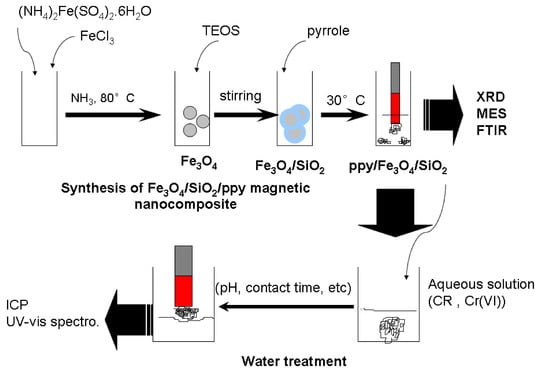
Synthesis of Polymer-Based Magnetic Nanocomposite for Multi-Pollutants Removal from Water
Abstract
Share and Cite
Alzahrani, F.M.; Alsaiari, N.S.; Katubi, K.M.; Amari, A.; Ben Rebah, F.; Tahoon, M.A. Synthesis of Polymer-Based Magnetic Nanocomposite for Multi-Pollutants Removal from Water. Polymers 2021, 13, 1742. https://doi.org/10.3390/polym13111742
Alzahrani FM, Alsaiari NS, Katubi KM, Amari A, Ben Rebah F, Tahoon MA. Synthesis of Polymer-Based Magnetic Nanocomposite for Multi-Pollutants Removal from Water. Polymers. 2021; 13(11):1742. https://doi.org/10.3390/polym13111742
Chicago/Turabian StyleAlzahrani, Fatimah Mohammed, Norah Salem Alsaiari, Khadijah Mohammedsaleh Katubi, Abdelfattah Amari, Faouzi Ben Rebah, and Mohamed A. Tahoon. 2021. "Synthesis of Polymer-Based Magnetic Nanocomposite for Multi-Pollutants Removal from Water" Polymers 13, no. 11: 1742. https://doi.org/10.3390/polym13111742
APA StyleAlzahrani, F. M., Alsaiari, N. S., Katubi, K. M., Amari, A., Ben Rebah, F., & Tahoon, M. A. (2021). Synthesis of Polymer-Based Magnetic Nanocomposite for Multi-Pollutants Removal from Water. Polymers, 13(11), 1742. https://doi.org/10.3390/polym13111742