Effect of Counterions on the Interaction among Concentrated Spherical Polyelectrolyte Brushes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of SPBs
2.3. Characterization
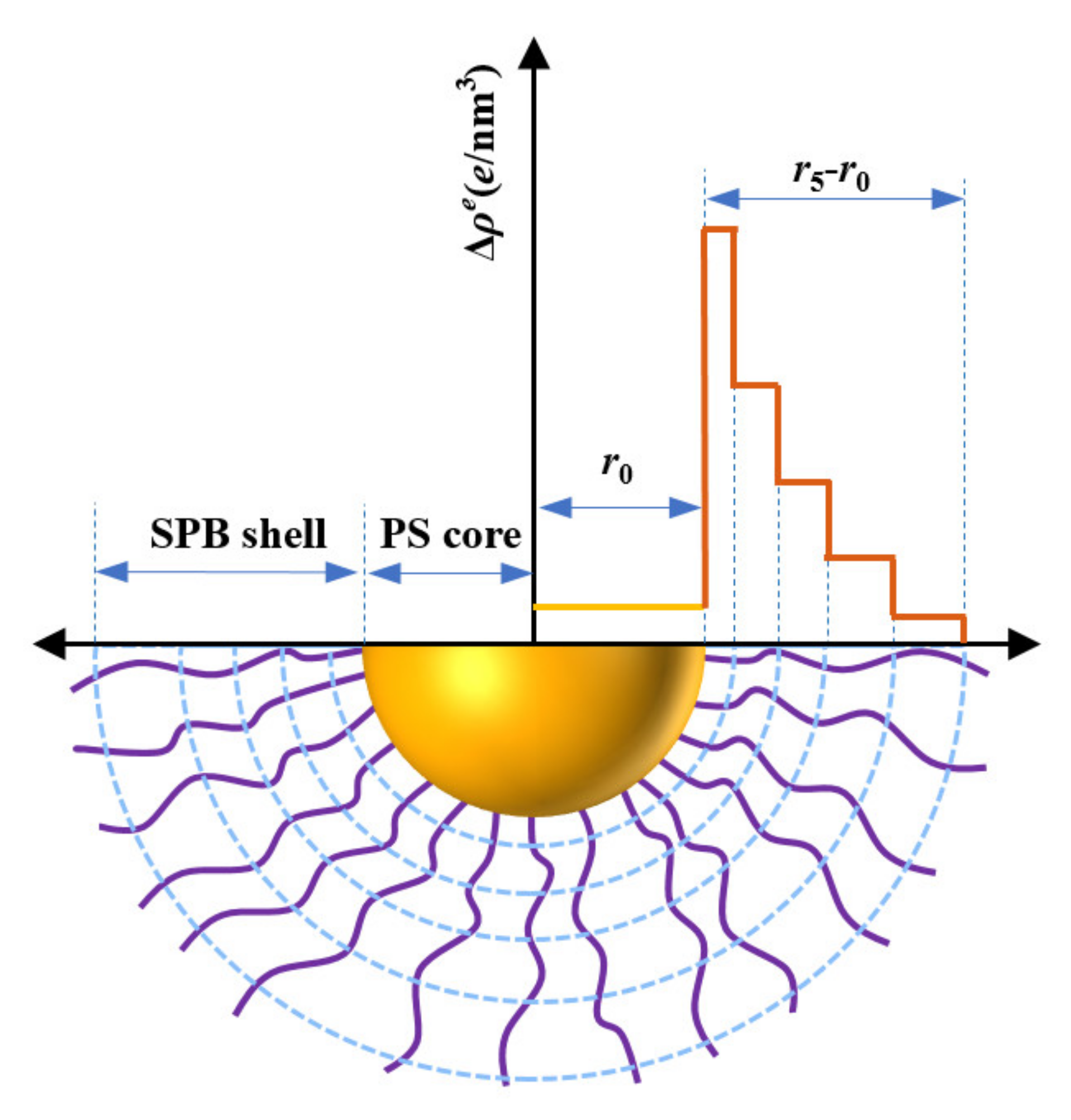
2.4. SAXS Theory
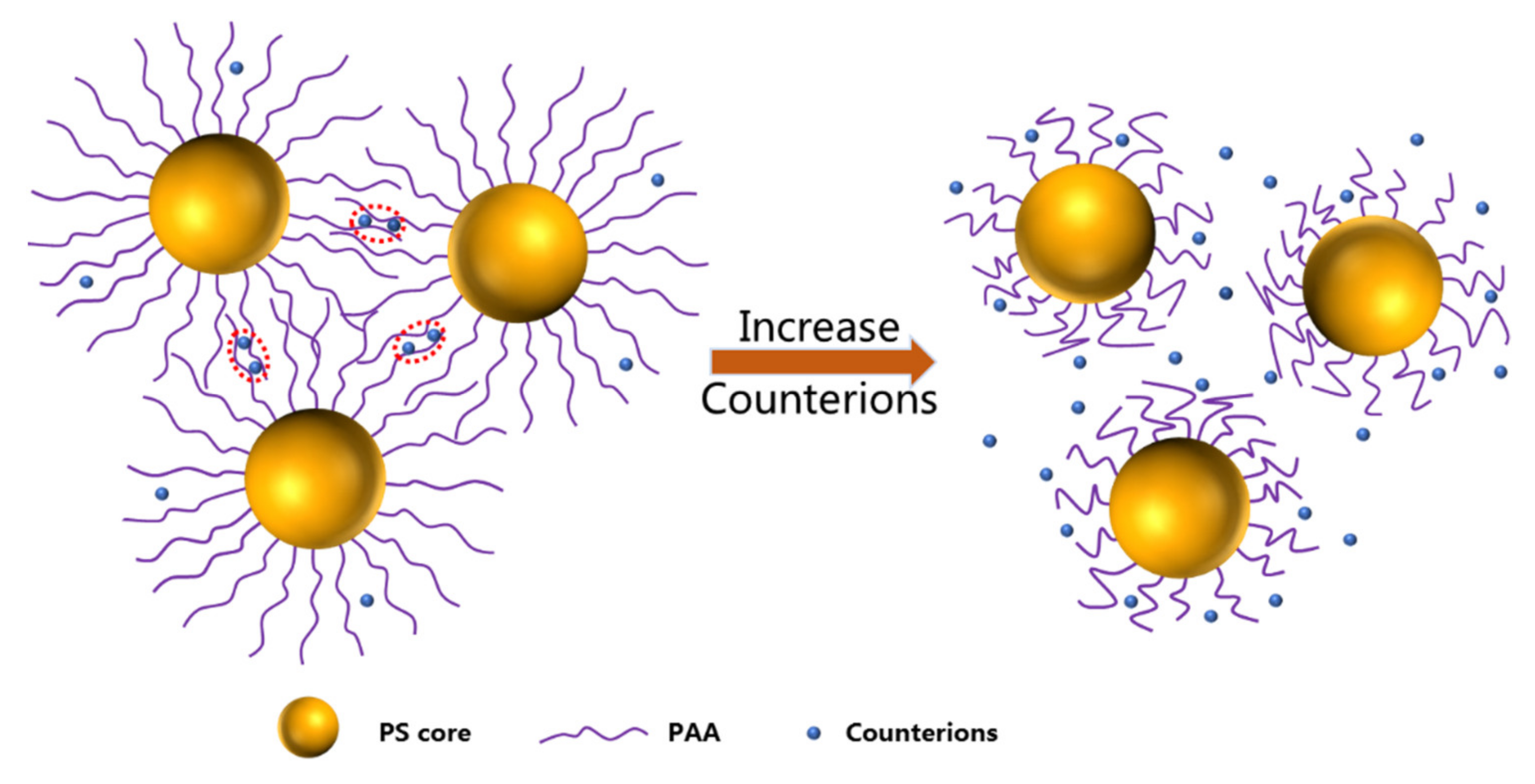
3. Results and Discussion
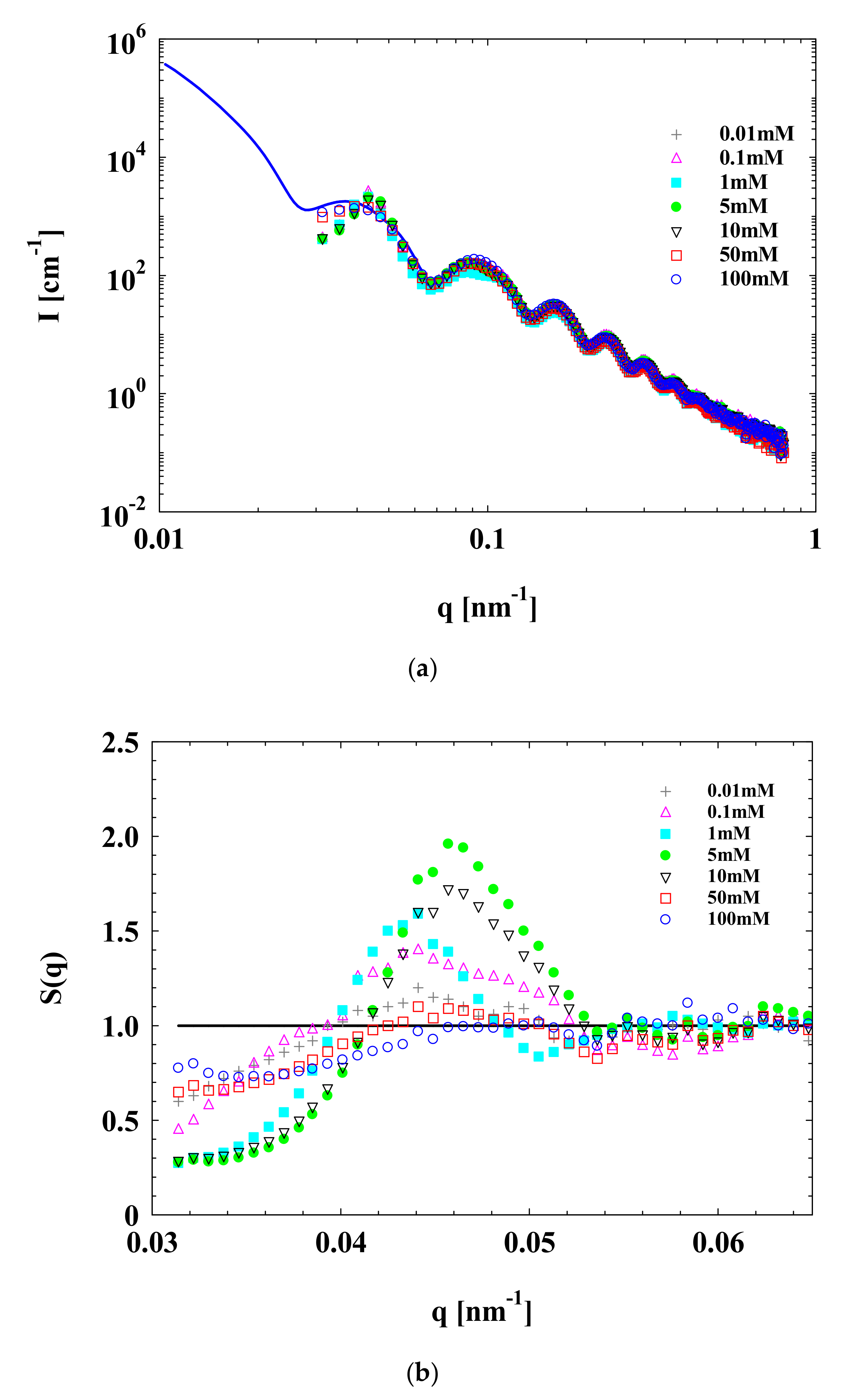
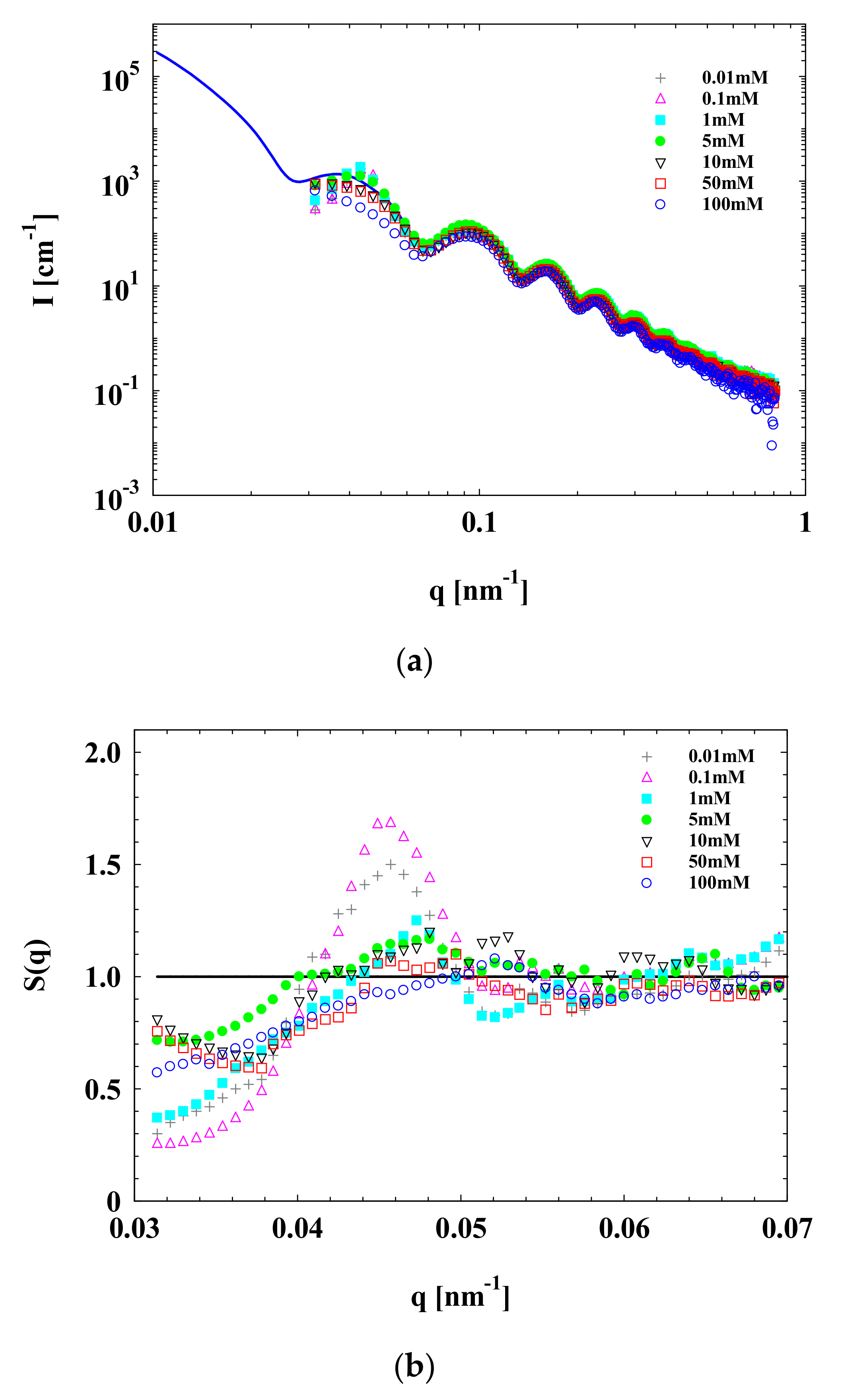
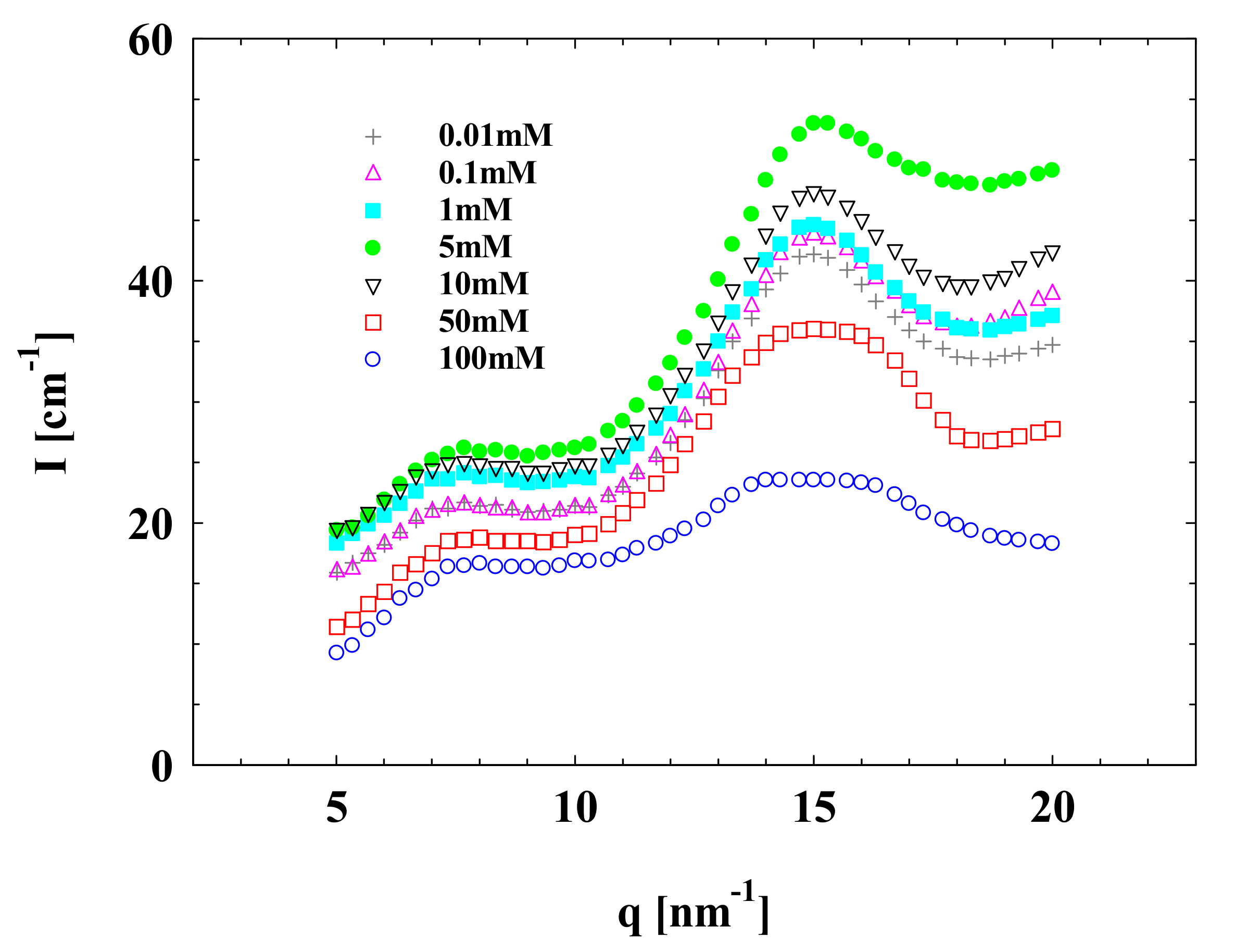
3.1. SAXS
3.2. WAXS
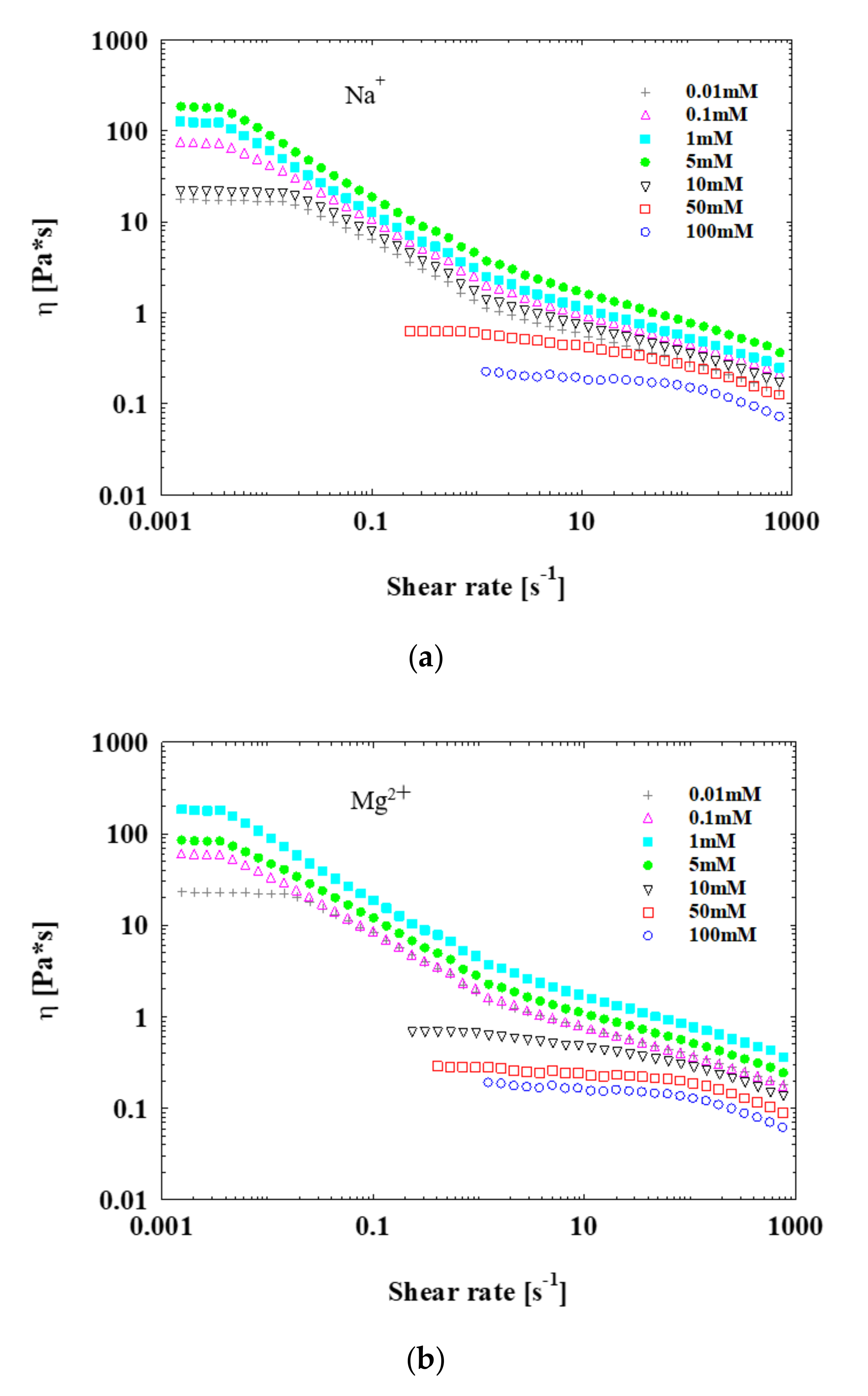
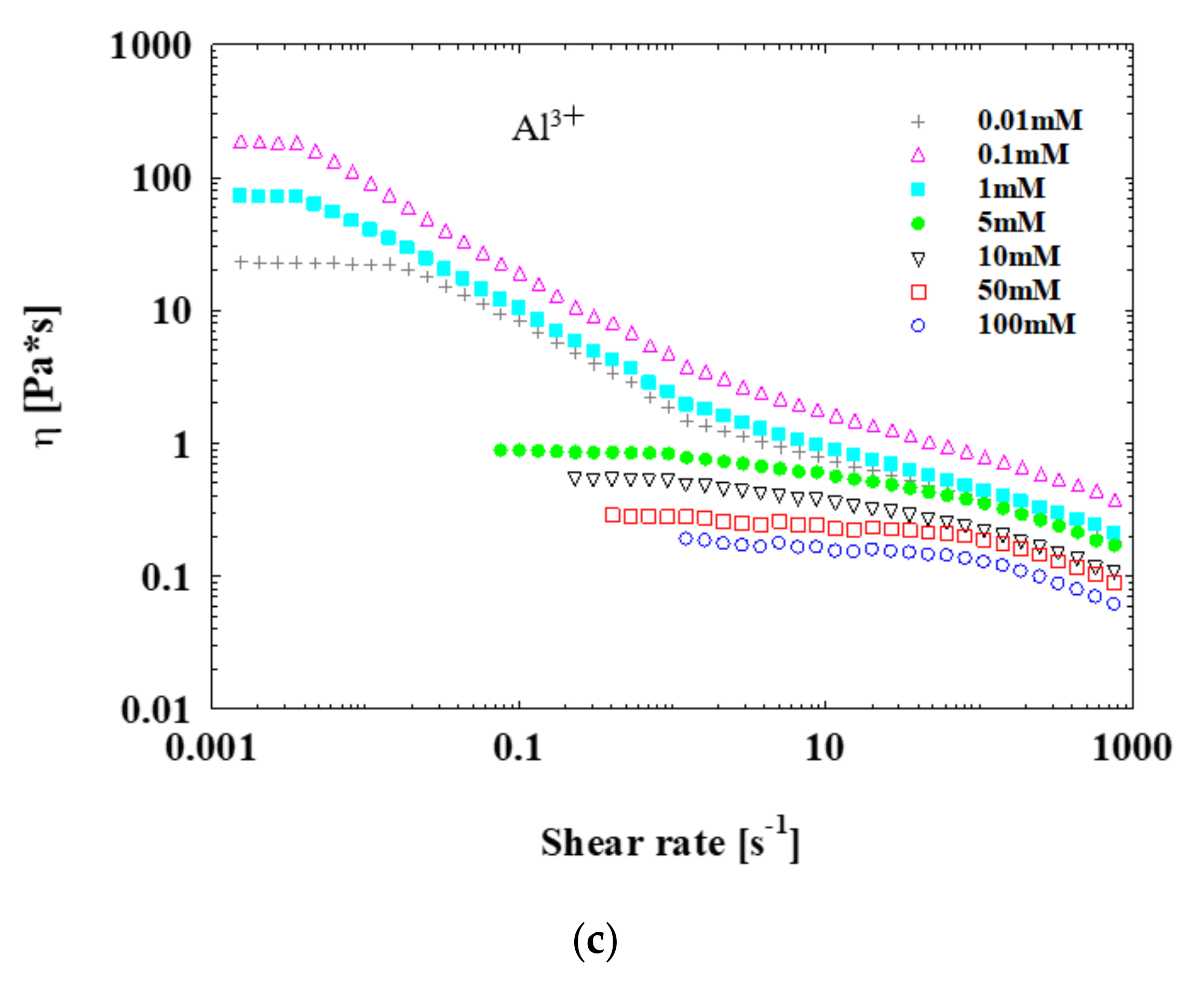
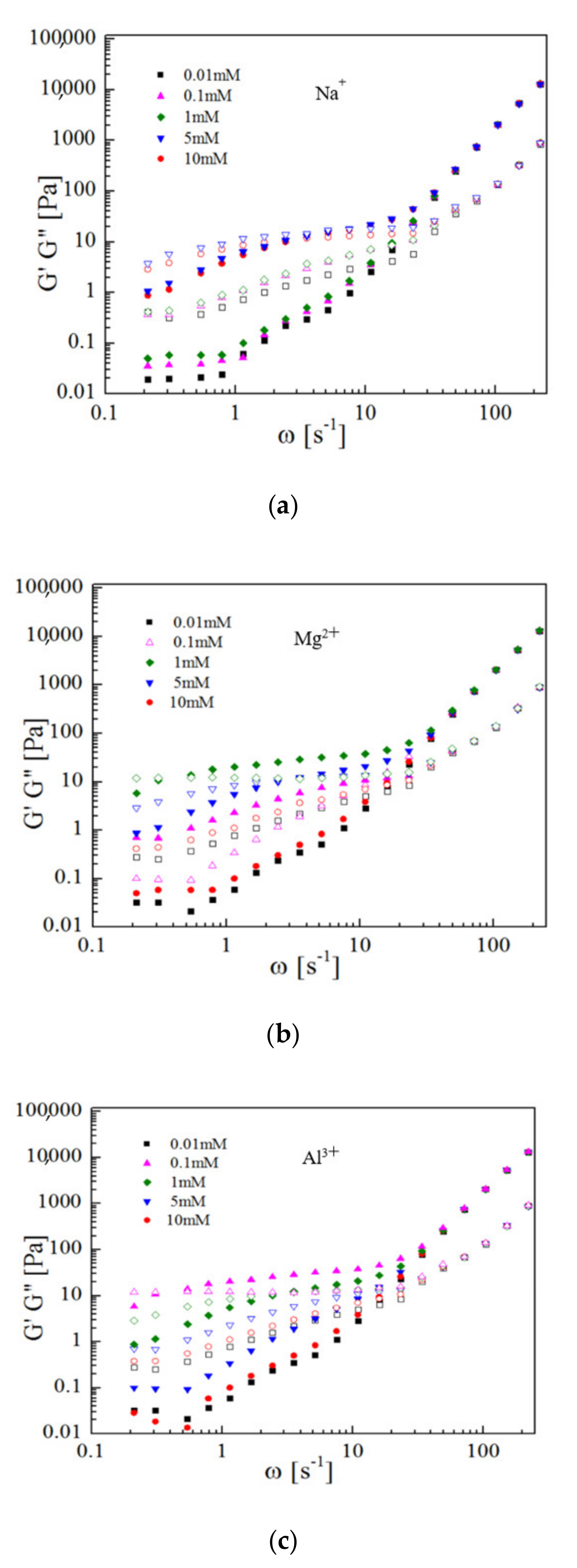
3.3. Rheology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, X.; Weiss, A.A.; Ballauff, M. Synthesis of Spherical Polyelectrolyte Brushes by Photoemulsion Polymerization. Macromolecules 1999, 32, 6043–6046. [Google Scholar] [CrossRef]
- Guo, X.; Ballauff, M. Spatial Dimensions of Colloidal Polyelectrolyte Brushes as Determined by Dynamic Light Scattering. Langmuir 2000, 16, 8719–8726. [Google Scholar] [CrossRef]
- Ballauff, M. Spherical polyelectrolyte brushes. Prog. Polym. Sci. 2007, 32, 1135–1151. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Li, L.; Wu, S.; Chen, Q.; Lu, Y.; Ballauff, M.; Guo, X. Synthesis of spherical polyelectrolyte brushes by ther-mo-controlled emulsion polymerization. Macromol. Rapid. Commun. 2010, 31, 1272–1275. [Google Scholar] [CrossRef]
- Wei, S.; Wang, Q.; Zhu, J.; Sun, L.; Lin, H.; Guo, Z. Multifunctional composite core–shell nanoparticles. Nanoscale 2011, 3, 4474–4502. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ballauff, M. Spherical polyelectrolyte brushes: Comparison between annealed and quenched brushes. Phys. Rev. E 2001, 64, 051406. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Sharma, G.; Lu, Y.; Ballauff, M. High catalytic activity of platinum nanoparticles immobilized on spherical polyelec-trolyte brushes. Langmuir 2005, 21, 12229–12234. [Google Scholar] [CrossRef] [PubMed]
- Ezhova, A.; Huber, K. Contraction and Coagulation of Spherical Polyelectrolyte Brushes in the Presence of Ag+, Mg2+, and Ca2+ Cations. Macromolecules 2016, 49, 7460–7468. [Google Scholar] [CrossRef]
- Wang, S.; Shah, H.; Zhang, F.; Lu, W. Silica-based nanocomposites via reverse microemulsions: Classifications, preparations, and applications. Nanoscale 2014, 6, 4418–4437. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, H.; Tian, Y.; Li, L.; Guo, X. Protein adsorption onto spherical polyelectrolyte brushes observed by small angle X-ray scattering. J. East China Univ. Sci. Technol. 2015, 41, 277–280. [Google Scholar]
- Yang, Q.; Li, L.; Zhao, F.; Wang, Y.; Ye, Z.; Guo, X. Generation of MnO2 nanozyme in spherical polyelectrolyte brush for col-orimetric detection of glutathione. Mater. Lett. 2019, 248, 89–92. [Google Scholar] [CrossRef]
- Ye, Z.; Li, L.; Zhao, F.; Tian, Y.; Wang, Y.; Yang, Q.; Dai, L.; Guo, X. Enrichment and distribution of counterions in spherical polyelectrolyte brushes probed by small angle X-ray scattering. J. Polym. Sci. B 2019, 57, 738–747. [Google Scholar] [CrossRef]
- Wittemann, A.; Haupt, B.; Ballauff, M. Adsorption of proteins on spherical polyelectrolyte brushes in aqueous solution. Phys. Chem. Chem. Phys. 2003, 5, 1671–1677. [Google Scholar] [CrossRef] [Green Version]
- Jayachandran, K.N.; Takacs-Cox, A.; Brooks, D. Synthesis and Characterization of Polymer Brushes of Poly(N,N-dimethylacrylamide) from Polystyrene Latex by Aqueous Atom Transfer Radical Polymerization. Macromolecules 2002, 35, 4247–4257. [Google Scholar] [CrossRef]
- Wang, W.; Li, L.; Henzler, K.; Lu, Y.; Wang, J.; Han, H.; Tian, Y.; Wang, Y.; Zhou, Z.; Lotze, G.; et al. Protein Immobilization onto Cationic Spherical Polyelectrolyte Brushes Studied by Small Angle X-ray Scattering. Biomacromolecules 2017, 18, 1574–1581. [Google Scholar] [CrossRef]
- Henzler, K.; Rosenfeldt, S.; Wittemann, A.; Harnau, L.; Finet, S.; Narayanan, T.; Ballauff, M. Directed Motion of Proteins along Tethered Polyelectrolytes. Phys. Rev. Lett. 2008, 100, 158301. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, L.; Han, H.; Wang, W.; Wang, Y.; Ye, Z.; Guo, X. Modification of spherical polyelectrolyte brushes by layer-by-layer self-assembly as observed by small angle X-ray scattering. Polymers 2016, 8, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhulina, B.; Birshtein, M.; Borisov, V. Curved polymer and polyelectrolyte brushes beyond the daoud-cotton model. Eur. Phys. J. E 2006, 20, 243–256. [Google Scholar] [CrossRef]
- Chen, L.; Merlitz, H.; He, S.-Z.; Wu, C.-X.; Sommer, J.-U. Polyelectrolyte Brushes: Debye Approximation and Mean-Field Theory. Macromolecules 2011, 44, 3109–3116. [Google Scholar] [CrossRef]
- Lu, Y.; Hoffmann, M.; Yelamanchili, R.S.; Terrenoire, A.; Schrinner, M.; Drechsler, M.; Möller, M.W.; Breu, J.; Ballauff, M. Well-Defined Crystalline TiO2Nanoparticles Generated and Immobilized on a Colloidal Nanoreactor. Macromol. Chem. Phys. 2009, 210, 377–386. [Google Scholar] [CrossRef]
- Dingenouts, N.; Norhausen, C.; Ballauff, M. Observation of the Volume Transition in Thermosensitive Core−Shell Latex Particles by Small-Angle X-ray Scattering. Macromolecules 1998, 31, 8912–8917. [Google Scholar] [CrossRef]
- Seelenmeyer, S.; Deike, I.; Rosenfeldt, S.; Norhausen, C.; Dingenouts, N.; Ballauff, M.; Narayanan, T.; Lindner, P. Small-angle x-ray and neutron scattering studies of the volume phase transition in thermosensitive core–shell colloids. J. Chem. Phys. 2001, 114, 10471–10478. [Google Scholar] [CrossRef]
- Xuan, Y.; Wei, W.; Li, L.; Xu, G.; Zhi, Z.; Fu, W. Analysis of spherical polyelectrolyte brushes by small angle X-ray scattering. Chin. J. Polym. Sci. 2014, 32, 778–785. [Google Scholar]
- Wang, W.; Li, L.; Han, H.; Tian, Y.; Zhou, Z.; Guo, X. Tunable immobilization of protein in anionic spherical polyelectrolyte brushes as observed by small-angle X-ray scattering. Colloid Polym. Sci. 2015, 293, 2789–2798. [Google Scholar] [CrossRef]
- Grunder, R.; Urban, G.; Ballauff, M. Small-angle x-ray analysis of latex particles with core-shell morphology. Colloid Polym. Sci. 1993, 271, 563–572. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, Y.; Zhang, Y.; Li, L.; Lu, Y.; Guo, X. Synthesis of Magnetic Spherical Polyelectrolyte Brushes. Macromolecules 2011, 44, 632–639. [Google Scholar] [CrossRef]
- Su, N.; Gu, P.; Zhao, J. Preparation and properties of polyaniline–anionic spherical polyelectrolyte brushes nanocomposite. Micro Nano Lett. 2015, 10, 175–178. [Google Scholar] [CrossRef]
- Koll, R.; Fruhner, L.; Heller, H.; Allgaier, J.; Pyckhout-Hintzen, W.; Kruteva, M. Creating a synthetic platform for the encap-sulation of nanocrystals with covalently bound polymer shells. Nanoscale 2019, 11, 3847–3854. [Google Scholar] [CrossRef] [Green Version]
- Qian, W.; Zhu, Q.; Duan, B.; Tang, W.; Yuan, Y.; Hu, A. Electrostatic self-assembled nanoparticles based on spherical poly-electrolyte brushes for magnetic resonance imaging. Dalton Trans. 2018, 47, 7663–7668. [Google Scholar] [CrossRef] [PubMed]
- Kremer, B.; Kirschhock, C.; Jacobs, A. Synthesis and characterization of zeogrid molecular sieves. C. R. Chim. 2005, 8, 379–390. [Google Scholar] [CrossRef]
- Martin, J.R.S.; Bihannic, I.; Santos, C.F.; Farinha, J.P.S.; Demé, B.; Leermakers, F.A.M.; Pinheiro, J.P.; Rotureau, E.; Duval, J.F.L. Structure of Multiresponsive Brush-Decorated Nanoparticles: A Combined Electrokinetic, DLS, and SANS Study. Langmuir 2015, 31, 4779–4790. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, Y.; Yang, Q.; Ye, Z.; Hua, C.; Tian, Y.; von Klitzing, R.; Guo, X. Interaction among Spherical Poly-electrolyte Brushes in Concentrated Aqueous Solution. Langmuir 2020, 36, 3104–3110. [Google Scholar] [CrossRef]
- Giulio, D.; Leonarda, L.; Alessandro, L.; Antonino, M. Structure of natural water-containing glasses from Lipari (Italy) and Eastern Rhodopes (Bulgaria): SAXS, WAXS and IR studies. J. Non–Cryst. Solids 1998, 232–234, 547–553. [Google Scholar]
- Nozue, Y.; Shinohara, Y.; Ogawa, Y. Deformation behavior of isotactic polypropylene spherulite during hot drawing inves-tigated by simultaneous microbeam SAXS-WAXS and POM measurement. Macromolecules 2007, 40, 2036–2045. [Google Scholar] [CrossRef]
- Polat, K.; Orujalipoor, I.; Semra, I. Nano and microstructures of SEBS/PP/wax blend membranes: SAXS and WAXS analyses. J. Polym. Eng. 2015, 35, 151–157. [Google Scholar] [CrossRef]
- De Robillard, Q.; Guo, X.; Ballauff, M.; Narayanan, T. Spatial Correlation of Spherical Polyelectrolyte Brushes in Salt-Free Solution As Observed by Small-Angle X-ray Scattering. Macromolecules 2000, 33, 9109–9114. [Google Scholar] [CrossRef]
- Ballauff, M. Nanoscopic Polymer Particles with a Well-Defined Surface: Synthesis, Characterization, and Properties. Macromol. Chem. Phys. 2003, 204, 220–234. [Google Scholar] [CrossRef]
- Ye, Z.; Li, L.; Dai, L.; Wang, Y.; Yang, Q.; Von Klitzing, R.; Guo, X. Selective uptake of different proteins by annealed and quenched cationic spherical polyelectrolyte brushes. J. Appl. Polym. Sci. 2020, 58, 3018–3030. [Google Scholar] [CrossRef]
- Weihua, W.; Fangfang, C.; Li, L. Interactions among spherical poly(acrylic acid) brushes: Observation by rheology and small angle x-ray scattering. J. Polym. Sci. Part B Polym. Phys. 2015, 54, 405–413. [Google Scholar]
- Carlos, R.; López, B.; Burghardt, W.R.; Kweon, M.S. Local and Global Stretching of Polymer Chains during Startup of Extensional Flow. ACS. Macro. Lett. 2019, 9, 26–31. [Google Scholar]
- Schrinner, M.; Ballauff, M.; Talmon, Y.; Kauffmann, Y.; Thun, J.; Möller, M.; Breu, J. Single Nanocrystals of Platinum Prepared by Partial Dissolution of Au-Pt Nanoalloys. Science 2009, 323, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Thünemann, A.; Kubowicz, S.; Burger, C. Alpha-helical-within-discotic columnar structures of a complex between poly(ethylene oxide)-block-poly(l-lysine) and a hexa-peri-hexabenzocoronene. J. Am. Chem. Soc. 2003, 125, 352–356. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, L.; Wang, Y.; Yang, Q.; Ye, Z.; Sun, L.; Yang, F.; Guo, X. Effect of Counterions on the Interaction among Concentrated Spherical Polyelectrolyte Brushes. Polymers 2021, 13, 1911. https://doi.org/10.3390/polym13121911
Wang Y, Li L, Wang Y, Yang Q, Ye Z, Sun L, Yang F, Guo X. Effect of Counterions on the Interaction among Concentrated Spherical Polyelectrolyte Brushes. Polymers. 2021; 13(12):1911. https://doi.org/10.3390/polym13121911
Chicago/Turabian StyleWang, Yunwei, Li Li, Yiming Wang, Qingsong Yang, Zhishuang Ye, Liang Sun, Fan Yang, and Xuhong Guo. 2021. "Effect of Counterions on the Interaction among Concentrated Spherical Polyelectrolyte Brushes" Polymers 13, no. 12: 1911. https://doi.org/10.3390/polym13121911






