Synthesis of Polymer-Based Magnetic Nanocomposite for Multi-Pollutants Removal from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
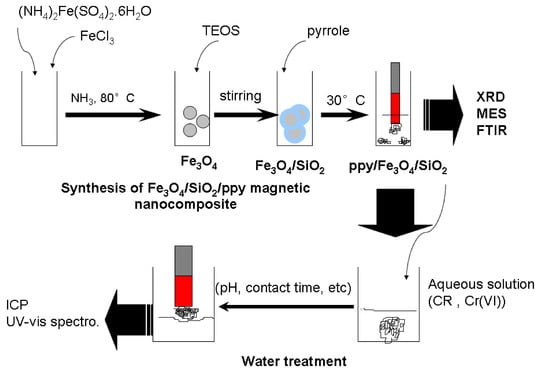
2.2. Synthesis of Fe3O4/SiO2/PPy Magnetic Nanocomposite
2.3. Material Characterization
2.4. Adsorption Studies
2.4.1. The Effect of Contact Time
2.4.2. The Effect of Adsorbent Dosage
2.4.3. The Effect of Solution pH
2.4.4. The Adsorption Isotherm
2.4.5. The Regeneration Study
2.4.6. Water Type Effect
3. Results and Discussion
3.1. Nanocomposite Characterization
3.2. Adsorption Studies
3.2.1. Effect of Contact Time
3.2.2. Effect of Adsorbent Dosage
3.2.3. Effect of Solution pH
3.2.4. Initial Concentration Effect
3.2.5. Adsorption Isotherm
3.2.6. The Regeneration Study
3.2.7. The Water Type Effect
3.2.8. Comparative Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddeeg, S.M.; Tahoon, M.A.; Alsaiari, N.S.; Shabbir, M.; Ben Rebah, F. Application of Functionalized Nanomaterials as Effective Adsorbents for the Removal of Heavy Metals from Wastewater: A Review. Curr. Anal. Chem. 2021, 17, 4–22. [Google Scholar] [CrossRef]
- Amari, A.; Elboughdiri, N.; Ghernaout, D.; Lajimi, R.H.; Alshahrani, A.M.; Tahoon, M.A.; Ben Rebah, F. Multifunctional crosslinked chitosan/nitrogen-doped graphene quantum dot for wastewater treatment. Ain Shams Eng. J. 2021. [Google Scholar] [CrossRef]
- Amari, A.; Alzahrani, F.M.; Mohammedsaleh Katubi, K.; Alsaiari, N.S.; Tahoon, M.A.; Ben Rebah, F. Clay-Polymer Nanocomposites: Preparations and Utilization for Pollutants Removal. Materials 2021, 14, 1365. [Google Scholar] [CrossRef] [PubMed]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, X.; Li, Z.; Lei, L. Biodegradation of Reactive blue 13 in a two-stage anaerobic/aerobic fluidized beds system with a Pseudomonas sp. isolate. Bioresour. Technol. 2010, 101, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-W.; Cho, H.; Lee, K.-W.; Won, E.-J.; Lee, Y.-M. Combined effects of heavy metals (Cd, As, and Pb): Comparative study using conceptual models and the antioxidant responses in the brackish water flea. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 239, 108863. [Google Scholar] [CrossRef] [PubMed]
- Suljević, D.; Sulejmanović, J.; Fočak, M.; Halilović, E.; Pupalović, D.; Hasić, A.; Alijagic, A. Assessing hexavalent chromium tissue-specific accumulation patterns and induced physiological responses to probe chromium toxicity in Coturnix japonica quail. Chemosphere 2021, 266, 129005. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Khan, A.A.P.; Jibali, B.M. Removal of Congo red, methylene blue and Cr (VI) ions from water using natural serpentine. J. Taiwan Inst. Chem. Eng. 2018, 82, 102–116. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, J.; Tian, C.; Gao, B.; Wang, L.; Jia, X. The degradation of methyl orange and membrane fouling behavior in anaerobic baffled membrane bioreactor. Chem. Eng. J. 2018, 338, 719–725. [Google Scholar] [CrossRef]
- Qiao, X.-Q.; Zhang, Z.-W.; Li, Q.-H.; Hou, D.; Zhang, Q.; Zhang, J.; Li, D.-S.; Feng, P.; Bu, X. In situ synthesis of n–n Bi 2 MoO 6 & Bi 2 S 3 heterojunctions for highly efficient photocatalytic removal of Cr (VI). J. Mater. Chem. A 2018, 6, 22580–22589. [Google Scholar]
- Feng, L.; Chen, W.-M.; Li, J.-L.; Day, G.; Drake, H.; Joseph, E.; Zhou, H.-C. Biological antagonism inspired detoxification: Removal of toxic elements by porous polymer networks. ACS Appl. Mater. Interfaces 2019, 11, 14383–14390. [Google Scholar] [CrossRef]
- Wang, R.; Ng, D.H.; Liu, S. Recovery of nickel ions from wastewater by precipitation approach using silica xerogel. J. Hazard. Mater. 2019, 380, 120826. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Troyer, L.D.; Catalano, J.G.; Giammar, D.E. Dynamics of chromium (VI) removal from drinking water by iron electrocoagulation. Environ. Sci. Technol. 2016, 50, 13502–13510. [Google Scholar] [CrossRef]
- Katubi, K.M.M.; Alsaiari, N.S.; Alzahrani, F.M.; M Siddeeg, S.; A Tahoon, M. Synthesis of Manganese Ferrite/Graphene Oxide Magnetic Nanocomposite for Pollutants Removal from Water. Processes 2021, 9, 589. [Google Scholar] [CrossRef]
- Amari, A.; Katubi, K.M.; Alsaiari, N.S.; Alzahrani, F.M.; Ben Rebah, F.; Tahoon, M.A. Magnetic metal organic framework immobilized laccase for wastewater decolorization. Processes 2021, 9, 774. [Google Scholar] [CrossRef]
- Delpiano, G.R.; Tocco, D.; Medda, L.; Magner, E.; Salis, A. Adsorption of Malachite Green and Alizarin Red S Dyes Using Fe-BTC Metal Organic Framework as Adsorbent. Int. J. Mol. Sci. 2021, 22, 788. [Google Scholar] [CrossRef]
- Ihlenburg, R.B.; Lehnen, A.-C.; Koetz, J.; Taubert, A. Sulfobetaine Cryogels for Preferential Adsorption of Methyl Orange from Mixed Dye Solutions. Polymers 2021, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Luca, P.D.; Chiodo, A.; Macario, A.; Siciliano, C.; B Nagy, J. Semi-Continuous Adsorption Processes with Multi-Walled Carbon Nanotubes for the Treatment of Water Contaminated by an Organic Textile Dye. Appl. Sci. 2021, 11, 1687. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Katubi, K.M.M.; Alzahrani, F.M.; Siddeeg, S.M.; Tahoon, M.A. The Application of Nanomaterials for the Electrochemical Detection of Antibiotics: A Review. Micromachines 2021, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Amari, A.; Al Mesfer, M.K.; Alsaiari, N.S.; Danish, M.; Alshahrani, A.M.; Tahoon, M.A.; Ben Rebah, F. Electrochemical and Optical Properties of Tellurium Dioxide (TeO2) Nanoparticles. Int. J. Electrochem. Sci. 2021, 16, 210235. [Google Scholar] [CrossRef]
- Amari, A.; Alalwan, B.; Siddeeg, S.M.; Tahoon, M.A.; Alsaiari, N.S.; Ben Rebah, F. Biomolecules Behavior on a Surface of Boron Doped/un-doped Graphene Nanosheets. Int. J. Electrochem. Sci. 2020, 15, 11427–11436. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Alsaiari, N.S.; Tahoon, M.A.; Ben Rebah, F. The application of nanomaterials as electrode modifiers for the electrochemical detection of ascorbic acid. Int. J. Electrochem. Sci. 2020, 15, 3327–3346. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Jiang, X.-P.; Li, Y.; Zeng, S.; Zhang, Y.-W. Preparation Fe3O4 chitosan magnetic particles for covalent immobilization of lipase from Thermomyces lanuginosus. Int. J. Biol. Macromol. 2015, 75, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted application of silica nanoparticles. A review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Cho, Y.; Shi, R.; Ivanisevic, A.; Borgens, R.B. A mesoporous silica nanosphere-based drug delivery system using an electrically conducting polymer. Nanotechnology 2009, 20, 275102. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Hu, Y.; Xu, J.; Qu, X.; Zhao, C. Nanocomposite with Polypyrrole Encapsulated within SBA-15 Mesoporous Silica: Preparation and Its Electrochemical Application. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 1792–1798. [Google Scholar] [CrossRef]
- Ansari, R.; Fahim, N.K. Application of polypyrrole coated on wood sawdust for removal of Cr (VI) ion from aqueous solutions. React. Funct. Polym. 2007, 67, 367–374. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, S.; Liu, W.; Du, Z.; Fang, H. Fe3O4/PPy composite nanospheres as anode for lithium-ion batteries with superior cycling performance. Electrochim. Acta 2014, 121, 428–433. [Google Scholar] [CrossRef]
- Pirsa, S.; Aghbolagh Sharifi, K. A review of the applications of bioproteins in the preparation of biodegradable films and polymers. J. Chem. Lett. 2020, 1, 47–58. [Google Scholar]
- Pirsa, S.; Asadzadeh, F.; Sani, I.K. Synthesis of Magnetic Gluten/Pectin/Fe3O4 Nano-hydrogel and Its Use to Reduce Environmental Pollutants from Lake Urmia Sediments. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3188–3198. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, X.; Shi, J.; Tang, X.; Jiang, J.; Liu, W. Highly selective fluorescent chemosensor for Zn 2+ derived from inorganic-organic hybrid magnetic core/shell Fe 3 O 4@ SiO 2 nanoparticles. Nanoscale Res. Lett. 2012, 7, 1–13. [Google Scholar]
- Wang, J.-G.; Wei, B.; Kang, F. Facile synthesis of hierarchical conducting polypyrrole nanostructures via a reactive template of MnO 2 and their application in supercapacitors. Rsc Adv. 2014, 4, 199–202. [Google Scholar] [CrossRef]
- Ayad, M.; Salahuddin, N.; Fayed, A.; Bastakoti, B.P.; Suzukic, N.; Yamauchi, Y. Chemical design of a smart chitosan-polypyrrole–magnetite nanocomposite toward efficient water treatment. Phys. Chem. Chem. Phys. 2014, 16, 21812. [Google Scholar] [CrossRef]
- Cheng, Y.; Gao, F.; An, L.; Li, X.; Wang, G. Different combinations of Fe3O4 microsphere, Polypyrrole and silver as core–shell nanocomposites for adsorption and photocatalytic application. Adv. Powder Technol. 2014, 25, 1600–1607. [Google Scholar] [CrossRef]
- Wang, G.; Chang, Y.; Wang, L.; Liu, C. Synthesis, characterization and microwave absorption properties of Fe3O4/Co core/shell-type nanoparticles. Adv. Powder Technol. 2012, 23, 861–865. [Google Scholar] [CrossRef]
- Aigbe, U.; Ho, W.; Maity, A.; Khenfouch, M.; Srinivasu, V. Removal of hexavalent chromium from wastewater using PPy/Fe3O4 magnetic nanocomposite influenced by rotating magnetic field from two pole three-phase induction motor. J. Phys. Conf. Ser. 2018, 948, 012008. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Su, Y.-S.; Manthiram, A. Sulfur-polypyrrole composite cathodes for lithium-sulfur batteries. J. Electrochem. Soc. 2012, 159, A1420. [Google Scholar] [CrossRef]
- Abbas, M.; Abdel-Hamed, M.; Chen, J. Efficient one-pot sonochemical synthesis of thickness-controlled silica-coated superparamagnetic iron oxide (Fe 3 O 4/SiO 2) nanospheres. Appl. Phys. A 2017, 123, 775. [Google Scholar] [CrossRef]
- Abbas, M.; Torati, S.R.; Kim, C. A novel approach for the synthesis of ultrathin silica-coated iron oxide nanocubes decorated with silver nanodots (Fe 3 O 4/SiO 2/Ag) and their superior catalytic reduction of 4-nitroaniline. Nanoscale 2015, 7, 12192–12204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirsa, S.; Alizadeh, N. Nanoporous conducting polypyrrole gas sensor coupled to a gas chromatograph for determination of aromatic hydrocarbons using dispersive liquid–liquid microextraction method. IEEE Sens. J. 2011, 11, 3400–3405. [Google Scholar] [CrossRef]
- Bahador, F.; Foroutan, R.; Esmaeili, H.; Ramavandi, B. Enhancement of the chromium removal behavior of Moringa oleifera activated carbon by chitosan and iron oxide nanoparticles from water. Carbohydr. Polym. 2021, 251, 117085. [Google Scholar] [CrossRef] [PubMed]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Biosorption performance of date palm empty fruit bunch wastes for toxic hexavalent chromium removal. Environ. Res. 2020, 187, 109694. [Google Scholar] [CrossRef] [PubMed]
- Hsini, A.; Benafqir, M.; Naciri, Y.; Laabd, M.; Bouziani, A.; Ez-zahery, M.; Lakhmiri, R.; El Alem, N.; Albourine, A. Synthesis of an arginine-functionalized polyaniline@ FeOOH composite with high removal performance of hexavalent chromium ions from water: Adsorption behavior, regeneration and process capability studies. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126274. [Google Scholar] [CrossRef]
- Hsini, A.; Naciri, Y.; Laabd, M.; El Ouardi, M.; Ajmal, Z.; Lakhmiri, R.; Boukherroub, R.; Albourine, A. Synthesis and characterization of arginine-doped polyaniline/walnut shell hybrid composite with superior clean-up ability for chromium (VI) from aqueous media: Equilibrium, reusability and process optimization. J. Mol. Liq. 2020, 316, 113832. [Google Scholar] [CrossRef]
- Tahar, L.B.; Oueslati, M.H.; Abualreish, M.J.A. Synthesis of magnetite derivatives nanoparticles and their application for the removal of chromium (VI) from aqueous solutions. J. Colloid Interface Sci. 2018, 512, 115–126. [Google Scholar] [CrossRef]
- Verma, A.; Chakraborty, S.; Basu, J.K. Adsorption study of hexavalent chromium using tamarind hull-based adsorbents. Sep. Purif. Technol. 2006, 50, 336–341. [Google Scholar] [CrossRef]
- Hsini, A.; Naciri, Y.; Benafqir, M.; Ajmal, Z.; Aarab, N.; Laabd, M.; Navío, J.; Puga, F.; Boukherroub, R.; Bakiz, B. Facile synthesis and characterization of a novel 1, 2, 4, 5-benzene tetracarboxylic acid doped polyaniline@ zinc phosphate nanocomposite for highly efficient removal of hazardous hexavalent chromium ions from water. J. Colloid Interface Sci. 2021, 585, 560–573. [Google Scholar] [CrossRef]
- Dognani, G.; Hadi, P.; Ma, H.; Cabrera, F.C.; Job, A.E.; Agostini, D.L.; Hsiao, B.S. Effective chromium removal from water by polyaniline-coated electrospun adsorbent membrane. Chem. Eng. J. 2019, 372, 341–351. [Google Scholar] [CrossRef]
- Siddeeg, S.M.; Amari, A.; Tahoon, M.A.; Alsaiari, N.S.; Ben Rebah, F. Removal of meloxicam, piroxicam and Cd+ 2 by Fe3O4/SiO2/glycidyl methacrylate-S-SH nanocomposite loaded with laccase. Alex. Eng. J. 2020, 59, 905–914. [Google Scholar] [CrossRef]
- Chigondo, M.; Paumo, H.K.; Bhaumik, M.; Pillay, K.; Maity, A. Magnetic arginine-functionalized polypyrrole with improved and selective chromium (VI) ions removal from water. J. Mol. Liq. 2019, 275, 778–791. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Amari, A.; Katubi, K.M.; Alzahrani, F.M.; Ben Rebah, F.; Tahoon, M.A. Innovative magnetite based polymeric nanocomposite for water treatment. Processes 2021, 9, 576. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Elanchezhiyan, S.S.; Preethi, J.; Meenakshi, S.; Park, C.M. Mechanistic performance of polyaniline-substituted hexagonal boron nitride composite as a highly efficient adsorbent for the removal of phosphate, nitrate, and hexavalent chromium ions from an aqueous environment. Appl. Surf. Sci. 2020, 511, 145543. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, Y.; Su, Q.; Chen, L.; Wang, Y.; Liu, J.; Sun, Y.; Ma, H. Removal of chromium (VI) from aqueous solutions using polypyrrole-based magnetic composites. Polym. Bull. 2017, 74, 1157–1174. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Chen, X.; Leng, L.; Wang, H.; Li, H.; Zeng, G. Facile synthesis of polypyrrole decorated reduced graphene oxide–Fe3O4 magnetic composites and its application for the Cr (VI) removal. Chem. Eng. J. 2015, 262, 597–606. [Google Scholar] [CrossRef]
- Ballav, N.; Choi, H.J.; Mishra, S.B.; Maity, A. Polypyrrole-coated halloysite nanotube clay nanocomposite: Synthesis, characterization and Cr (VI) adsorption behaviour. Appl. Clay Sci. 2014, 102, 60–70. [Google Scholar] [CrossRef]
- Bhaumik, M.; Maity, A.; Srinivasu, V.; Onyango, M.S. Removal of hexavalent chromium from aqueous solution using polypyrrole-polyaniline nanofibers. Chem. Eng. J. 2012, 181, 323–333. [Google Scholar] [CrossRef]
- Ballav, N.; Maity, A.; Mishra, S.B. High efficient removal of chromium (VI) using glycine doped polypyrrole adsorbent from aqueous solution. Chem. Eng. J. 2012, 198, 536–546. [Google Scholar] [CrossRef]
- Bhaumik, M.; Maity, A.; Srinivasu, V.; Onyango, M.S. Enhanced removal of Cr (VI) from aqueous solution using polypyrrole/Fe3O4 magnetic nanocomposite. J. Hazard. Mater. 2011, 190, 381–390. [Google Scholar] [CrossRef]
- Ballav, N.; Choi, H.; Mishra, S.; Maity, A. Synthesis, characterization of Fe3O4@ glycine doped polypyrrole magnetic nanocomposites and their potential performance to remove toxic Cr (VI). J. Ind. Eng. Chem. 2014, 20, 4085–4093. [Google Scholar] [CrossRef]
- Amalraj, A.; Selvi, M.K.; Rajeswari, A.; Pius, A. Preparation and characterization of aspartic acid doped polypyrrole for the efficient removal of Cr (VI) from aqueous solution. J. Water Process Eng. 2016, 11, 162–173. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, W.; Wang, R.; Zhang, L.; Zhu, L.; Zhang, Q. Ionothermal confined self-organization for hierarchical porous magnesium borate superstructures as highly efficient adsorbents for dye removal. J. Mater. Chem. A 2014, 2, 19167–19179. [Google Scholar] [CrossRef]
- Lorenc-Grabowska, E.; Gryglewicz, G. Adsorption characteristics of Congo Red on coal-based mesoporous activated carbon. Dye. Pigment. 2007, 74, 34–40. [Google Scholar] [CrossRef]
- Zhao, J.; Tan, Y.; Su, K.; Zhao, J.; Yang, C.; Sang, L.; Lu, H.; Chen, J. A facile homogeneous precipitation synthesis of NiO nanosheets and their applications in water treatment. Appl. Surf. Sci. 2015, 337, 111–117. [Google Scholar] [CrossRef]
- Li, X.; Xiao, W.; He, G.; Zheng, W.; Yu, N.; Tan, M. Pore size and surface area control of MgO nanostructures using a surfactant-templated hydrothermal process: High adsorption capability to azo dyes. Colloids Surf. A Physicochem. Eng. Asp. 2012, 408, 79–86. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sharma, A. Azadirachta indica leaf powder as an effective biosorbent for dyes: A case study with aqueous Congo Red solutions. J. Environ. Manag. 2004, 71, 217–229. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhai, J.; Zhou, M.; Dong, S. Ordered magnetic core–manganese oxide shell nanostructures and their application in water treatment. J. Mater. Chem. 2009, 19, 7030–7035. [Google Scholar] [CrossRef]
- Wang, L.; Wang, A. Adsorption characteristics of Congo Red onto the chitosan/montmorillonite nanocomposite. J. Hazard. Mater. 2007, 147, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Netto, M.S.; Oliveira, M.L.S.; Seliem, M.K.; Dotto, G.L.; Bonilla-Petriciolet, A.; Li, Q. Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: Experiments, characterization and physical interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
- Cseri, L.; Topuz, F.; Abdulhamid, M.A.; Alammar, A.; Budd, P.M.; Szekely, G. Electrospun Adsorptive Nanofibrous Membranes from Ion Exchange Polymers to Snare Textile Dyes from Wastewater. Adv. Mater. Technol. 2021, 2000955. [Google Scholar] [CrossRef]






| Pollutant | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qmax (mg.g−1) | KL (L.mg.g−1) | R2 | N | KF (L.mg.g−1) | R2 | B (J.mol−1) | A (L.g−1) | R2 | |
| CR dye | 361.43 | 0.017 | 0.946 | 0.487 | 2.1360 | 0.842 | 69.44 | 4.33 | 0.931 |
| Cr(VI) | 298.22 | 0.013 | 0.892 | 0.537 | 1.2720 | 0.785 | 57.12 | 4.52 | 0.835 |
| Adsorbent | Pollutant | qm (mg.g−1) | Ref. |
|---|---|---|---|
| PPy/Fe3O4/SiO2 | CR dye and Cr(VI) | 361.43 and 298.22 | This study |
| PPy/Fe3O4/AgCl | Cr(VI) | 111 | [52] |
| PPy-rGO/Fe3O4 | Cr(VI) | 227 | [53] |
| PPy-coated halloysite nanotubes | Cr(VI) | 149 | [54] |
| PPy-PANI fibers | Cr(VI) | 227 | [55] |
| Glycine-doped PPy | Cr(VI) | 217 | [56] |
| PPy/Fe3O4 | Cr(VI) | 169 | [57] |
| Fe3O4 glycine-doped PPy | Cr(VI) | 238 | [58] |
| Aspartic acid-doped PPy | Cr(VI) | 177 | [59] |
| Hierarchical porous MgBO2(OH) microspheres | CR dye | 228 | [60] |
| Mesoporous activated carbon | CR dye | 189 | [61] |
| NiO nanosheets | CR dye | 168 | [62] |
| MgO powders | CR dye | 105 | [63] |
| Neem leaf powder2 | CR dye | 41 | [64] |
| Magnetic core–manganese oxide shell | CR dye | 42 | [65] |
| Chitosan/montmorillonite nanocomposite | CR dye | 55 | [66] |
| Ashitaba waste-based activated carbons | CR dye | 289–381 | [67] |
| Walnut shell-based activated carbons | CR dye | 314–400 | [68] |
| Nanofibrous membranes from ion polymers | CR dye | 70.8 | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, F.M.; Alsaiari, N.S.; Katubi, K.M.; Amari, A.; Ben Rebah, F.; Tahoon, M.A. Synthesis of Polymer-Based Magnetic Nanocomposite for Multi-Pollutants Removal from Water. Polymers 2021, 13, 1742. https://doi.org/10.3390/polym13111742
Alzahrani FM, Alsaiari NS, Katubi KM, Amari A, Ben Rebah F, Tahoon MA. Synthesis of Polymer-Based Magnetic Nanocomposite for Multi-Pollutants Removal from Water. Polymers. 2021; 13(11):1742. https://doi.org/10.3390/polym13111742
Chicago/Turabian StyleAlzahrani, Fatimah Mohammed, Norah Salem Alsaiari, Khadijah Mohammedsaleh Katubi, Abdelfattah Amari, Faouzi Ben Rebah, and Mohamed A. Tahoon. 2021. "Synthesis of Polymer-Based Magnetic Nanocomposite for Multi-Pollutants Removal from Water" Polymers 13, no. 11: 1742. https://doi.org/10.3390/polym13111742
APA StyleAlzahrani, F. M., Alsaiari, N. S., Katubi, K. M., Amari, A., Ben Rebah, F., & Tahoon, M. A. (2021). Synthesis of Polymer-Based Magnetic Nanocomposite for Multi-Pollutants Removal from Water. Polymers, 13(11), 1742. https://doi.org/10.3390/polym13111742