Insight into the Alcohol-Free Ring-Opening Polymerization of TMC Catalyzed by TBD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
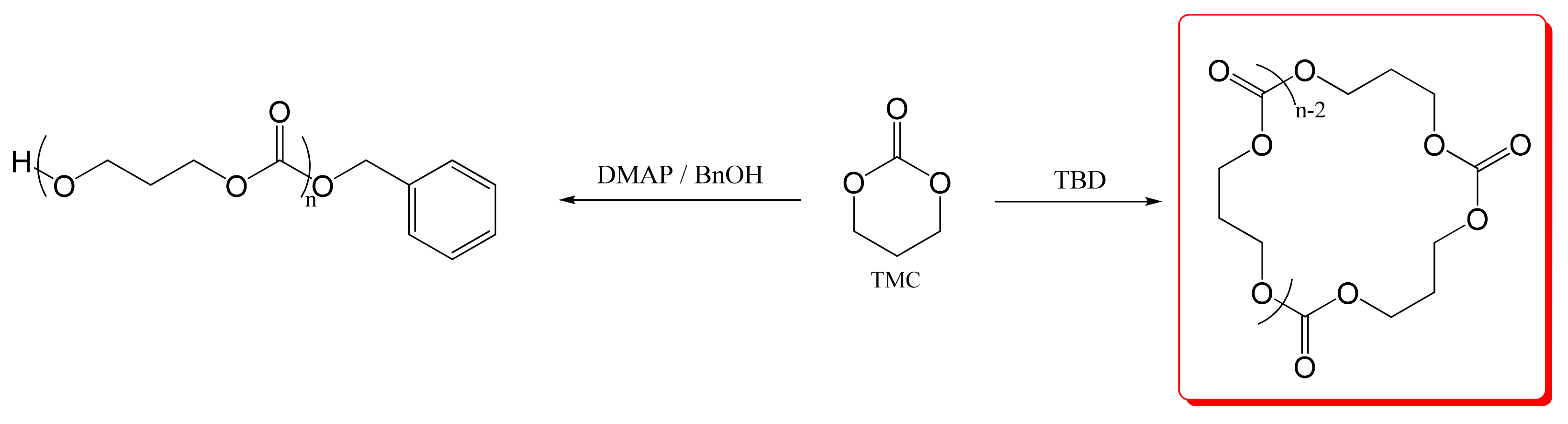
2.2. Syntheses
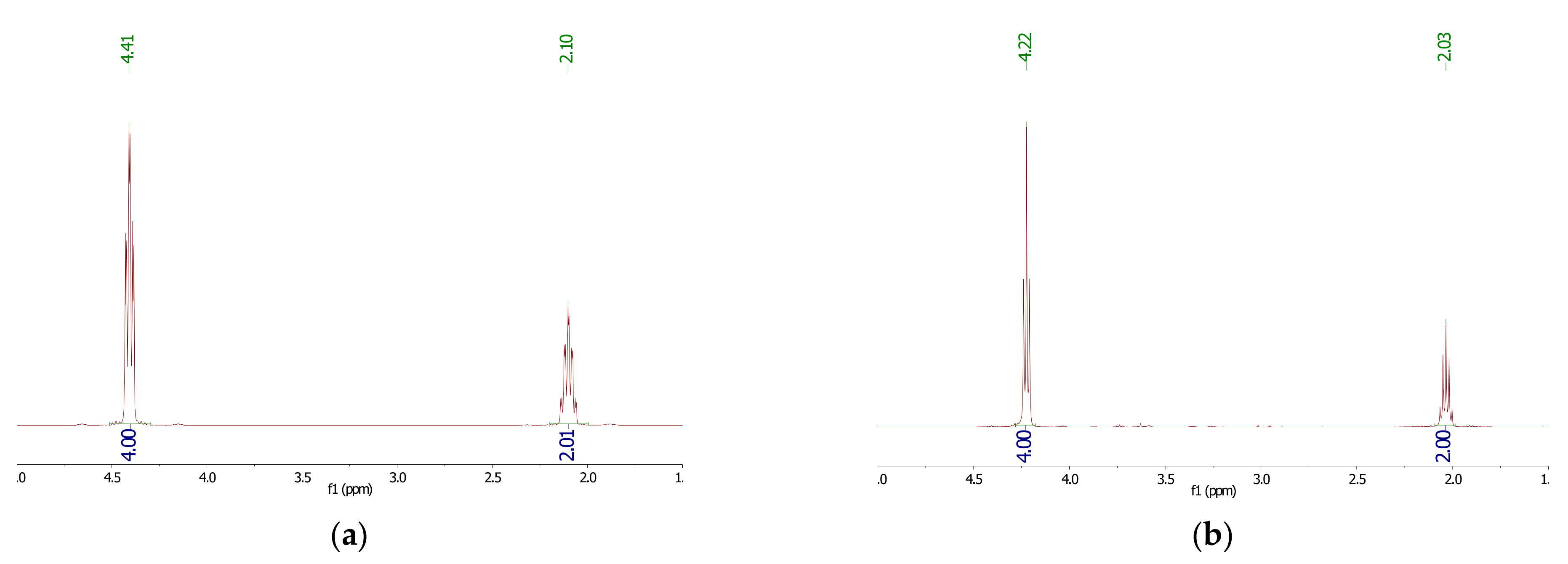
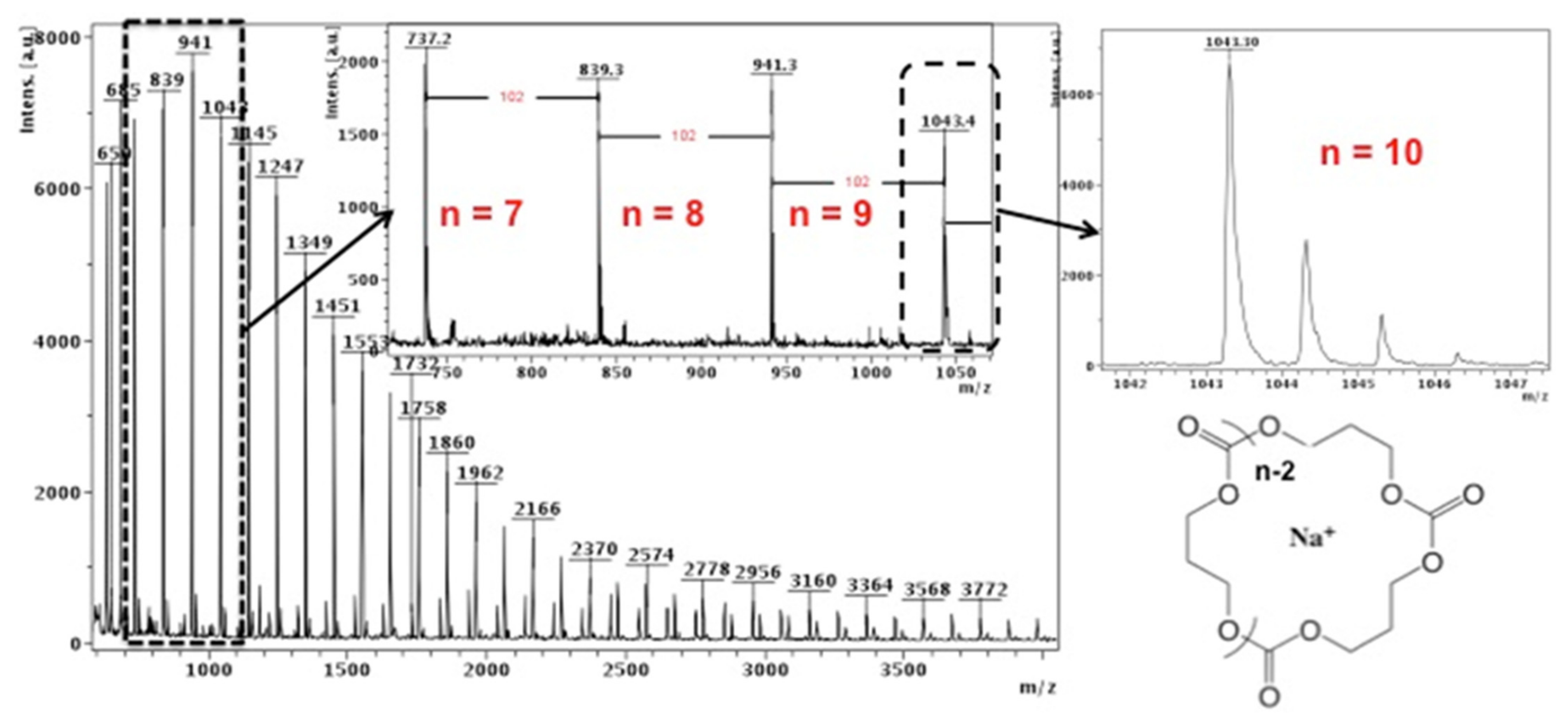
2.3. Characterizations
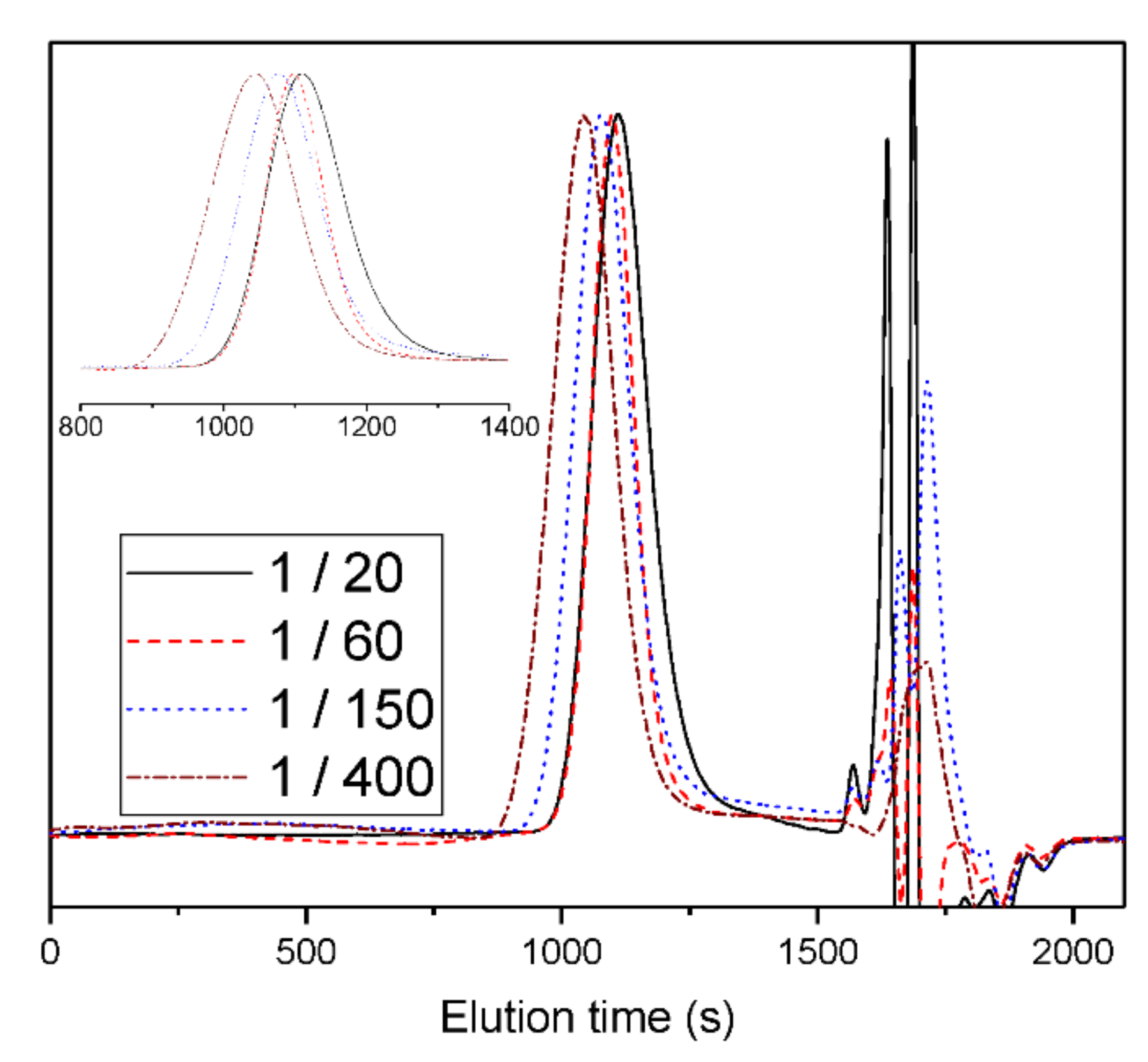
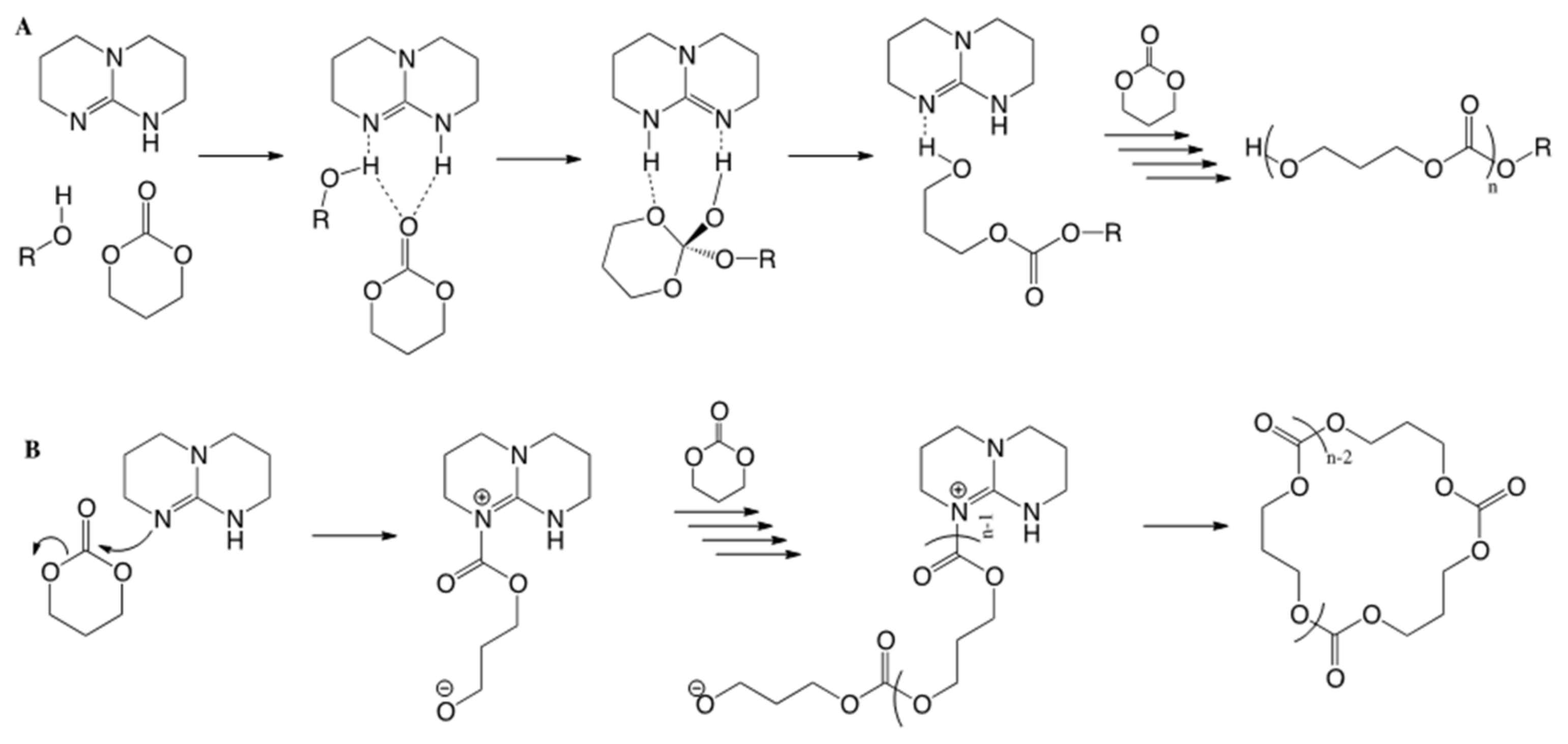
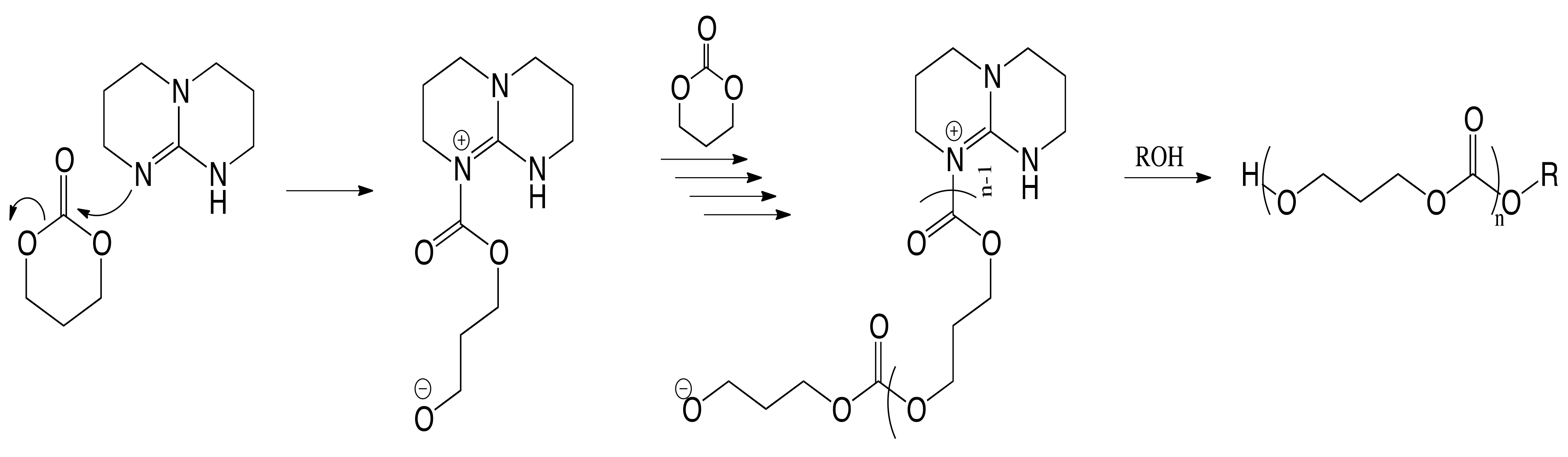
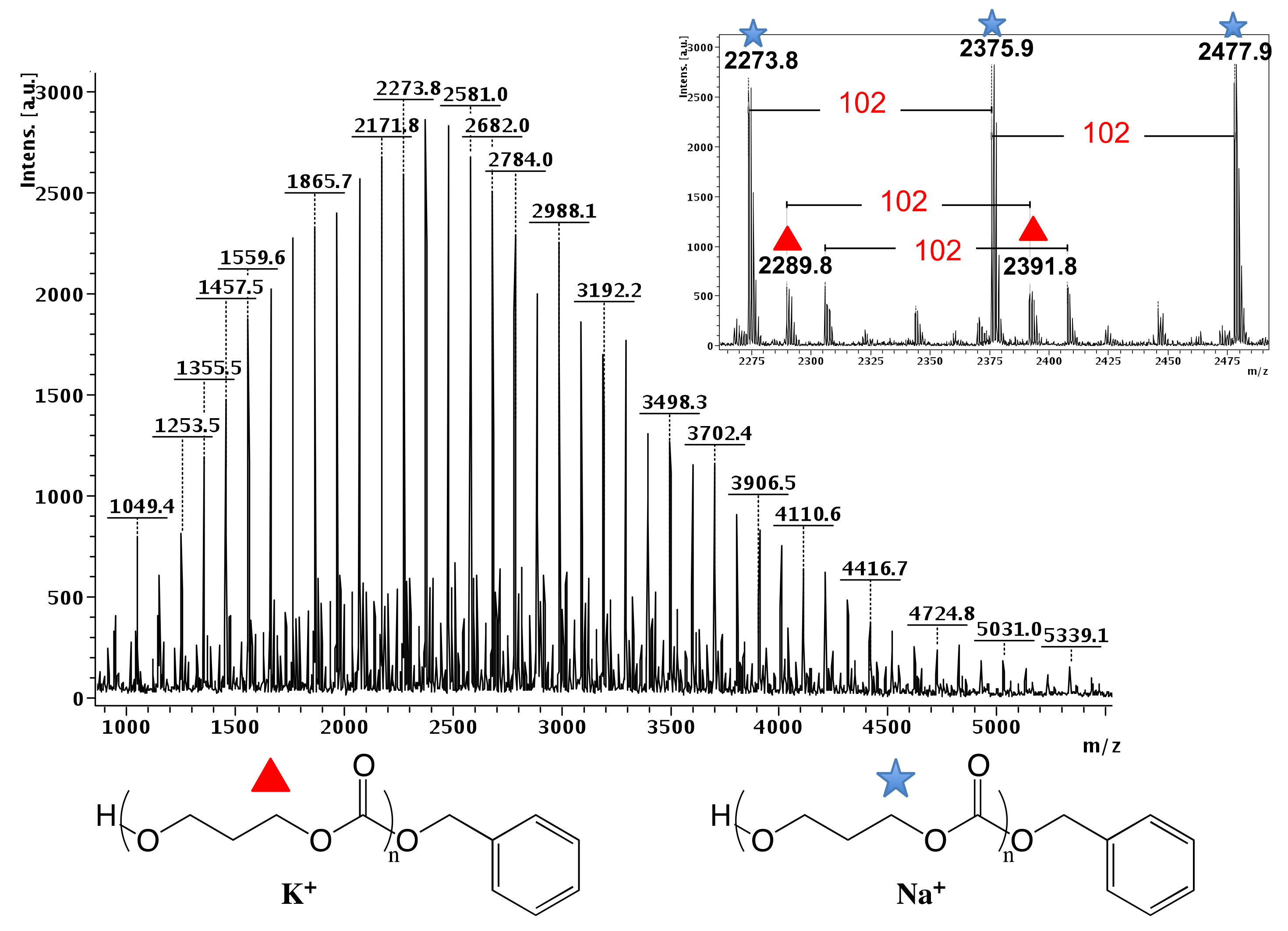
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.W.; Feng, E.; Song, J. Renaissance of aliphatic polycarbonates: New techniques and biomedical applications. J. Appl. Polym. Sci. 2014, 131, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carothers, W.H.; Van Natta, F.J. Studies on polymerization and ring formation. III. Glycol esters of carbonic acid. J. Am. Chem. Soc. 1930, 52, 314–326. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, X.J. Recent development of functional aliphatic polycarbonates for the construction of amphiphilic polymers. Polym. Chem. 2017, 8, 7429–7437. [Google Scholar] [CrossRef]
- Suriano, F.; Coulembier, O.; Hedrick, J.L.; Dubois, P. Functionalized cyclic carbonates: From synthesis and metal-free catalyzed ring-opening polymerization to applications. Polym. Chem. 2011, 2, 528–533. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, X.J. Cationic polycarbonates via ring-opening polymerization: Design, synthesis, and applications. Polym. Chem. 2019, 10, 296–305. [Google Scholar] [CrossRef]
- Bexis, P.; De Winter, J.; Arno, M.C.; Coulembier, O.; Dove, A.P. Organocatalytic synthesis of alkyne-functional aliphatic polycarbonates via ring-opening polymerization of an eight-membered-N-cyclic carbonate. Macromol. Rapid Commun. 2021, 42. [Google Scholar] [CrossRef]
- Geschwind, J.; Wurm, F.; Frey, H. From CO2-based multifunctional polycarbonates with a controlled number of functional groups to graft polymers. Macromol. Chem. Phys. 2013, 214, 892–901. [Google Scholar] [CrossRef]
- Mespouille, L.; Coulembier, O.; Kawalec, M.; Dove, A.P.; Dubois, P. Implementation of metal-free ring-opening polymerization in the preparation of aliphatic polycarbonate materials. Prog. Polym. Sci. 2014, 39, 1144–1164. [Google Scholar] [CrossRef]
- Feng, J.; Zhuo, R.X.; Zhang, X.Z. Construction of functional aliphatic polycarbonates for biomedical applications. Prog. Polym. Sci. 2012, 37, 211–236. [Google Scholar] [CrossRef]
- Yan, B.K.; Hou, J.Q.; Wei, C.; Xiao, Y.; Lang, M.D.; Huang, F.R. Facile preparation of long-chain aliphatic polycarbonates containing block copolycarbonates via one-pot sequential organic catalyzed polymerization of macrocyclic carbonates and trimethylene carbonates. Polym. Chem. 2020, 11, 2166–2172. [Google Scholar] [CrossRef]
- Zhang, Z.; Kuijer, R.; Bulstra, S.K.; Grijpma, D.W.; Feijen, J. The in vivo and in vitro degradation behavior of poly(trimethylene carbonate). Biomaterials 2006, 27, 1741–1748. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Eklund, M. Influence of molecular-structure on the degradation mechanism of degradable polymers–In-vitro degradation of poly(trimethylene carbonate), poly(trimethylene carbonate-co-caprolactone), and poly(adipic anhydride). J. Appl. Polym. Sci. 1995, 57, 87–103. [Google Scholar] [CrossRef]
- Pego, A.P.; Poot, A.A.; Grijpma, D.W.; Feijen, J. In vitro degradation of trimethylene carbonate based (Co)polymers. Macromol. Biosci. 2002, 2, 411–419. [Google Scholar] [CrossRef]
- Barouti, G.; Khalil, A.; Orione, C.; Jarnouen, K.; Cammas-Marion, S.; Loyer, P.; Guillaume, S.M. Poly(trimethylene carbonate)/Poly(malic acid) amphiphilic diblock copolymers as biocompatible nanoparticles. Chem. Eur. J. 2016, 22, 2819–2830. [Google Scholar] [CrossRef]
- Diallo, A.K.; Guerin, W.; Slawinski, M.; Brusson, J.M.; Carpentier, J.F.; Guillaume, S.M. Block and random copolymers of 1,2-cyclohexyl cyclocarbonate and L-lactide or trimethylene carbonate synthesized by ring-opening polymerization. Macromolecules 2015, 48, 3247–3256. [Google Scholar] [CrossRef]
- Fang, J.Y.; Lin, Y.K.; Wang, S.W.; Li, Y.C.; Lee, R.S. Synthesis and characterization of dual-stimuli-responsive micelles based on poly(N-isopropylacrylamide) and polycarbonate with photocleavable moieties. React. Funct. Polym. 2015, 95, 46–54. [Google Scholar] [CrossRef]
- Nederberg, F.; Lohmeijer, B.G.G.; Leibfarth, F.; Pratt, R.C.; Choi, J.; Dove, A.P.; Waymouth, R.M.; Hedrick, J.L. Organocatalytic ring opening polymerization of trimethylene carbonate. Biomacromolecules 2007, 8, 153–160. [Google Scholar] [CrossRef]
- Toshikj, N.; Robin, J.J.; Blanquer, S. A simple and general approach for the synthesis of biodegradable triblock copolymers by organocatalytic ROP from poly(lactide) macroinitiators. Eur. Polym. J. 2020, 127. [Google Scholar] [CrossRef]
- Pendergraph, S.A.; Klein, G.; Johansson, M.K.G.; Carlmark, A. Mild and rapid surface initiated ring-opening polymerisation of trimethylene carbonate from cellulose. Rsc. Adv. 2014, 4, 20737–20743. [Google Scholar] [CrossRef] [Green Version]
- Samuel, C.; Chalamet, Y.; Boisson, F.; Majeste, J.C.; Becquart, F.; Fleury, E. Highly efficient metal-free organic catalysts to design new environmentally friendly starch-based blends. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 493–503. [Google Scholar] [CrossRef]
- Bat, E.; Grijpma, D.W.; Feijen, J. Thermoreversible gelation behaviour of PTMC-PEG-PTMC triblock copolymers. J. Control. Release 2008, 132, E37–E39. [Google Scholar] [CrossRef]
- Fukushima, K. Poly(trimethylene carbonate)-based polymers engineered for biodegradable functional biomaterials. Biomater. Sci. 2016, 4, 9–24. [Google Scholar] [CrossRef]
- Guney, A.; Malda, J.; Dhert, W.J.A.; Grijpma, D.W. Triblock copolymers based on epsilon-caprolactone and trimethylene carbonate for the 3D printing of tissue engineering scaffolds. Int. J. Artif. Organs 2017, 40, 176–184. [Google Scholar] [CrossRef]
- Lebleu, C.; Rodrigues, L.; Guigner, J.M.; Brulet, A.; Garanger, E.; Lecommandoux, S. Self-assembly of PEG-b-PTMC copolymers: Micelles and polymersomes size control. Langmuir 2019, 35, 13364–13374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi, S.; Blanquer, S.B.G.; van Kooten, T.G.; Grijpma, D.W. Biodegradable nanocomposite hydrogel structures with enhanced mechanical properties prepared by photo-crosslinking solutions of poly(trimethylene carbonate)-poly(ethylene glycol)-poly(trimethylene carbonate) macromonomers and nanoclay particles. Acta Biomater. 2012, 8, 4233–4243. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Jie, S.Y.; Braunstein, P.; Li, B.G. Pyridyl-urea catalysts for the solvent-free ring-opening polymerization of lactones and trimethylene carbonate. Eur. Polym. J. 2019, 121. [Google Scholar] [CrossRef]
- Li, X.; Mignard, N.; Taha, M.; Fernandez-de-Alba, C.; Chen, J.D.; Zhang, S.M.; Fort, L.; Becquart, F. Synthesis of Poly(trimethylene carbonate) oligomers by ring-opening polymerization in bulk. Macromol. Chem. Phys. 2020, 221. [Google Scholar] [CrossRef]
- Helou, M.; Miserque, O.; Brusson, J.M.; Carpentier, J.F.; Guillaume, S.M. Organocatalysts for the controlled “immortal” ring-opening polymerization of six-membered-ring cyclic carbonates: A metal-free, green process. Chem. Eur. J. 2010, 16, 13805–13813. [Google Scholar] [CrossRef]
- Chang, Y.A.; Rudenko, A.E.; Waymouth, R.M. Zwitterionic ring-opening polymerization of N-substituted eight-membered cyclic carbonates to generate cyclic poly(carbonate)s. ACS Macro Lett. 2016, 5, 1162–1166. [Google Scholar] [CrossRef]
- Murayama, M.; Sanda, F.; Endo, T. Anionic ring-opening polymerization of a cyclic carbonate having a norbornene structure with amine initiators. Macromolecules 1998, 31, 919–923. [Google Scholar] [CrossRef]
- Ottou, W.N.; Sardon, H.; Mecerreyes, D.; Vignolle, J.; Taton, D. Update and challenges in organo-mediated polymerization reactions. Prog. Polym. Sci. 2016, 56, 64–115. [Google Scholar] [CrossRef] [Green Version]
- Brown, H.A.; Waymouth, R.M. Zwitterionic ring-opening polymerization for the synthesis of high molecular weight cyclic polymers. Acc. Chem. Res. 2013, 46, 2585–2596. [Google Scholar] [CrossRef]
- Roovers, J. Cyclic Polymers, 2nd ed.; Semlyen, J.A., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Wei, J.J.; Meng, H.; Guo, B.B.; Zhong, Z.Y.; Meng, F.H. Organocatalytic ring-opening copolymerization of trimethylene carbonate and dithiolane trimethylene carbonate: Impact of organocatalysts on copolymerization kinetics and copolymer microstructures. Biomacromolecules 2018, 19, 2294–2301. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Q.; Liao, S.R.; Zhao, C.S.; Xie, X.Y. Understanding cyclic by-products and ether linkage formation pathways in the transesterification synthesis of aliphatic polycarbonates. Eur. Polym. J. 2017, 97, 253–262. [Google Scholar] [CrossRef]
- Kiesewetter, M.K.; Scholten, M.D.; Kirn, N.; Weber, R.L.; Hedrick, J.L.; Waymouth, R.M. Cyclic guanidine organic catalysts: What is magic about triazabicyclodecene? J. Org. Chem. 2009, 74, 9490–9496. [Google Scholar] [CrossRef]
- Jones, G.O.; Chang, Y.A.; Horn, H.W.; Acharya, A.K.; Rice, J.E.; Hedrick, J.L.; Waymouth, R.M. N-heterocyclic carbene-catalyzed ring opening polymerization of epsilon-caprolactone with and without alcohol initiators: Insights from theory and experiment. J. Phys. Chem. B 2015, 119, 5728–5737. [Google Scholar] [CrossRef]
- Nederberg, F.; Connor, E.F.; Moller, M.; Glauser, T.; Hedrick, J.L. New paradigms for organic catalysts: The first organocatalytic living polymerization. Angew. Chem. Int. Ed. 2001, 40, 2712–2715. [Google Scholar] [CrossRef]



| Exp. | ncata/nTMC | Catalyst | Alcohol | Temp. (°C) | Reaction Time (min) | Conv. (%) a | Mn (g.mol−1) b | Ð b |
|---|---|---|---|---|---|---|---|---|
| 1 | 1/20 | TBD | without | 25 | 30 | 98 | 14,800 | 1.31 |
| 2 | 1/60 | TBD | without | 25 | 60 | 98 | 20,400 | 1.19 |
| 3 | 1/150 | TBD | without | 25 | 90 | 95 | 23,100 | 1.45 |
| 4 | 1/400 | TBD | without | 25 | 480 | 92 | 33,700 | 1.55 |
| 5 | 1/120 | TBD | BuOH | 25 | 30 | 93 | 5200 | 1.17 |
| 6 | 1/160 | TBD | BuOH | 25 | 360 | 92 | 7900 | 1.5 |
| 7 | 1/220 | DMAP | BnOH | 130 | 140 | --- | 14,600 | 1.60 |
| 8 | 1/440 | DMAP | BnOH | 130 | 110 | --- | 20,600 | 1.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azemar, F.; Gimello, O.; Pinaud, J.; Robin, J.-J.; Monge, S. Insight into the Alcohol-Free Ring-Opening Polymerization of TMC Catalyzed by TBD. Polymers 2021, 13, 1589. https://doi.org/10.3390/polym13101589
Azemar F, Gimello O, Pinaud J, Robin J-J, Monge S. Insight into the Alcohol-Free Ring-Opening Polymerization of TMC Catalyzed by TBD. Polymers. 2021; 13(10):1589. https://doi.org/10.3390/polym13101589
Chicago/Turabian StyleAzemar, Fabrice, Olinda Gimello, Julien Pinaud, Jean-Jacques Robin, and Sophie Monge. 2021. "Insight into the Alcohol-Free Ring-Opening Polymerization of TMC Catalyzed by TBD" Polymers 13, no. 10: 1589. https://doi.org/10.3390/polym13101589







