetable Oils. Sustain. Polym. Biomass 2017, 209–226. [Google Scholar] [CrossRef]
- Saleh, A.F.; Kamarudin, E.; Yaacob, A.B.; Yussof, A.W.; Abdullah, M.A. Optimization of -biomethane production by anaerobic digestion of palm oil mill effluent using response surface methodology. Asia Pac. J. Chem. Eng. 2012, 7, 353–360. [Google Scholar] [CrossRef]
- Thames, S.F.; Yu, H.; Subramanian, R. Cationic ultraviolet curable coatings from castor oil. J. Appl. Polym. Sci. 2000, 77, 8–13. [Google Scholar] [CrossRef]
- Decker, C.; Viet, T.N.T.; Thi, H.P. Photoinitiated cationic polymerization of epoxides. Polym. Int. 2001, 50, 986–997. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, Y.; Lei, D.; Yang, J.; Yang, Y.; Su, J. Facile method to prepared photoinitiated cationic curable plant oil and its enhancement on the impact strength of cycloaliphatic diepoxide. Ind. Crop. Prod. 2019, 135, 72–80. [Google Scholar] [CrossRef]
- Rosli, W.W.; Kumar, R.; Zah, S.M.; Hilmi, M. UV radiation curing of epoxidized palm oil–cycloaliphatic diepoxide system induced by cationic photoinitiators for surface coatings. Eur. Polym. J. 2003, 39, 593–600. [Google Scholar] [CrossRef]
- Chen, J.; Soucek, M.D.; Simonsick, W.J.; Celikay, R.W. Synthesis and photopolymerization of norbornyl epoxidized linseed oil. Polymers 2002, 43, 5379–5389. [Google Scholar] [CrossRef]
- Crivello, J.V.; Narayan, R.; Sternstein, S. Fabrication and mechanical characterization of glass fiber reinforced UV-cured composites from epoxidized vegetable oils. J. Appl. Polym. Sci. 1997, 64, 2073–2087. [Google Scholar] [CrossRef]
- Crivello, J.V.; Bulut, U. Curcumin: A naturally occurring long-wavelength photosensitizer for diaryliodonium salts. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5217–5231. [Google Scholar] [CrossRef]
- Jiratumnukul, N.; Intarat, R. UV Radiation Energy Consumption in Curing Process of Epoxidized Sunflower Oil-Organoclay Hybrid Coatings. J. Met. Mater. Miner. 2006, 16, 53–56. [Google Scholar]
- Shibata, M.; Teramoto, N.; Someya, Y.; Suzuki, S. Bio-based nanocomposites composed of photo-cured epoxidized soybean oil and supramolecular hydroxystearic acid nanofibers. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 669–673. [Google Scholar] [CrossRef]
- Ahn, B.K.; Sung, J.; Kim, N.; Kraft, S.; Sun, X.S. UV-curable pressure-sensitive adhesives derived from functionalized soybean oils and rosin ester. Polym. Int. 2012, 62, 1293–1301. [Google Scholar] [CrossRef]
- Lalevée, J.; Fouassier, J.P. Recent advances in sunlight induced polymerization: Role of new photoinitiating systems based on the silyl radical chemistry. Polym. Chem. 2011, 2, 1107–1113. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lalevée, J.; Gigmes, D.; Fouassier, J.P. Green Chemistry: Sunlight-Induced Cationic Polymerization of Renewable Epoxy Monomers Under Air. Macromolecules 2010, 43, 1364–1370. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Lalevée, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P. A Breakthrough toward Long Wavelength Cationic Photopolymerization: Initiating Systems Based on Violanthrone Derivatives and Silyl Radicals. Macromolecules 2011, 44, 8374–8379. [Google Scholar] [CrossRef]
- Chen, Z.; Chisholm, B.J.; Webster, D.C.; Zhang, Y.; Patel, S. Study of epoxidized-cardanol containing cationic UV curable materials. Prog. Org. Coat. 2009, 65, 246–250. [Google Scholar] [CrossRef]
- Kanehashi, S.; Tamura, S.; Kato, K.; Honda, T.; Ogino, K.; Miyakoshi, T. Photopolymerization of Bio-Based Epoxy Prepolymers Derived from Cashew Nut Shell Liquid (CNSL). J. Fiber Sci. Technol. 2017, 73, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Vacche, S.D.; Vitale, A.; Bongiovanni, R. Photocuring of Epoxidized Cardanol for Biobased Composites with Microfibrillated Cellulose. Molecules 2019, 24, 3858. [Google Scholar] [CrossRef] [Green Version]
- Noè, C.; Malburet, S.; Milani, E.; Bouvet-Marchand, A.; Graillot, A.; Sangermano, M. Cationic UV-curing of epoxidized cardanol derivatives. Polym. Int. 2020, 69, 668–674. [Google Scholar] [CrossRef]
- Cheon, J.; Cho, D.; Song, B.K.; Park, J.; Kim, B.; Lee, B.C. Thermogravimetric and Fourier-transform infrared analyses on the cure behavior of polycardanol containing epoxy groups cured by electron beam. J. Appl. Polym. Sci. 2014, 132, 1–10. [Google Scholar] [CrossRef]
- Cheon, J.; Cho, D. Effects of the electron-beam absorption dose on the glass transition, thermal expansion, dynamic mechanical properties, and water uptake of polycardanol containing epoxy groups cured by an electron beam. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Han, T.L.; Kumar, R.N.; Rozman, H.; Noor, M.M. GMA grafted sago starch as a reactive component in ultra violet radiation curable coatings. Carbohydr. Polym. 2003, 54, 509–516. [Google Scholar] [CrossRef]
- Cho, J.K.; Lee, J.-S.; Jeong, J.; Kim, B.; Kim, B.; Kim, S.; Shin, S.; Kim, H.-J.; Lee, S.-H. Synthesis of carbohydrate biomass-based furanic compounds bearing epoxide end group(s) and evaluation of their feasibility as adhesives. J. Adhes. Sci. Technol. 2013, 27, 2127–2138. [Google Scholar] [CrossRef]
- Nameer, S.; Larsen, D.B.; Duus, J.Ø.; Daugaard, A.E.; Johansson, M. Biobased Cationically Polymerizable Epoxy Thermosets from Furan and Fatty Acid Derivatives. ACS Sustain. Chem. Eng. 2018, 6, 9442–9450. [Google Scholar] [CrossRef] [Green Version]
- Crivello, J.V.; Yang, B. Studies of synthesis and cationic photopolymerization of three isomeric monoterpene diepoxides. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 1881–1890. [Google Scholar] [CrossRef]
- Park, H.J.; Ryu, C.Y.; Crivello, J.V. Photoinitiated cationic polymerization of limonene 1,2-oxide and α-pinene oxide. J. Polym. Sci. Part A Polym. Chem. 2012, 51, 109–117. [Google Scholar] [CrossRef]
- Breloy, L.; Ouarabi, C.A.; Brosseau, A.; Dubot, P.; Brezova, V.; Andaloussi, S.A.; Malval, J.-P.; Versace, D.-L. β-Carotene/Limonene Derivatives/Eugenol: Green Synthesis of Antibacterial Coatings under Visible-Light Exposure. ACS Sustain. Chem. Eng. 2019, 7, 19591–19604. [Google Scholar] [CrossRef]
- Ortiz, R.A.; Valdez, A.E.G.; Cruz, D.H.; Jiménez, G.N.; Jiménez, A.I.H.; Padilla, J.G.T.; Guerrero-Santos, R. Highly reactive novel biobased cycloaliphatic epoxy resins derived from nopol and a study of their cationic photopolymerization. J. Polym. Res. 2020, 27, 1–16. [Google Scholar] [CrossRef]
- Nguyen, Q.; Nguyen, N.; De Anda, A.R.; Nguyen, V.; Versace, D.; Langlois, V.; Naili, S.; Renard, E. Photocurable bulk epoxy resins based on resorcinol derivative through cationic polymerization. J. Appl. Polym. Sci. 2020, 137, 1–10. [Google Scholar] [CrossRef]


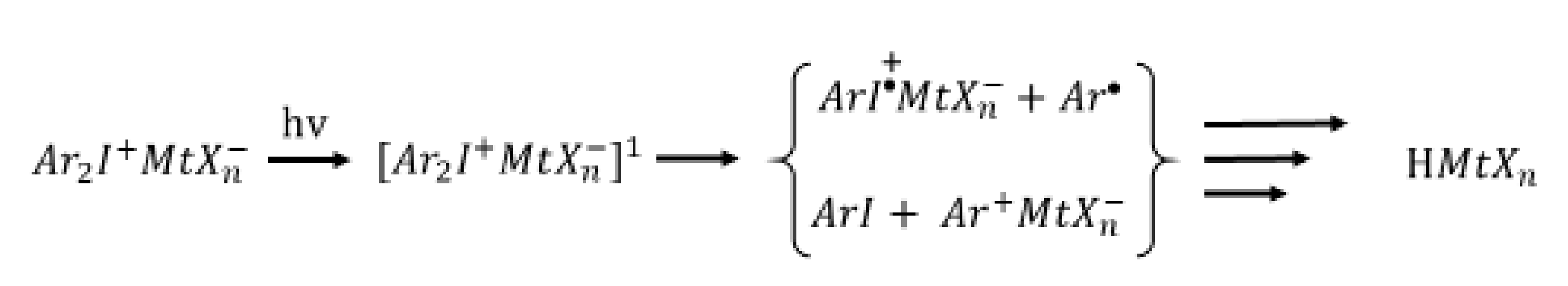

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noè, C.; Hakkarainen, M.; Sangermano, M. Cationic UV-Curing of Epoxidized Biobased Resins. Polymers 2021, 13, 89. https://doi.org/10.3390/polym13010089
Noè C, Hakkarainen M, Sangermano M. Cationic UV-Curing of Epoxidized Biobased Resins. Polymers. 2021; 13(1):89. https://doi.org/10.3390/polym13010089
Chicago/Turabian StyleNoè, Camilla, Minna Hakkarainen, and Marco Sangermano. 2021. "Cationic UV-Curing of Epoxidized Biobased Resins" Polymers 13, no. 1: 89. https://doi.org/10.3390/polym13010089
APA StyleNoè, C., Hakkarainen, M., & Sangermano, M. (2021). Cationic UV-Curing of Epoxidized Biobased Resins. Polymers, 13(1), 89. https://doi.org/10.3390/polym13010089






