Residual Birch Wood Lignocellulose after 2-Furaldehyde Production as a Potential Feedstock for Obtaining Fiber
Abstract
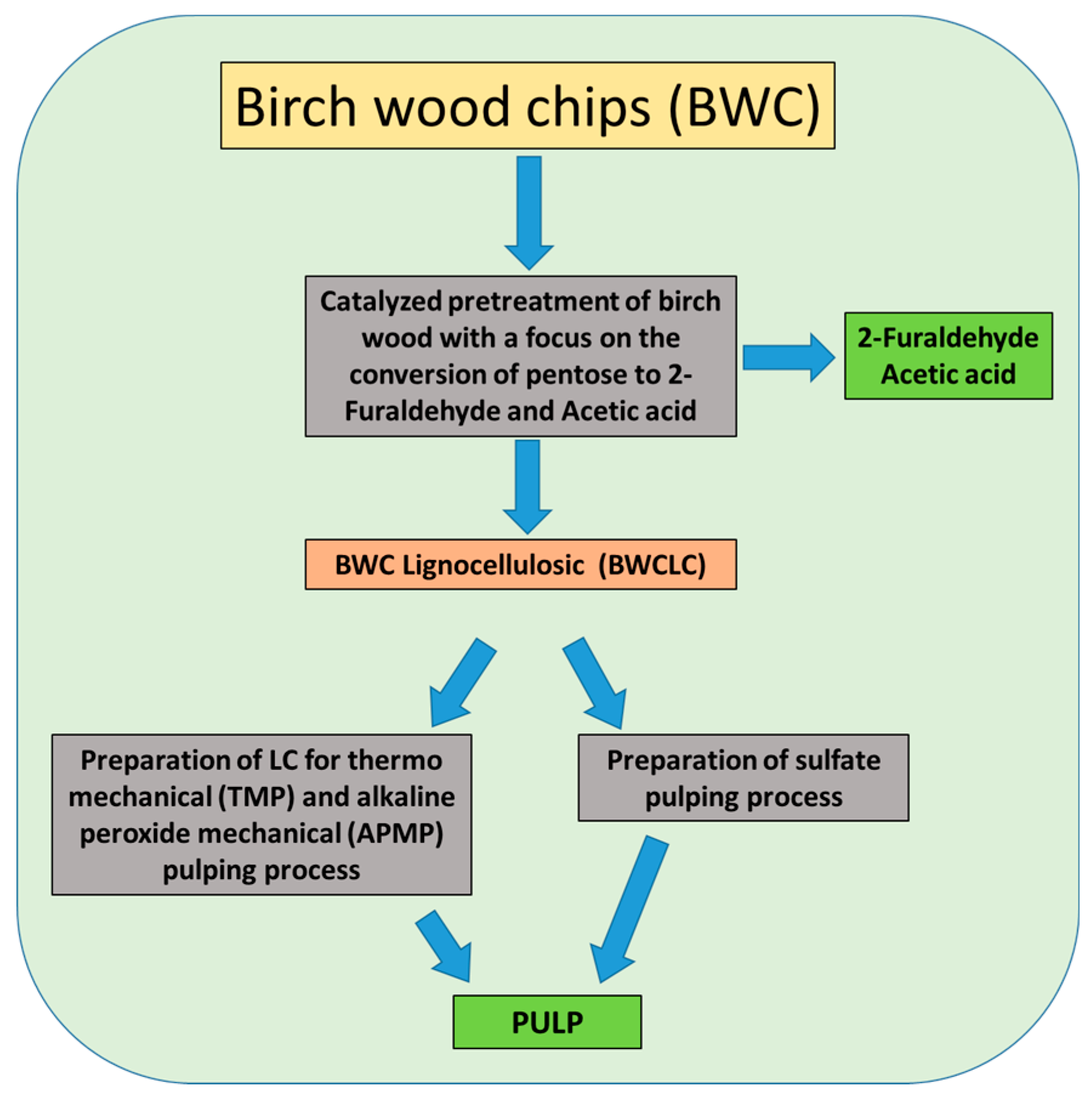
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Samples
2.3. Experimental Design
2.4. Catalyzed Pre-Treatment of Birch Wood Chips
2.5. HPLC Analysis
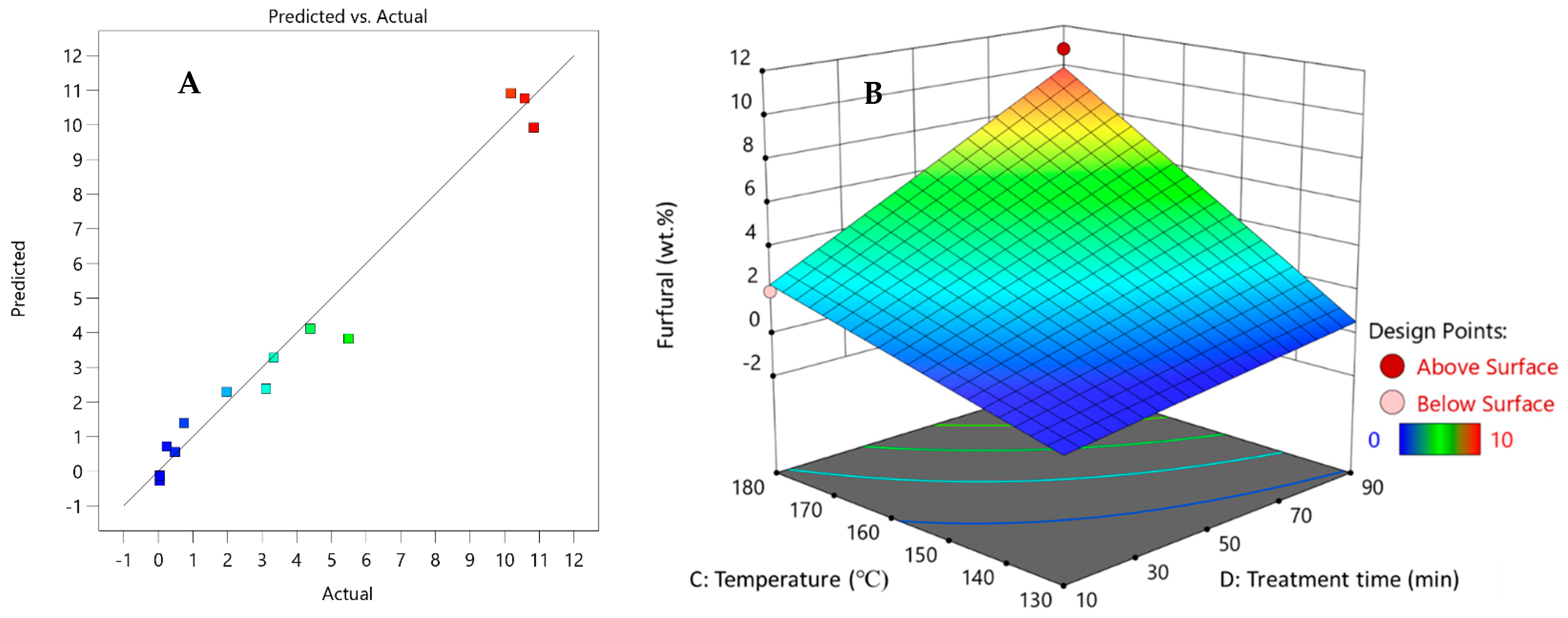
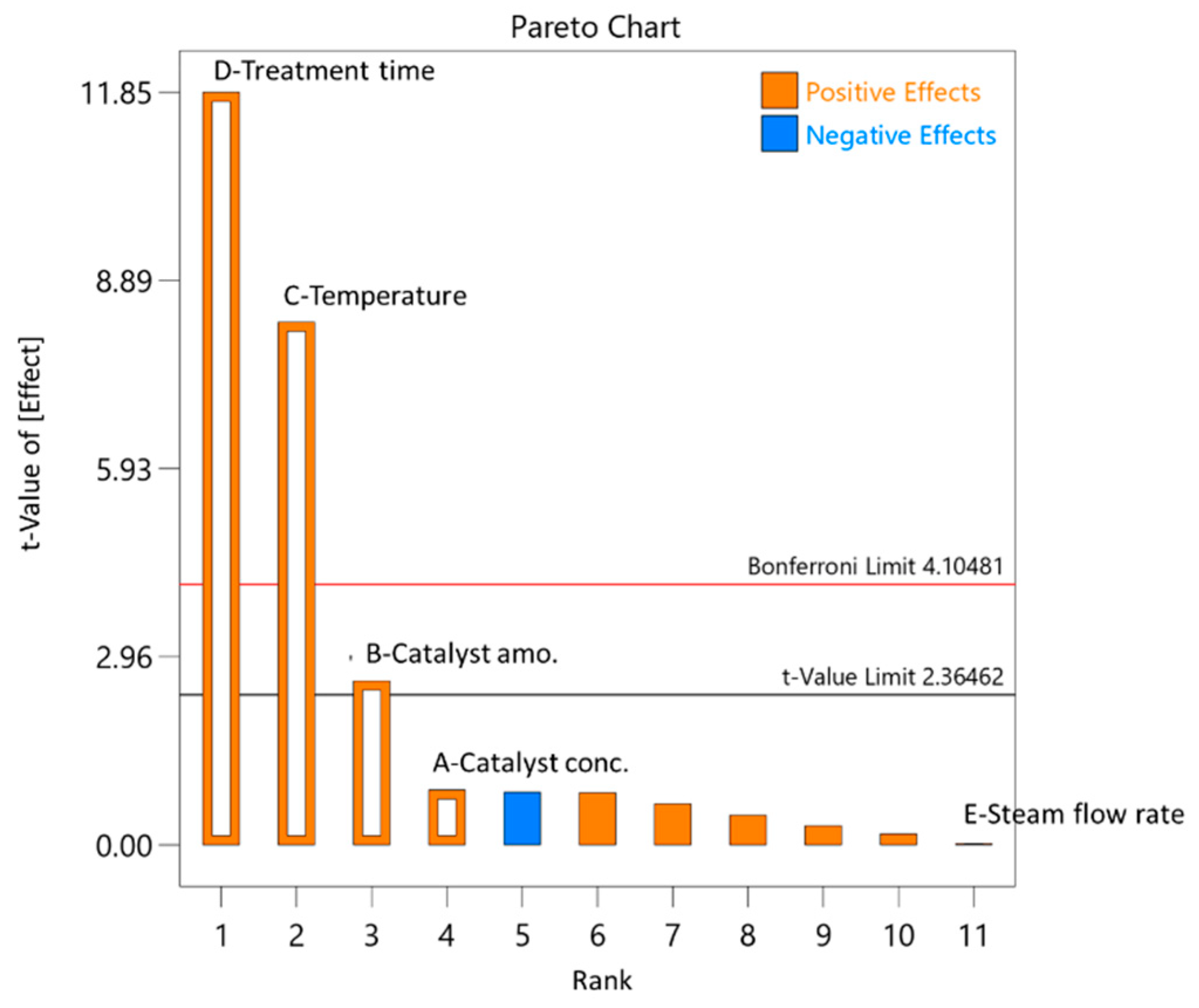
3. Results and Discussion
3.1. Analysis of the Raw Material
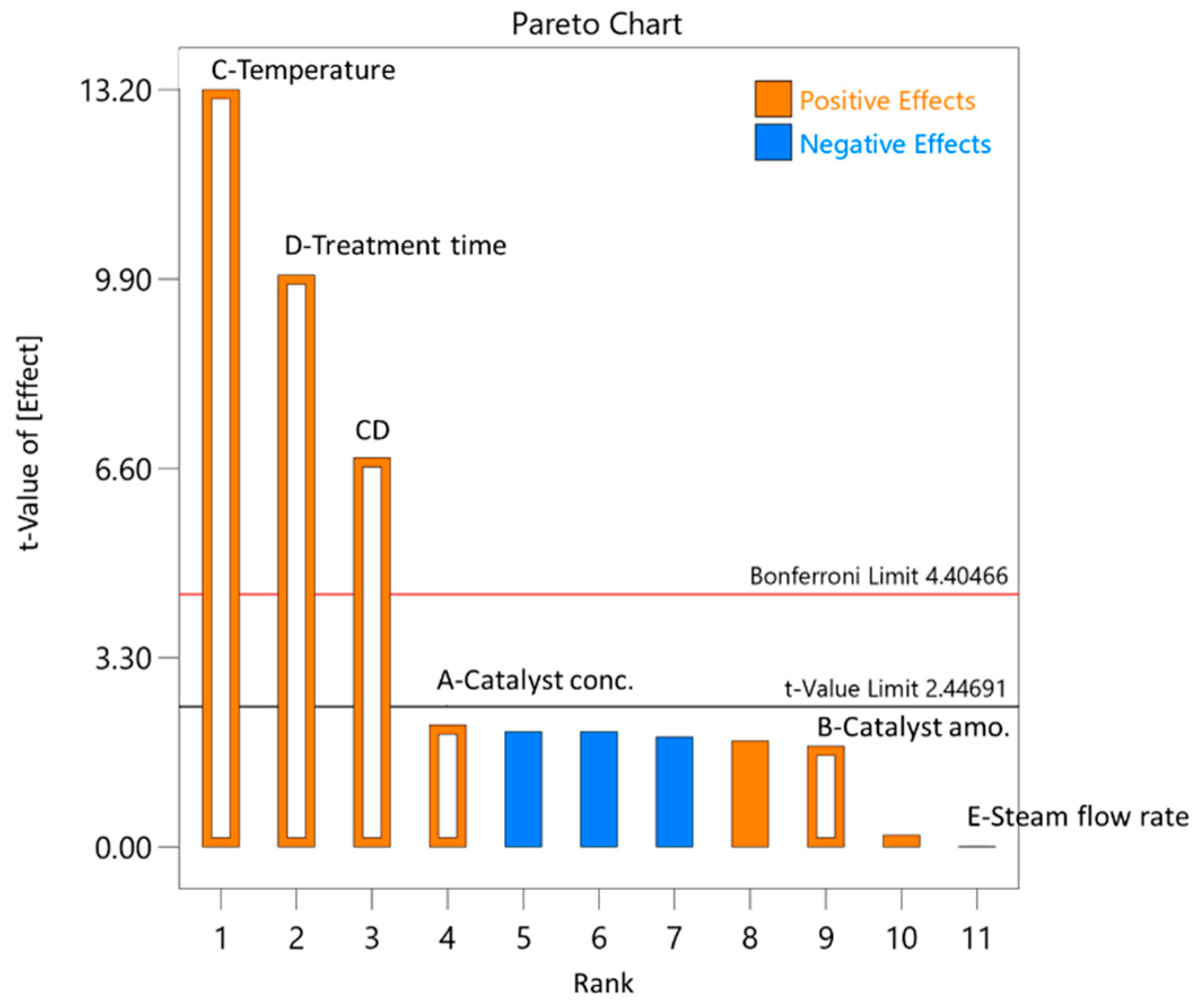
3.2. Selection of the Initial Pre-Treatment Process Parameters for the Experimental Plan
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, R.G.; Sorrell, S.R. The future of oil supply. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20130179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucia, L.A. Lignocellulosic biomass: A potential feedstock to replace petroleum. BioResources 2008, 3, 981–982. [Google Scholar] [CrossRef]
- UNDP. Pathways To Sustainable; UNDP: New York, NY, USA, 2020; Volume 41, ISBN 9789211172287. [Google Scholar]
- Balan, V. Current Challenges in Commercially Producing Biofuels from Lignocellulosic Biomass. ISRN Biotechnol. 2014, 2014, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Appels, L.; Tan, T.; Dewil, R. Bioethanol from Lignocellulosic Biomass: Current Findings Determine Research Priorities. Sci. World J. 2014, 2014, 298153. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P. Structure of Lignocellulosic Biomass; Springer: Singapore, 2016; pp. 7–12. [Google Scholar]
- Dias, A.S.; Lima, S.; Pillinger, M.; Valente, A.A. Acidic cesium salts of 12-tungstophosphoric acid as catalysts for the dehydration of xylose into furfural. Carbohydr. Res. 2006, 341, 2946–2953. [Google Scholar] [CrossRef] [PubMed]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and challenges of biomass as a suitable feedstock for bioenergy and biochemical production: A review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Clauser, N.M.; González, G.; Mendieta, C.M.; Kruyeniski, J.; Area, M.C.; Vallejos, M.E. Biomass waste as sustainable raw material for energy and fuels. Sustainability 2021, 13, 794. [Google Scholar] [CrossRef]
- Xie, H.; Liu, W.; Beadham, I.; Gathergood, N. Biorefinery with Ionic Liquids. In The Role of Green Chemistry in Biomass Processing and Conversion; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 75–133. [Google Scholar]
- Donate, P.M. Green synthesis from biomass. Chem. Biol. Technol. Agric. 2014, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- Calcio Gaudino, E.; Cravotto, G.; Manzoli, M.; Tabasso, S. From waste biomass to chemicals and energy via microwave-assisted processes. Green Chem. 2019, 21, 1202–1235. [Google Scholar] [CrossRef]
- Gomez, L.D.; Steele-King, C.G.; McQueen-Mason, S.J. Sustainable liquid biofuels from biomass: The writing’s on the walls. New Phytol. 2008, 178, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Competitive liquid biofuels from biomass. Appl. Energy 2011, 88, 17–28. [Google Scholar] [CrossRef]
- Margeot, A.; Hahn-Hagerdal, B.; Edlund, M.; Slade, R.; Monot, F. New improvements for lignocellulosic ethanol. Curr. Opin. Biotechnol. 2009, 20, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, P.; Bo, D.; Chang, H. The optimization of formic acid hydrolysis of xylose in furfural production. Carbohydr. Res. 2012, 357, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, P.; Bermudez, L.; Bakker, R. Literature Review of Physical and Chemical Pretreatment Processes for Lignocellulosic Biomass. Biomass 2010, 1–49. Available online: https://library.wur.nl/WebQuery/wurpubs/reports/396201 (accessed on 25 May 2021).
- Anthonia, E.E.; Philip, H.S. An overview of the applications of furfural and its derivatives. Int. J. Adv. Chem. 2015, 3, 42. [Google Scholar] [CrossRef]
- Vedernikovs, N.; Puke, M.; Furfural, I.K. Bioethanol Production from Hardwood and Agricultural Waste. In Proceedings of the 5th UEAA General Assembly and the Associated Workshop on Renewable Energy Resources, Production and Technologies, Furfural and Bioethanol Production from Hardwood and Agricultural Waste, Riga, Latvia, 28–31 May 2008; pp. 176–191. [Google Scholar]
- Vedernikovs, N. Method for Obtaining Furfural and Ethanol. Latvian Republic Patent No. 13676, 20 July 2008. [Google Scholar]
- NGO Zalas Majas Latvian Forest Sector in Facts and Figures. Available online: https://www.zm.gov.lv/en/mezi/statiskas-lapas/publications?nid=2566#jump (accessed on 31 May 2021).
- Lachowicz, H.; Wróblewska, H.; Sajdak, M.; Komorowicz, M.; Wojtan, R. The chemical composition of silver birch (Betula pendula Roth.) wood in Poland depending on forest stand location and forest habitat type. Cellulose 2019, 26, 3047–3067. [Google Scholar] [CrossRef] [Green Version]
- Brazdausks, P.; Puke, M.; Vedernikovs, N.; Kruma, I. Influence of Biomass Pretreatment Process Time on Furfural Extraction from Birch Wood. Sci. J. Riga Technol. Univ. Environ. Clim. Technol. 2013, 11, 5–11. [Google Scholar] [CrossRef] [Green Version]



| Temperature | Catalyst (H3PO4) Concentration | Process Duration | Steam Flow Rate | Catalyst Amount |
|---|---|---|---|---|
| (T) | (c) | (τ) | (v) | (m) |
| 130–180 °C | 10–95% | 10–90 min | 100–240 g·min−1 | 3–10% |
| Standard | Calibration Curve Equation | R2 |
|---|---|---|
| Xylose | y = 1.34 × 1011x + 2188 | 0.99998 |
| Arabinose | y = 1.33 × 1011x + 676 | 0.999990 |
| Glucose | y = 1.42 × 1011x + 1491 | 0.99998 |
| Galactose | y = 1.28 × 1011x | 0.99994 |
| Mannose | y = 1.45 × 1011x | 0.99997 |
| Cellobiose | y = 1.42 × 1011x | 0.99994 |
| 2-Furaldehyde | y = 1.60 × 1011x − 927,950 | 0.999991 |
| 5-HMF | y = 1.67 × 1011x − 114,575 | 0.9999990 |
| Acetic acid | y = 6.12 × 105x − 631 | 0.999996 |
| Levulinic acid | y = 9.56 × 105x + 307 | 0.99998 |
| Formic acid | y = 4.08 × 105x + 803 | 0.999993 |
| Compound | Amount (% from o.d.m.) |
|---|---|
| Extractives (ethanol-benzene) | 4.24 ± 0.06 |
| Extractives (hot water) | 1.60 ± 0.40 |
| Glucose | 37.84 ± 0.05 |
| Xylose | 21.96 ± 0.06 |
| Galactose | 0.83 ± 0.05 |
| Arabinose | 0.66 ± 0.06 |
| Mannose | 1.60 ± 0.50 |
| Acid-insoluble lignin | 19.42 ± 0.04 |
| Acid-soluble lignin | 3.71 ± 0.06 |
| Ash | 0.60 ± 0.010 |
| Other unidentified compounds | 1.32 ± 0.05 |
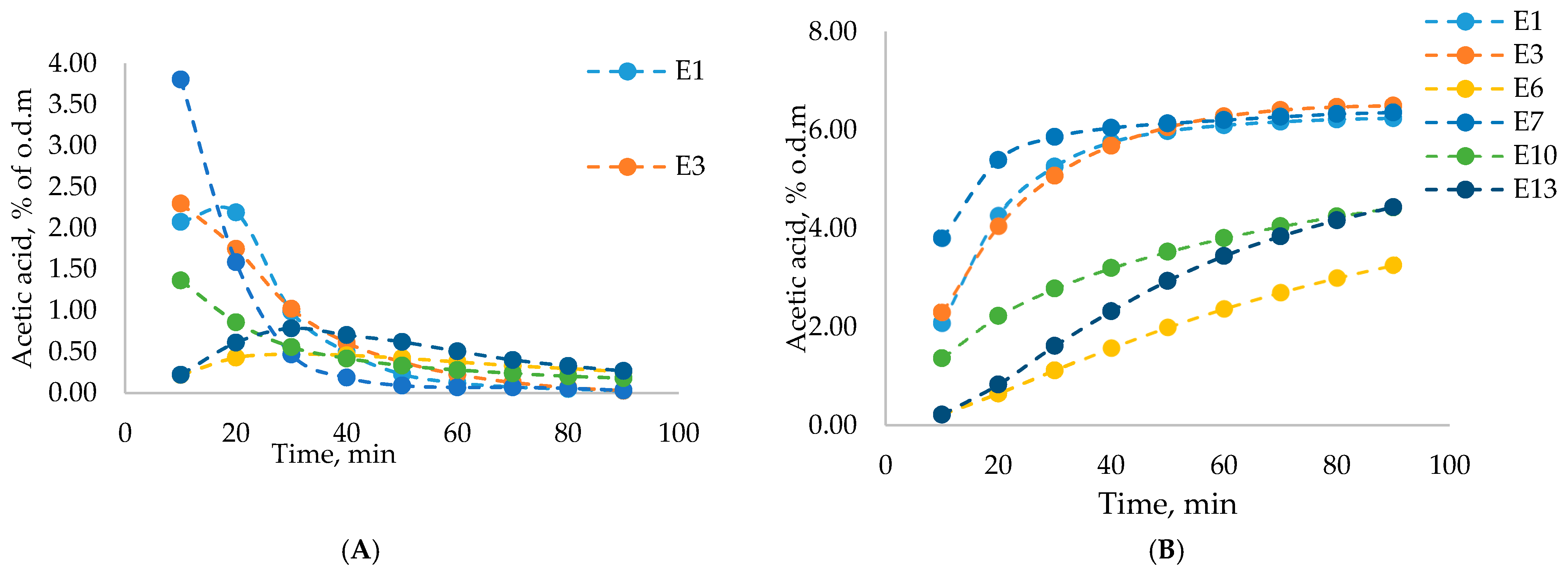
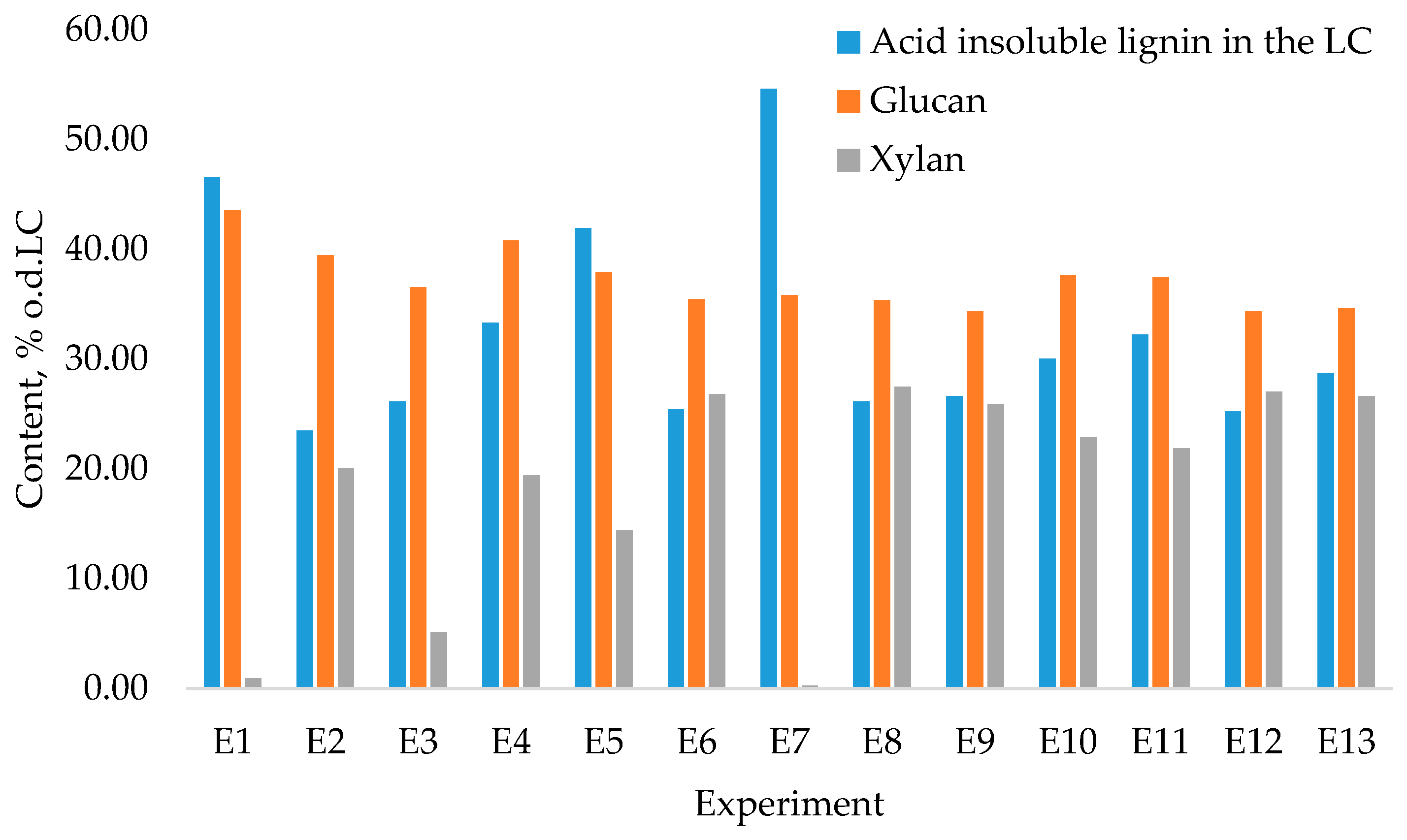
| Exp. No. | Variable Parameters, c/T/m/τ/ v | 2-Furaldehyde Yield * | Acetic Acid Yield * | Formic Acid Yield * | Levulinic Acid Yield * | 5-HMF Yield * | Lignocellulose Yield * |
|---|---|---|---|---|---|---|---|
| E1 | LC/10/180/3/85/100 | 10.84 ± 0.06 | 6.24 ± 0.04 | 1.00 ± 0.04 | 0.041 ± 0.012 | 0.15 ± 0.02 | 69.09 ± 0.06 |
| E2 | LC/10/180/3/10/100 | 1.97 ± 0.02 | 2.35 ± 0.04 | 0.24 ± 0.02 | 0.031 ± 0.002 | 0.050 ± 0.012 | 86.98 ± 0.07 |
| E3 | LC/85/180/3/85/100 | 10.18 ± 0.04 | 6.50 ± 0.06 | 1.32 ± 0.07 | 0.17 ± 0.02 | 0.34 ± 0.05 | 68.13 ± 0.08 |
| E4 | LC/47.5/155/6.5/50/170 | 5.49 ± 0.03 | 5.02 ± 0.04 | 0.50 ± 0.04 | 0 | 0.031 ± 0.009 | 82.62 ± 0.05 |
| E5 | LC/85/180/10/10/100 | 4.39 ± 0.02 | 3.40 ± 0.02 | 0.39 ± 0.03 | 0 | 0.011 ± 0.002 | 77.85 ± 0.10 |
| E6 | LC/10/130/3/85/140 | 0.48 ± 0.03 | 3.26 ± 0.03 | 0.19 ± 0.02 | 0 | 0.030 ± 0.011 | 95.18 ± 0.12 |
| E7 | LC/10/180/10/85/240 | 10.59 ± 0.08 | 6.36 ± 0.05 | 1.52 ± 0.06 | 0.24 ± 0.02 | 0.41 ± 0.06 | 82.31 ± 0.06 |
| E8 | LC/85/130/10/10/140 | 0.24 ± 0.02 | 1.39 ± 0.04 | 0.10 ± 0.02 | 0 | 0.021 ± 0.008 | 94.34 ± 0.14 |
| E9 | LC/10/130/10/10/140 | 0.040 ± 0.010 | 1.76 ± 0.03 | 0.040 ± 0.010 | 0 | 0 | 97.68 ± 0.16 |
| E10 | LC/85/130/10/85/140 | 3.11 ± 0.04 | 4.42 ± 0.02 | 0.38 ± 0.04 | 0 | 0.010 ± 0.004 | 88.30 ± 0.10 |
| E11 | LC/85/180/3/10/240 | 3.33 ± 0.03 | 3.22 ± 0.07 | 0.38 ± 0.02 | 0.020 ± 0.011 | 0.041 ± 0.002 | 88.59 ± 0.06 |
| E12 | LC/85/130/3/10/100 | 0.039 ± 0.002 | 0.51 ± 0.04 | 0.040 ± 0.011 | 0 | 0 | 98.07 ± 0.07 |
| E13 | LC/10/130/10/85/100 | 0.79 ± 0.06 | 4.43 ± 0.08 | 0.26 ± 0.03 | 0 | 0 | 92.11 ± 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puke, M.; Godina, D.; Kirpluks, M.; Rizikovs, J.; Brazdausks, P. Residual Birch Wood Lignocellulose after 2-Furaldehyde Production as a Potential Feedstock for Obtaining Fiber. Polymers 2021, 13, 1816. https://doi.org/10.3390/polym13111816
Puke M, Godina D, Kirpluks M, Rizikovs J, Brazdausks P. Residual Birch Wood Lignocellulose after 2-Furaldehyde Production as a Potential Feedstock for Obtaining Fiber. Polymers. 2021; 13(11):1816. https://doi.org/10.3390/polym13111816
Chicago/Turabian StylePuke, Maris, Daniela Godina, Mikelis Kirpluks, Janis Rizikovs, and Prans Brazdausks. 2021. "Residual Birch Wood Lignocellulose after 2-Furaldehyde Production as a Potential Feedstock for Obtaining Fiber" Polymers 13, no. 11: 1816. https://doi.org/10.3390/polym13111816
APA StylePuke, M., Godina, D., Kirpluks, M., Rizikovs, J., & Brazdausks, P. (2021). Residual Birch Wood Lignocellulose after 2-Furaldehyde Production as a Potential Feedstock for Obtaining Fiber. Polymers, 13(11), 1816. https://doi.org/10.3390/polym13111816






