Advances in Low-Density Flexible Polyurethane Foams by Optimized Incorporation of High Amount of Recycled Polyol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumental Analysis Methods
2.1.1. FTIR Analysis
2.1.2. Thermogravimetric Analysis (TGA)
2.1.3. Scanning Electron Microscopy (SEM)
2.2. Physical Properties of the Polyols
2.3. Formulation of the Flexible Polyether Polyurethane Foam
Amine Catalysts Used for the Synthesis of PU Foams
2.4. Testing Methods of the Physical Properties of the Foams
3. Results and Discussion
3.1. Characterization of the Recycled and Reference Polyol
3.1.1. Physical Properties
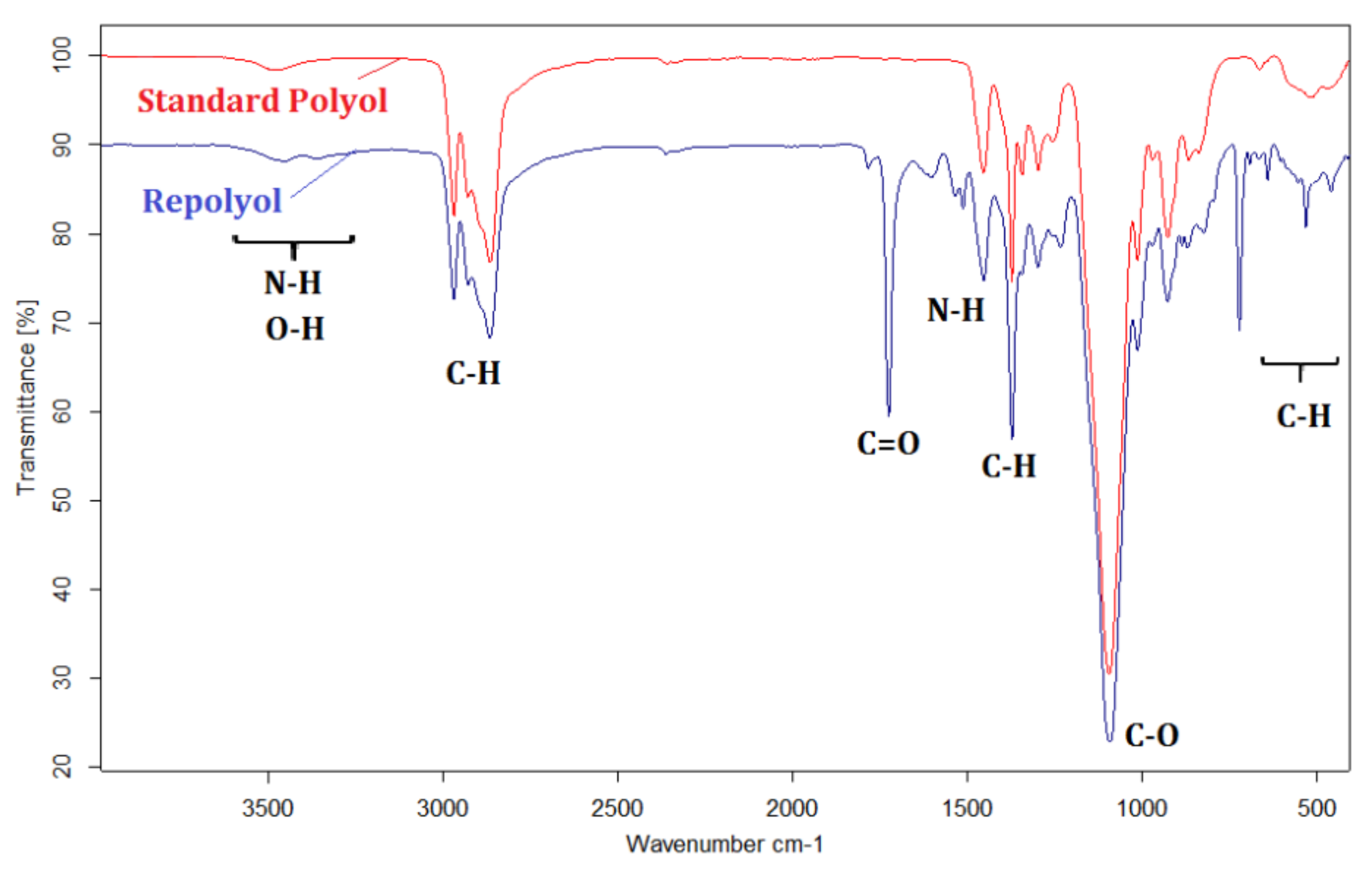
3.1.2. FTIR Analysis
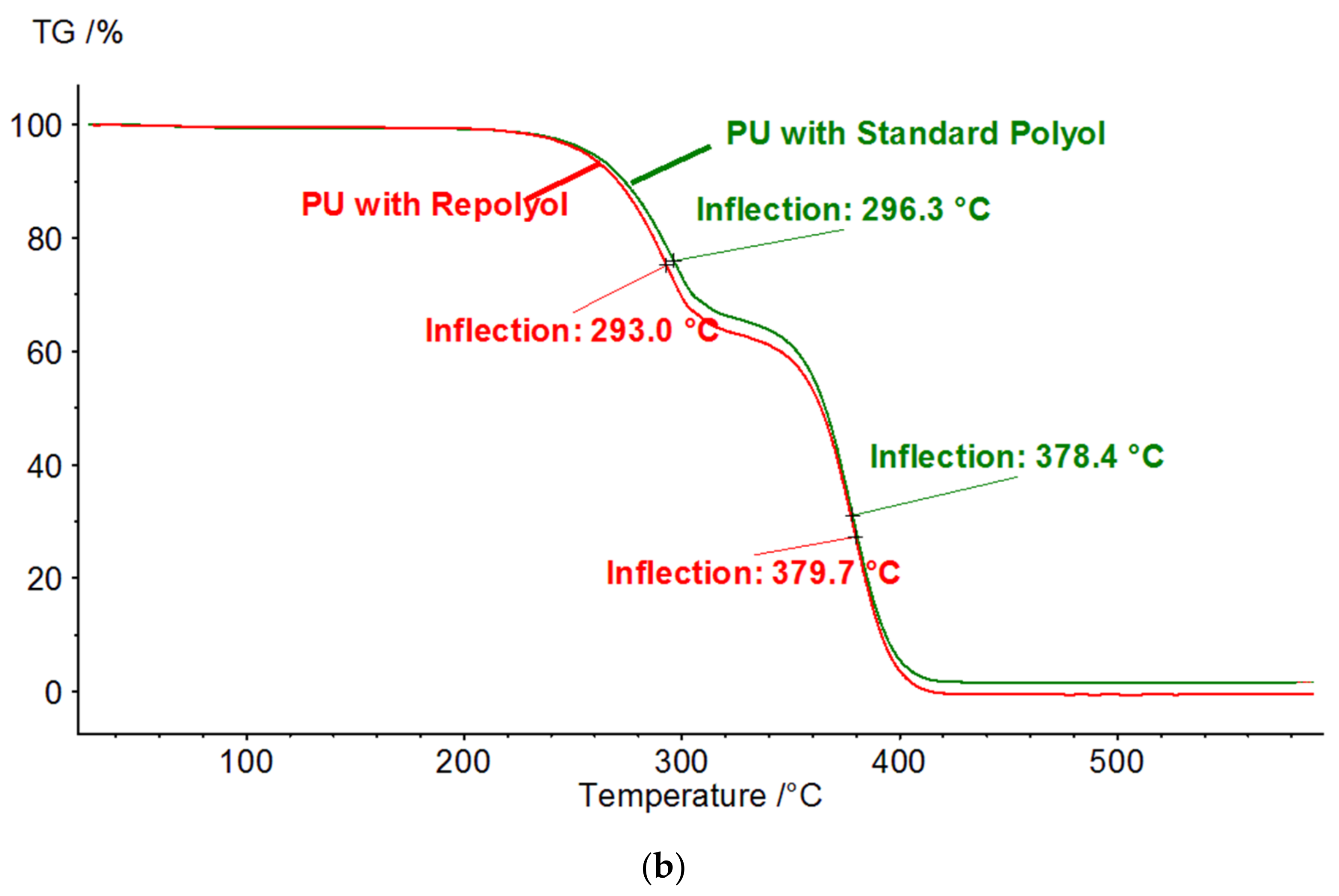
3.1.3. Thermogravimetric Analysis (TGA)
3.2. Production of Flexible Polyurethane Foams with Increasing Amounts of Recycled Polyol
3.2.1. Influence of the Recycled Polyol Amount
3.2.2. Optimization of the Polyurethane Foam Properties by Appropriate Selection of the Tertiary Amine Catalyst
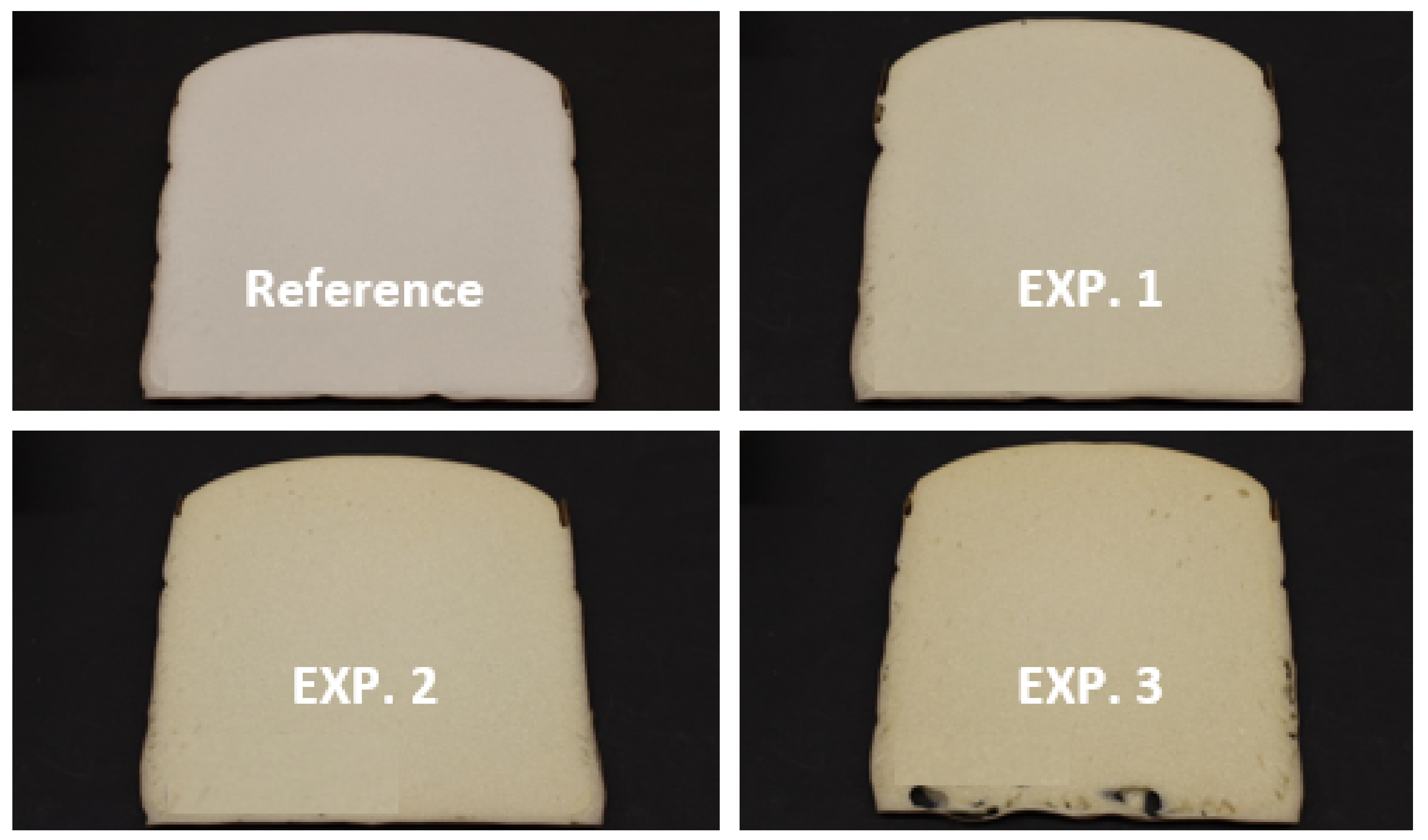
3.2.3. SEM Analysis
3.2.4. Foam Emission Test Assessment for Evaluation of the Environmental Impact
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miao, Y.; von Jouanne, A.; Yokochi, A. Current technologies in depolymerization process and the road ahead. Polymers 2021, 13, 449. [Google Scholar] [CrossRef] [PubMed]
- Polyurethane Foam Market by Type (Rigid Foam, Flexible Foam, Spray Foam), End-Use Industry (Building & Construction, Bedding & Furniture, Automotive, Electronics, Footwear, Packaging, Others), and Region—Global Forecast to 2025. Available online: https://www.marketsandmarkets.com/Market-Reports/polyurethane-foams-market-1251.html (accessed on 3 December 2020).
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Recycling of polyurethanes from laboratory to industry, a journey towards the sustainability. Waste Manag. 2018, 76, 147–171. [Google Scholar] [CrossRef] [PubMed]
- Alavi Nikje, M.M.; Garmarudi, A.B.; Idris, A.B. Polyurethane waste reduction and recycling: From bench to pilot scales. Des. Monomers Polym. 2011, 14, 395–421. [Google Scholar] [CrossRef]
- Datta, J.; Włoch, M. Chapter 14—Recycling of Polyurethanes. In Polyurethane Polymers. Blends and Interpenetrating Polymer Networks; Thomas, S., Datta, J., Haponiuk, J.T., Reghunadhan, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 323–358. ISBN 978-0-128-04039-3. [Google Scholar]
- Serrano, L.; Rincón, E.; García, A.; Rodríguez, J.; Briones, R. Bio-degradable polyurethane foams produced by liquefied polyol from wheat straw biomass. Polymers 2020, 12, 2646. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Dong, Q.; Liu, S.; Xie, H.; Liu, L.; Li, J. Recycling and disposal methods for polyurethane foam wastes. Procedia Environ. Sci. 2012, 16, 167–175. [Google Scholar] [CrossRef]
- Deng, Y.; Dewil, R.; Appels, L.; Ansart, R.; Baeyens, J.; Kang, Q. Reviewing the thermo-chemical recycling of waste polyurethane foam. J. Environ. Manag. 2021, 278, 111527. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, R.V.; Srivastava, S.; Mahanwar, P.A.; Gadekar, P.T. Recycling and disposal methods for polyurethane wastes: A review. Open J. Polym. Chem. 2019, 9, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Gama, N.; Godinho, B.; Marques, G.; Silva, R.; Barros-Timmons, A.; Ferreira, A. Recycling of polyurethane scraps via acidolysis. Chem. Eng. J. 2020, 395, 125102. [Google Scholar] [CrossRef]
- Godinho, B.; Gama, N.; Barros-Timmons, A.; Ferreira, A. Recycling of polyurethane wastes using different carboxylic acids via acidolysis to produce wood adhesives. J. Polym. Sci. 2021, 59, 697–705. [Google Scholar] [CrossRef]
- Gama, N.; Godinho, B.; Marques, G.; Silva, R.; Barros-Timmons, A.; Ferreira, A. Recycling of polyurethane by acidolysis: The effect of reaction conditions on the properties of the recovered polyol. Polymer 2021, 219, 123561. [Google Scholar] [CrossRef]
- Sołtysiński, M.; Piszczek, K.; Romecki, D.; Narożniak, S.; Tomaszewska, J.; Skórczewska, K. Conversion of polyurethane technological foam waste and post-consumer polyurethane mattresses into polyols—Industrial applications. Polimery 2018, 63, 234–238. [Google Scholar] [CrossRef]
- Rees, J.J.M.; Fowler, G. Rebound Polyurethane Foam Comprising Reclaimed Carpet Material and Methods for the Manufacture. U.S. Patent 9410026B1, 9 August 2016. [Google Scholar]
- Münzmay, T.; Resshofer, W.; Meckel, W. Process for the Production of Hydroxyl-Group-Containing Compounds from Polyurethane Polyurea and or Polyurea Wastes. U.S. Patent 5635542, 3 June 1997. [Google Scholar]
- Kettemann, B.; Melciorre, M. Process for Recovering Secondary Polyols from Polyadducts Mixed with Nonglycolysable Materials. U.S. Patent 5684054, 4 November 1997. [Google Scholar]
- Kierkus, P.; You, K. Process for the Preparation of Recyclate Polyols Having a Low Amine Content. U.S. Patent 5763692, 9 June 1998. [Google Scholar]
- Münzmay, T.; Meckel, W.; Nefzger, H.; Dörner, K.-H. Process for the Production of Hydroxy Functional Compounds from Polyurethane Polyurea Waste. U.S. Patent 6020386, 1 February 2000. [Google Scholar]
- Naber, B.; Neiss, V.; Gassan, M.; Deutsch, W. Recycling of Plastic Waste Containing a Mixture of Polyurethanes with Other Plastics. U.S. Patent 6069182, 30 May 2000. [Google Scholar]
- Behrendt, G. Method for Producing Polyols and Polyols. U.S. Patent 6683119B1, 27 January 2004. [Google Scholar]
- Tucker, B.; Ulrich, H. Novel Process of Reclaiming Polyurethane Foam. U.S. Patent 3983087, 28 September 2001. [Google Scholar]
- Sendijarevic, V. Process for Chemical Recycling of Polyurethane-Containing Scrap. U.S. Patent 6750260B2, 15 June 2004. [Google Scholar]
- Kurlis, Z.; Horak, Z.; Benes, H. Method of Recycling Waste Polyurethane Foams. EP Patent 2183311B1, 3 December 2014. [Google Scholar]
- UTECH Polyurethane Report Recycling Polyols Back into Flexible foam, Information. Available online: https://www.utech-polyurethane.com/node/807346/printable/print (accessed on 3 December 2020).
- Kiss, G.; Rusu, G.; Peter, F.; Tănase, I.; Bandur, G. Recovery of Flexible Polyurethane Foam Waste for Efficient Reuse in Industrial Formulations. Polymers 2020, 12, 1533. [Google Scholar] [CrossRef] [PubMed]
- Eling, B.; Tomović, Z.; Schädler, V. Current and future trends in polyurethanes: An industrial perspective. Macromol. Chem. Phys. 2020, 221, 2000114. [Google Scholar] [CrossRef]
- Muuronen, M.; Deglmann, P.; Tomovic, Z. Design principles for rational polyurethane catalyst development. J. Org. Chem. 2019, 84, 8202–8209. [Google Scholar] [CrossRef] [PubMed]
- Dworakowska, S.; Bogdał, D.; Zaccheria, F.; Ravasio, N. The role of catalysis in the synthesis of polyurethane foams based on renewable raw materials. Cat. Today 2014, 223, 148–156. [Google Scholar] [CrossRef]
- German Institute for Standardisation (Deutsches Institut für Normung). DIN EN ISO Standard No. 845:2009, Cellular Plastics and Rubbers—Determination of Apparent Density. 2009. Available online: https://infostore.saiglobal.com/en-gb/standards/DIN-EN-ISO-845-2009-449695_SAIG_DIN_DIN_1014045/ (accessed on 14 February 2021).
- International Organization for Standardization. ISO Standard No. 3386-1:1986. Polymeric Materials, Cellular Flexible—Determination of Stress-Strain Characteristics in Compression—Part 1: Low-Density Materials. 1986. Available online: https://www.iso.org/standard/8683.html (accessed on 14 February 2021).
- International Organization for Standardization. ISO Standard No. 7231:2010. Polymeric Materials, Cellular, Flexible—Determination of Air Flow Value at Constant Pressure-Drop. 2010. Available online: https://www.iso.org/standard/43778.html (accessed on 14 February 2021).
- Kiss, G.; Heisler, L.; Luo, Y.; Pickrell, G. New additives for polyester and flame lamination foam. In Proceedings of the Polyurethanes 2012 Technical Conference Proceedings, Atlanta, GA, USA, 24–26 September 2012; American Chemistry Council: Washington, DC, USA, 2012; pp. 419–432, ISBN 978-1-62748-278-3. [Google Scholar]
- VDA 278 Thermodesorptionsanalyse Organischer Emissionen zur Charakterisierung Nichtmetallischer KFZ-Werkstoffe. Available online: https://www.vda.de/de/services/Publikationen/vda-278-thermodesorptionsanalyse-organischer-emissionen.html (accessed on 3 December 2020).
- Allan, D.; Daly, J.; Liggat, J.J. Thermal volatilisation analysis of TDI-based flexible polyurethane foam. Polym. Degrad. Stab. 2013, 98, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Kiss, G.; Heisler, L.; Fiore, L. New developments in silicone surfactants for high resilience slabstock foams. In Proceedings of the UTECH Technical Program, Maastricht, The Netherlands, April 2015; Available online: https://www.utech-polyurethane.com/node/858881/printable/print (accessed on 3 December 2020).
- Defonseka, C. Flexible Polyurethane Foams. A Practical Guide; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2019. [Google Scholar]
- Kraitape, N.; Thongpin, C. Influence of recycled polyurethane polyol on the properties of flexible polyurethane foams. Energy Procedia 2016, 89, 186–197. [Google Scholar] [CrossRef] [Green Version]
- Product Technical Data Sheet and Detailed Description of Niax Catalyst EF-700. Available online: https://www.momentive.com/en-us/categories/urethane-additives/niax-catalyst-ef-700 (accessed on 3 December 2020).



| Component | Pbw a |
|---|---|
| Reference polyol (Voranol 3322) | 70–100 |
| Repolyol | 0–30 |
| Water | 4.50 |
| Niax Silicone L-895 | 1.00 |
| Niax Stannous Octoate | 0.16 |
| Amine Catalyst(s) b | 0.06–0.30 |
| Isocyanate index c (TDI 80/20) | 108 |
| Catalyst | Chemical Name | Chemical Structure |
|---|---|---|
| Amine 1 | Bis-[2-(N,N-dimethylamino)-ethyl]-ether | 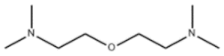 |
| Amine 2 | 1,4-Diazabicyclo[2.2.2]octane | 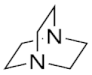 |
| Amine 3 | N,N′-Bis[3-(dimethylamino)propyl]urea | 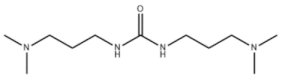 |
| Characteristic | Repolyol | Reference Polyol |
|---|---|---|
| Color | Brown | Colorless |
| Water content (%) | 0.05 ± 0.02 | 0.05 ± 0.02 |
| Viscosity (cSt) | 12,500 ± 110 | 550 ± 8.7 |
| Hydroxyl number (mg KOH/g) | 46.90 ± 1.02 | 48.00 ± 1.11 |
| Acid number (mg KOH/g) | 0.22 ± 0.01 | 0.05 ± 0.005 |
| Formulation Changes vs. Table 1 | Reference Foam | EXP. 1 | EXP. 2 | EXP. 3 |
|---|---|---|---|---|
| Reference polyol (pbw) | 100.0 | 90.0 | 80.0 | 70.0 |
| Repolyol (pbw) | 0.0 | 10.0 | 20.0 | 30.0 |
| Amine 1: Amine 2 at 1:3 weight ratio (pbw) | 0.06 | 0.06 | 0.06 | 0.06 |
| Foam Physical Properties | ||||
| Rise time (s) | 102 | 100 | 100 | 111 |
| Foam settling (%) | - | - | - | - |
| Density (kg/m3) | 22.60 | 22.50 | 22.50 | 23.20 |
| Relative density | 1.00 | 0.995 | 0.995 | 1.026 |
| Compression set 75%, 22 h, 70 °C (%) | 12.34 | 14.92 | 24.22 | 38.07 |
| Hardness CFD-40% (kPa) | 3.26 | 4.06 | 4.15 | 5.17 |
| SAG | 2.45 | 2.49 | 2.69 | 3.77 |
| Airflow (L/min) | 123.00 | 91.00 | 20.00 | 1.00 |
| Cell structure (fine 1… coarse 8) | 2 | 2 | 3 | 3 |
| Formulation Changes vs. Table 1 | EXP. 3 | EXP. 4 | EXP. 5 | EXP. 6 |
|---|---|---|---|---|
| Reference polyol (pbw) | 70.0 | 70.0 | 70.0 | 70.0 |
| Repolyol (pbw) | 30.0 | 30.0 | 30.0 | 30.0 |
| Amine 1: Amine 2, at 1:3 weight ratio (pbw) | 0.06 | |||
| Amine 1 (pbw) | 0.06 | |||
| Amine 2 (pbw) | 0.06 | |||
| Amine 3 (pbw) | 0.30 | |||
| Foam Physical Properties | ||||
| Rise time (s) | 111 | 111 | 111 | 99 |
| Foam settling (%) | - | - | - | - |
| Density (kg/m3) | 21.57 | 21.21 | 21.75 | 22.70 |
| Relative density | 1.00 | 0.98 | 1.01 | 1.05 |
| Hardness CFD-40% (kPa) | 5.17 | 5.57 | 4.82 | 4.06 |
| Compression set 75%, 22 h, 70 °C (%) | 38.07 | 39.55 | 34.21 | 13.40 |
| SAG | 3.77 | 4.19 | 3.36 | 2.67 |
| Airflow (L/min) | 1.00 | 1.00 | 1.00 | 87.00 |
| Cell structure (fine 1… coarse 8) | 3 | 3 | 3 | 2 |
| Formulation Changes vs. Table 1 | EXP. 1 | EXP. 6 |
|---|---|---|
| Reference Polyol (pbw) | 100.0 | 70.0 |
| Repolyol (pbw) | - | 30.0 |
| Amine 1: Amine 2 at ratio 1:3 (pbw) | 0.06 | - |
| Amine 3 (pbw.) | - | 0.30 |
| Stannous octoate (pbw.) | 0.16 | 0.16 |
| Emission Test VOC and FOG Results (ppm) | ||
| Total VOC | 102.0 | 41.0 |
| Catalyst package contribution | 93.0 | 32.0 |
| Total FOG | 194.0 | 187.0 |
| Catalyst package contribution | 44.0 | 33.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, G.; Rusu, G.; Bandur, G.; Hulka, I.; Romecki, D.; Péter, F. Advances in Low-Density Flexible Polyurethane Foams by Optimized Incorporation of High Amount of Recycled Polyol. Polymers 2021, 13, 1736. https://doi.org/10.3390/polym13111736
Kiss G, Rusu G, Bandur G, Hulka I, Romecki D, Péter F. Advances in Low-Density Flexible Polyurethane Foams by Optimized Incorporation of High Amount of Recycled Polyol. Polymers. 2021; 13(11):1736. https://doi.org/10.3390/polym13111736
Chicago/Turabian StyleKiss, Gabriel, Gerlinde Rusu, Geza Bandur, Iosif Hulka, Daniel Romecki, and Francisc Péter. 2021. "Advances in Low-Density Flexible Polyurethane Foams by Optimized Incorporation of High Amount of Recycled Polyol" Polymers 13, no. 11: 1736. https://doi.org/10.3390/polym13111736
APA StyleKiss, G., Rusu, G., Bandur, G., Hulka, I., Romecki, D., & Péter, F. (2021). Advances in Low-Density Flexible Polyurethane Foams by Optimized Incorporation of High Amount of Recycled Polyol. Polymers, 13(11), 1736. https://doi.org/10.3390/polym13111736









