Synthesis and Characterization of a Fe3O4@PNIPAM-Chitosan Nanocomposite and Its Potential Application in Vincristine Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of NpFe3O4
2.3. NIPAM Polymerization on NpFe3O4 in Presence CS (Fe3O4@PNIPAM-CS)
2.4. Characterization
2.5. Vincristine Sulfate Loading
2.6. Vincristine Sulfate Release Study Mathematical Release Model
3. Results
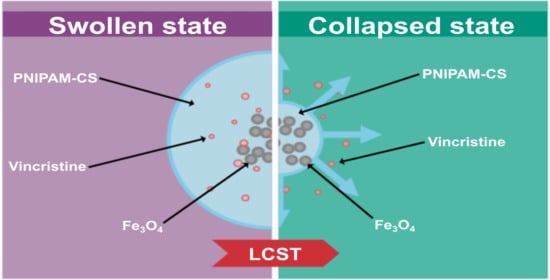
3.1. Swelling Kinetics and LCST
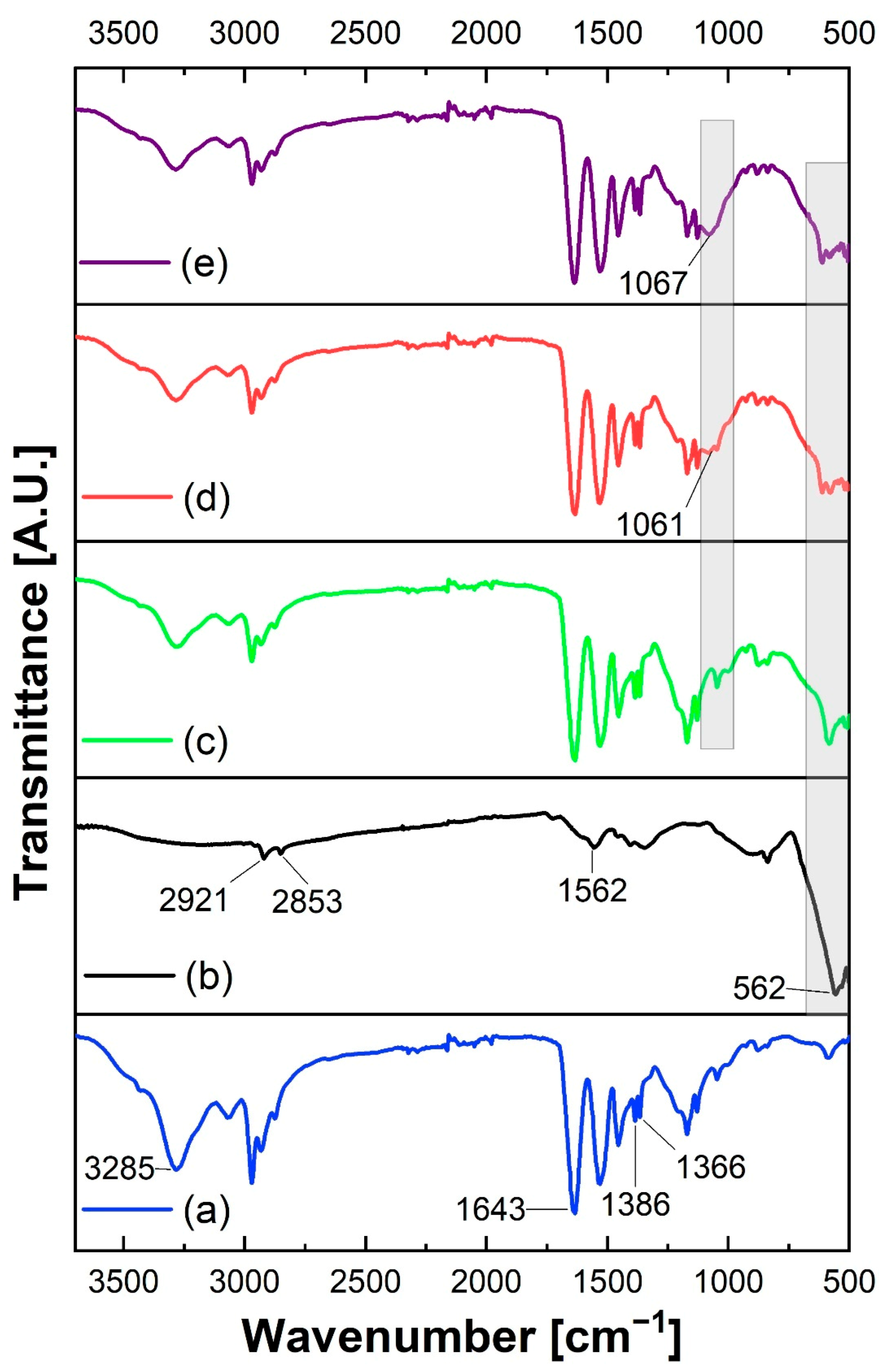
3.2. Infrared Spectroscopy Analysis
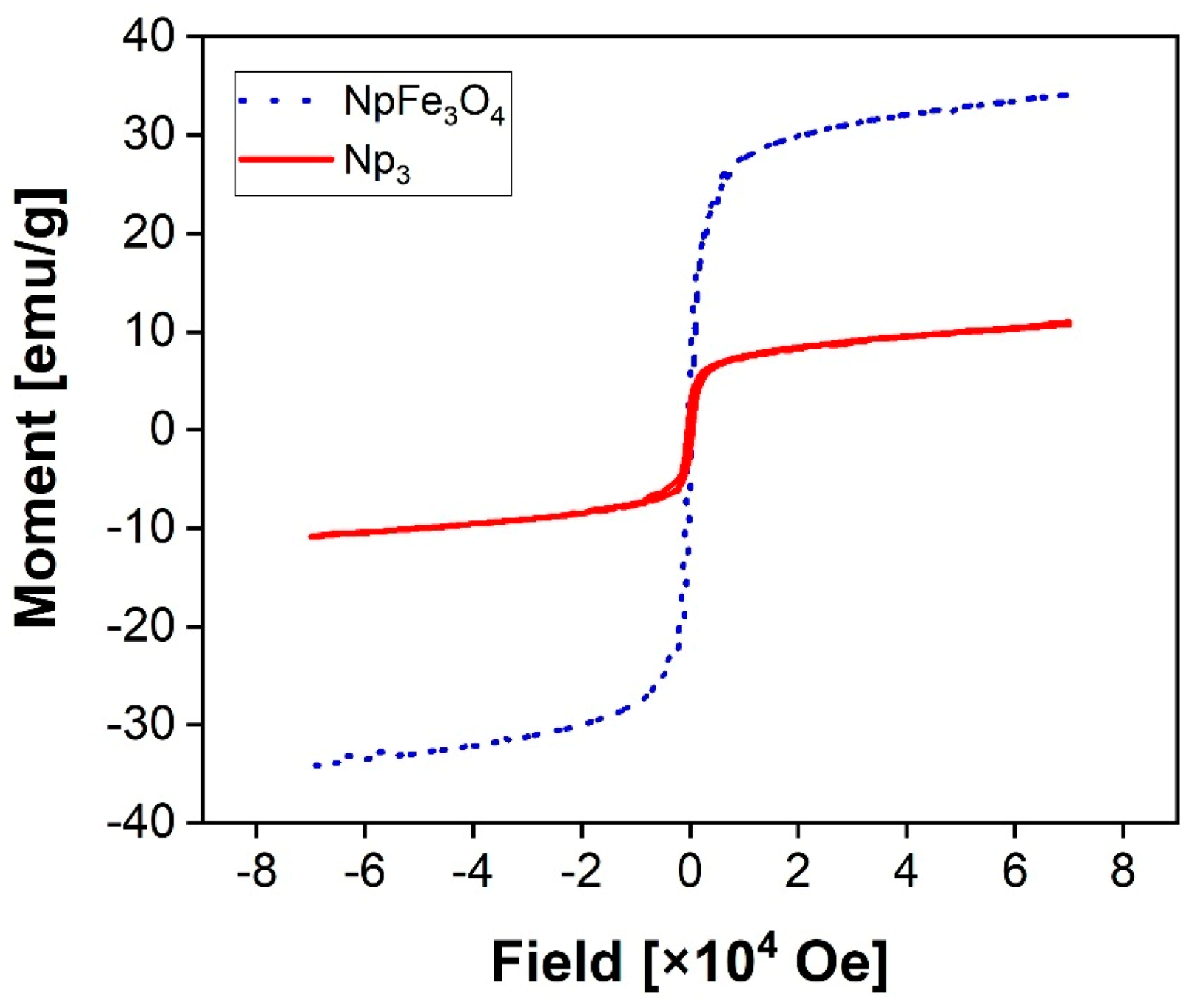
3.3. Magnetic Properties
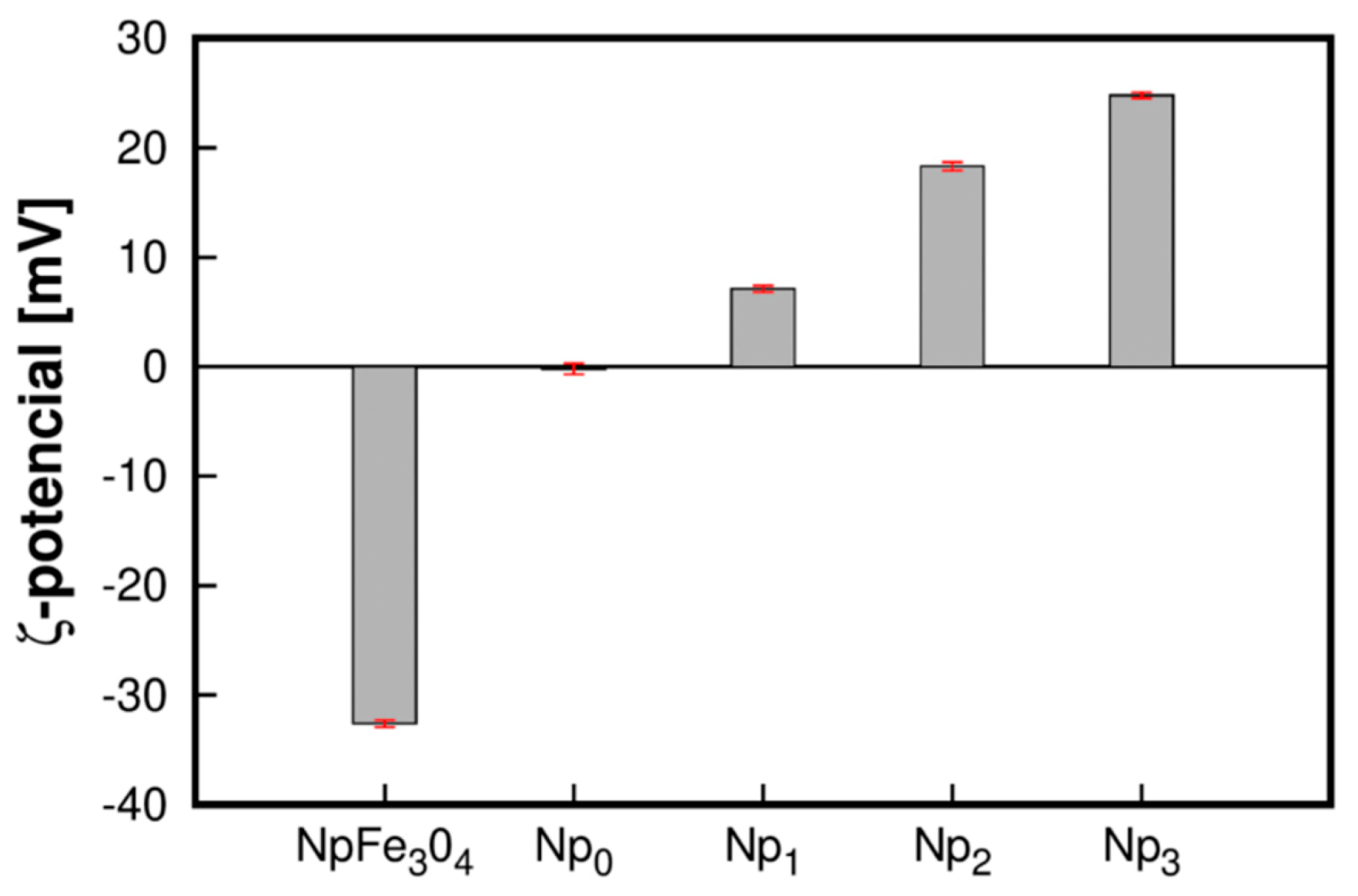
3.4. ζ-Potential
3.5. Morphology Analysis
3.6. Drug Loading (DL) and Encapsulation Efficiency (%EE)
3.7. Effect of Temperature on Drug Delivery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grande, A.H. Nanotecnología y nanopartículas magnéticas: La física actual en lucha contra la enfermedad. Rev. R. Acad. Cienc. Exact. Fís. Nat. 2007, 101, 321–327. [Google Scholar]
- Wang, J.; Huang, N.; Peng, Q.; Cheng, X.; Li, W. Temperature/pH dual-responsive and luminescent drug carrier based on PNIPAM-MAA/lanthanide-polyoxometalates for controlled drug delivery and imaging in HeLa cells. Mater. Chem. Phys. 2020, 239. [Google Scholar] [CrossRef]
- Coral, D.F.; Jenny, A.; Mera, J.A.M. Una guía para el estudio de nanopartículas magnéticas de óxidos de hierro con aplicaciones biomédicas. Parte II. Ing. Cienc. 2017, 13, 207–232. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, W.; Wang, Y.; Wang, X.; Long, S.; Yang, J. Novel PNIPAm-based electrospun nanofibres used directly as a drug carrier for “on-off” switchable drug release. Colloids Surf. B Biointerfaces 2019, 182. [Google Scholar] [CrossRef]
- Umapathi, R.; Reddy, P.M.; Kumar, A.; Venkatesu, P.; Chang, C.J. The biological stimuli for governing the phase transition temperature of the ‘smart’ polymer PNIPAM in water. Colloids Surf. B Biointerfaces 2015, 135, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Matsui, I. Nanoparticles for electronic device applications: A brief review. J. Chem. Eng. Jpn. 2005, 38, 535–546. [Google Scholar] [CrossRef]
- Alarcón-Payán, D.A.; Koyani, R.D.; Vazquez-Duhalt, R. Chitosan-based biocatalytic nanoparticles for pollutant removal from wastewater. Enzyme Microb. Technol. 2017, 100, 71–78. [Google Scholar] [CrossRef]
- Kokardekar, R.R.; Shah, V.K.; Mody, H.R. PNIPAM Poly (N-isopropylacrylamide): A thermoresponsive “smart” polymer in novel drug delivery system. Med. Update 2012, 7, 60–63. [Google Scholar]
- Bahl, S.; Nagar, H.; Singh, I.; Sehgal, S. Smart materials types, properties and applications: A review. Mater. Today Proc. 2020, 28, 1302–1306. [Google Scholar] [CrossRef]
- Nasseri, R.; Deutschman, C.P.; Han, L.; Pope, M.A.; Tam, K.C. Cellulose nanocrystals in smart and stimuli-responsive materials: A review. Mater. Today Adv. 2020, 5. [Google Scholar] [CrossRef]
- Kurakula, M.; Naveen, N.R. Prospection of recent chitosan biomedical trends: Evidence from patent analysis (2009–2020). Int. J. Biol. Macromol. 2020, 165, 1924–1938. [Google Scholar] [CrossRef] [PubMed]
- Sanoj Rejinold, N.; Sreerekha, P.R.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Biocompatible, biodegradable and thermo-sensitive chitosan-g-poly (N-isopropylacrylamide) nanocarrier for curcumin drug delivery. Int. J. Biol. Macromol. 2011, 49, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Larson, N.; Ghandehari, H. Polymeric conjugates for drug delivery. Chem. Mater. 2012, 24, 840–853. [Google Scholar] [CrossRef] [Green Version]
- Häfeli, U.O.; Sweeney, S.M.; Beresford, B.A.; Humm, J.L.; Macklis, R.M. Effective targeting of magnetic radioactive90Y-microspheres to tumor cells by an externally applied magnetic field. Preliminary in vitro and in vivo results. Nucl. Med. Biol. 1995, 22, 147–155. [Google Scholar] [CrossRef]
- Sadighian, S.; Rostamizadeh, K.; Hosseini-Monfared, H.; Hamidi, M. Doxorubicin-conjugated core-shell magnetite nanoparticles as dual-targeting carriers for anticancer drug delivery. Colloids Surf. B Biointerfaces 2014, 117, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Venkatasubramanian, R.; Hein, S.; Misra, R.D.K. A stimulus-responsive magnetic nanoparticle drug carrier: Magnetite encapsulated by chitosan-grafted-copolymer. Acta Biomater. 2008, 4, 1024–1037. [Google Scholar] [CrossRef]
- Alicia, J.D.; Javier, C.H. Nanopartículas magnéticas de zinc y calcio para aplicaciones en hipertermia magnética. Rev. Fac. Ing. 2016, 25, 89–98. [Google Scholar]
- Honey Priya, J.; Rijo, J.; Anju, A.; Anoop, K.R. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebadi, M.; Buskaran, K.; Bullo, S.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Synthesis and cytotoxicity study of magnetite nanoparticles coated with polyethylene glycol and sorafenib–zinc/aluminium layered double hydroxide. Polymers 2020, 12, 2716. [Google Scholar] [CrossRef]
- Petters, C.; Irrsack, E.; Koch, M.; Dringen, R. Uptake and metabolism of iron oxide nanoparticles in brain cells. Neurochem. Res. 2014, 39, 1648–1660. [Google Scholar] [CrossRef]
- Hernández, P.; Lucero-Acuña, A.; Moreno-Cortez, I.E.; Esquivel, R.; Álvarez-Ramos, E. Thermo-magnetic properties of Fe 3 O 4 @poly(N-Isopropylacrylamide) core–shell nanoparticles and their cytotoxic effects on HeLa and MDA-MB-231 cell lines. J. Nanosci. Nanotechnol. 2019, 20, 2063–2071. [Google Scholar] [CrossRef]
- Shi, S.F.; Jia, J.F.; Guo, X.K.; Zhao, Y.P.; Chen, D.S.; Guo, Y.Y.; Cheng, T.; Zhang, X.L. Biocompatibility of chitosan-coated iron oxide nanoparticles with osteoblast cells. Int. J. Nanomed. 2012, 7, 5593–5602. [Google Scholar]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Islam, N.; Dmour, I.; Taha, M.O. Degradability of chitosan micro/nanoparticles for pulmonary drug delivery. Heliyon 2019, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briceño, S.; Hernandez, A.C.; Sojo, J.; Lascano, L.; Gonzalez, G. Degradation of magnetite nanoparticles in biomimetic media. J. Nanopart. Res. 2017, 19. [Google Scholar] [CrossRef]
- Kumar, B.; Jalodia, K.; Kumar, P.; Gautam, H.K. Recent advances in nanoparticle-mediated drug delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 260–268. [Google Scholar] [CrossRef]
- Das, M.; Shim, K.H.; An, S.S.A.; Yi, D.K. Review on gold nanoparticles and their applications. Toxicol. Environ. Health Sci. 2011, 3, 193–205. [Google Scholar] [CrossRef]
- Alkhalil, A.; Strand, S.; Mucker, E.; Huggins, J.W.; Jahrling, P.B.; Ibrahim, S.M. Inhibition of Monkeypox virus replication by RNA interference. Virol. J. 2009, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández Paredes, H.G. Efecto Antiproliferativo de la Betanina y Coadyuvante con Vincristina en Células de Leucemia Lingoblástica Aguda Tipo T. Master’s Thesis, Universidad Autónoma de Querétaro, Santiago de Querétaro, Mexico, October 2018. [Google Scholar]
- Fawcett, S.L.; Grant, I.; Hall, P.N.; Kelsall, A.W.R.; Nicholson, J.C. Vincristine as a treatment for a large haemangioma threatening vital functions. Br. J. Plast. Surg. 2004, 57, 168–171. [Google Scholar] [CrossRef]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esquivel, R.; Canale, I.; Ramirez, M.; Hernández, P.; Zavala-Rivera, P.; Álvarez-Ramos, E.; Lucero-Acuña, A. Poly(N-isopropylacrylamide)-coated gold nanorods mediated by thiolated chitosan layer: Thermo-pH responsiveness and optical properties. E-Polymers 2018, 18, 163–174. [Google Scholar] [CrossRef]
- Lammel, T.; Thit, A.; Cui, X.; Mouneyrac, C.; Baun, A.; Valsami-Jones, E.; Sturve, J.; Selck, H. Trophic transfer of CuO NPs from sediment to worms (Tubifex tubifex) to fish (Gasterosteus aculeatus): A comparative study of dissolved Cu and NPs enriched with a stable isotope tracer (65Cu). Environ. Sci. Nano 2020, 7. [Google Scholar] [CrossRef]
- Kerli, S.; Alver, U.; Göğebakan, M. Investigation of the electrical properties of Al85Y9Ni6 metallic glass and formulation of the results. Glass Phys. Chem. 2020, 46, 189–193. [Google Scholar] [CrossRef]
- Jiao, Y.; Gong, X.; Han, H.; Gao, Y.; Lu, W.; Liu, Y.; Xian, M.; Shuang, S.; Dong, C. Facile synthesis of orange fluorescence carbon dots with excitation independent emission for pH sensing and cellular imaging. Anal. Chim. Acta 2018, 1042, 125–132. [Google Scholar] [CrossRef]
- Chen, G.; Hoffman, A.S. Graft copolymers that exhibit temperature-induced phase transitions over a wide range of pH. Nature 1995, 373. [Google Scholar] [CrossRef]
- Huang, C.H.; Chuang, T.J.; Ke, C.J.; Yao, C.H. Doxorubicin-gelatin/Fe3O4-Alginate dual-layer magnetic nanoparticles as targeted anticancer drug delivery vehicles. Polymers 2020, 12, 1747. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Quinto, C.A.; Zhang, L.; Mohindra, P.; Bao, G. Size-dependent heating of magnetic iron oxide nanoparticles. ACS Nano 2017, 11, 6808–6816. [Google Scholar] [CrossRef]
- Schwertmann, U.; Cornell, R.M. The Iron Oxides: Structure, Properties, Reactions, Occurrence, and Uses; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- Atabaev, T.S.; Kim, H.K.; Hwang, Y.H. Fabrication of bifunctional core-shell Fe3O4 particles coated with ultrathin phosphor layer. Nanoscale Res. Lett. 2013, 8, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Shagholani, H.; Ghoreishi, S.M.; Mousazadeh, M. Improvement of interaction between PVA and chitosan via magnetite nanoparticles for drug delivery application. Int. J. Biol. Macromol. 2015, 78, 130–136. [Google Scholar] [CrossRef]
- Bruniaux, J.; Ben Djemaa, S.; Aubert, K.H.; Marchais, H.; Chourpa, I.; David, S. Stealth magnetic nanocarriers of siRNA as platform for breast cancer theranostics. Int. J. Pharm. 2017, 532, 660–668. [Google Scholar] [CrossRef]
- Ali, E.M.M.; Elashkar, A.A.; El-Kassas, H.Y.; Salim, E.I. Methotrexate loaded on magnetite iron nanoparticles coated with chitosan: Biosynthesis, characterization, and impact on human breast cancer MCF-7 cell line. Int. J. Biol. Macromol. 2018, 120, 1170–1180. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, J.C. FITC-dextran releases from chitosan microgel coated with poly(N-isopropylacrylamide-co-methacrylic acid). Polym. Test. 2010, 29, 784–792. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, Q.L.; Zhu, A.M.; Zhang, Q.G. One-pot synthesis of poly(N-isopropylacrylamide)/chitosan composite microspheres via microemulsion. Carbohydr. Polym. 2012, 90, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Dawes, G.J.S.; Fratila-Apachitei, L.E.; Mulia, K.; Apachitei, I.; Witkamp, G.J.; Duszczyk, J. Size effect of PLGA spheres on drug loading efficiency and release profiles. J. Mater. Sci. Mater. Med. 2009, 20, 1089–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, K.; He, X.; Song, Z.; Yin, Q.; Zhang, Y.; Uckun, F.M.; Jiang, C.; Cheng, J. Dimeric drug polymeric nanoparticles with exceptionally high drug loading and quantitative loading efficiency. J. Am. Chem. Soc. 2015, 137, 3458–3461. [Google Scholar] [CrossRef]
- Yar, Y.; Khodadust, R.; Akkoc, Y.; Utkur, M.; Saritas, E.U.; Gozuacik, D.; Acar, H.Y. Development of tailored SPION-PNIPAM nanoparticles by ATRP for dually responsive doxorubicin delivery and MR imaging. J. Mater. Chem. B 2018, 6, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Ahmad, I.; Umar, S.; Iqbal, Z.; Samim, M.; Ahmad, F.J. PNIPAM nanoparticles for targeted and enhanced nose-to-brain delivery of curcuminoids: UPLC/ESI-Q-ToF-MS/MS-based pharmacokinetics and pharmacodynamic evaluation in cerebral ischemia model. Drug Deliv. 2014, 7544, 1–20. [Google Scholar] [CrossRef] [PubMed]





| Temperature (°C) | K1 | N | R2 |
|---|---|---|---|
| 25 | 0.1168 | 0.2749 | 0.9754 |
| 37 | 0.0447 | 0.3929 | 0.9781 |
| 41 | 0.0985 | 0.3035 | 0.8911 |
| Temperature (°C) | A1 | A2 | Logx01 | Logx02 | h1 | h2 | R2 |
|---|---|---|---|---|---|---|---|
| 25 | - | - | - | - | - | - | - |
| 37 | −9.7686 | 0.9931 | −526.4825 | 1304.0414 | 0.0037 | 0.0037 | 0.99639 |
| 41 | −66.5933 | 0.9977 | −619.7001 | 715.7139 | 0.1503 | 0.1503 | 0.99853 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Téllez, C.N.; Luque-Alcaraz, A.G.; Plascencia-Jatomea, M.; Higuera-Valenzuela, H.J.; Burgos-Hernández, M.; García-Flores, N.; Álvarez-Ramos, M.E.; Iriqui-Razcon, J.L.; Gonzalez, R.E.; Hernández-Abril, P.A. Synthesis and Characterization of a Fe3O4@PNIPAM-Chitosan Nanocomposite and Its Potential Application in Vincristine Delivery. Polymers 2021, 13, 1704. https://doi.org/10.3390/polym13111704
Hernández-Téllez CN, Luque-Alcaraz AG, Plascencia-Jatomea M, Higuera-Valenzuela HJ, Burgos-Hernández M, García-Flores N, Álvarez-Ramos ME, Iriqui-Razcon JL, Gonzalez RE, Hernández-Abril PA. Synthesis and Characterization of a Fe3O4@PNIPAM-Chitosan Nanocomposite and Its Potential Application in Vincristine Delivery. Polymers. 2021; 13(11):1704. https://doi.org/10.3390/polym13111704
Chicago/Turabian StyleHernández-Téllez, Cynthia N., Ana G. Luque-Alcaraz, Maribel Plascencia-Jatomea, Hiram J. Higuera-Valenzuela, Mabeth Burgos-Hernández, Nadia García-Flores, Mario E. Álvarez-Ramos, Jorge L. Iriqui-Razcon, Reynaldo Esquivel Gonzalez, and Pedro A. Hernández-Abril. 2021. "Synthesis and Characterization of a Fe3O4@PNIPAM-Chitosan Nanocomposite and Its Potential Application in Vincristine Delivery" Polymers 13, no. 11: 1704. https://doi.org/10.3390/polym13111704