The Effect of Pollutant Gases on Surfactant Migration in Acrylic Emulsion Films: A Comparative Study and Preliminary Evaluation of Surface Cleaning
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Weathering Experiments
2.3. Cleaning Methods
2.4. Optical 3D Microscopy
2.5. Atomic Force Microscopy (AFM) Combined with Raman Spectroscopy
2.6. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3. Results and Discussion
3.1. Three-Dimensional Optical Microscopy
3.1.1. After Aging
3.1.2. After Cleaning
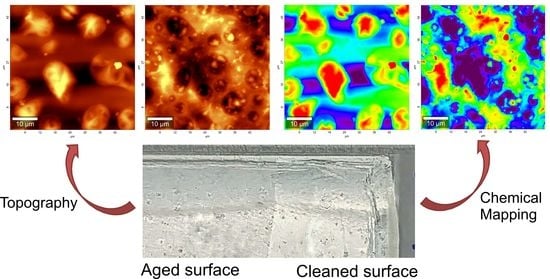
3.2. AFM Combined with Raman Spectroscopy
3.2.1. After Aging
3.2.2. After Cleaning
3.3. ATR-FTIR Spectroscopy
3.3.1. After Aging
3.3.2. After Cleaning
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jablonski, E.; Learner, T.; Hayes, J.; Golden, M. Conservation concerns for acrylic emulsion paints. Stud. Conserv. 2003, 48, 3–12. [Google Scholar] [CrossRef]
- Learner, T. A review of synthetic binding media in twentieth-century paints. Conservator 2000, 24, 96–103. [Google Scholar] [CrossRef]
- Learner, T.J.S.; Smithen, P.; Krueger, J.W.; Schilling, M.R. Modern Paints Uncovered; The Getty Conservation Institute: Los Angeles, CA, USA, 2007. [Google Scholar]
- Ormsby, B.; Learner, T. The effects of wet surface cleaning treatments on acrylic emulsion artists’ paints—A review of recent scientific research. Stud. Conserv. 2010, 55, 29–41. [Google Scholar] [CrossRef]
- Crook, J.; Learner, T. The Impact of Modern Paints; Tate Gallery: London, UK, 2000. [Google Scholar]
- Agarwal, N.; Farris, R.J. Water absorption by acrylic-based latex blend films and its effect on their properties. J. Appl. Polym. Sci. 1999, 72, 1407–1419. [Google Scholar] [CrossRef]
- Owen, L.; Ploeger, R.; Murray, A. The effects of water exposure on surface characteristics of acrylic emulsion paints. J. Can. Assoc. Conserv. 2004, 29, 8–25. [Google Scholar]
- Ploeger, R.; Murray, A.; Hesp, S.; Scalarone, D. Morphological changes and rates of leaching of water-soluble material from artists’ acrylic paint films during aqueous immersions. Mod. Paint. Uncovered Proc. Mod. Paint. Uncovered Symp. 2007, 201–207. [Google Scholar]
- Digney-Peer, S.; Burnstock, A.; Learner, T.; Khanjian, H.; Hoogland, F.; Boon, J. The Migration of Surfactants in Acrylic Emulsion Paint Films. Stud. Conserv. 2004, 49, 202–207. [Google Scholar] [CrossRef]
- Chiantore, O.; Scalarone, D.; Learner, T. Characterization of Artists’ Acrylic Emulsion Paints. Int. J. Polym. Anal. Charact. 2003, 8, 67–82. [Google Scholar] [CrossRef]
- Pagnin, L.; Calvini, R.; Wiesinger, R.; Schreiner, M. SO2− and NOx− initiated atmospheric degradation of polymeric films: Morphological and chemical changes, influence of relative humidity and inorganic pigments. Microchem. J. 2021, 164, 106087. [Google Scholar] [CrossRef]
- Hamilton, R.; Kucera, V.; Tidblad, J.; Watt, J. The Effects of Air Pollution on Cultural Heritage; Springer: London, UK, 2009. [Google Scholar] [CrossRef]
- De Santis, F.; Di Palo, V.; Allegrini, I. Determination of some atmospheric pollutants inside a museum: Relationship with the concentration outside. Sci. Total Environ. 1992, 127, 211–223. [Google Scholar] [CrossRef]
- Fardi, T.; Pintus, V.; Kampasakali, E.; Pavlidou, E.; Papaspyropoulos, K.G.; Schreiner, M.; Kyriacou, G.; Kampasakali, E. A novel methodological approach for the assessment of surface cleaning of acrylic emulsion paints. Microchem. J. 2018, 141, 25–39. [Google Scholar] [CrossRef]
- Ormsby, B.; Kampasakali, E.; Learner, T. Surfactants and Acrylic Emulsion Paints: Evaluating Changes Induced by Wet Surface Cleaning Treatments. In New Insights into the Cleaning of Paintings (Proceedings from the Cleaning 2010 International Conference, Universidad Politécnica de Valencia and Museum Conservation Institute); The Smithsonian Institution: Washington, DC, USA, 2010; pp. 159–164. [Google Scholar]
- Ormsby, B.; Barker, R.; Hellen, R.; Smithen, P. Cleaning Acrylic Emulsion Paintings: Case Study Treatments, Evaluation and Reflections. In What’s Changing: Theories and Practices in the Restoration of Contemporary Art; Castello di Rivoli Museo D’Arte: Turin, Italy, 2012. [Google Scholar]
- Ziraldo, I.; Watts, K.; Luk, A.; Lagalante, A.F.; Wolbers, R.C. The influence of temperature and humidity on swelling and surfactant migration in acrylic emulsion paint films. Stud. Conserv. 2016, 61, 209–221. [Google Scholar] [CrossRef]
- Ormsby, B.; Learner, T.; Foster, G.; Druzik, J.; Schilling, M. Wet–cleaning Acrylic Emulsion Paint Films: An Evaluation of Physical, Chemical and Optical Changes. In Modern Paints Uncovered; Institute GC: Los Angeles, CA, USA, 2007; pp. 187–198. [Google Scholar]
- Scalarone, D.; Lazzari, M.; Castelvetro, V.; Chiantore, O. Surface Monitoring of Surfactant Phase Separation and Stability in Waterborne Acrylic Coatings. Chem. Mater. 2007, 19, 6107–6113. [Google Scholar] [CrossRef]
- Riedo, C.; Rollo, G.; Chiantore, O.; Scalarone, D. Detection and Identification of Possible Gel Residues on the Surface of Paintings after Cleaning Treatments. Heritage 2021, 4, 304–315. [Google Scholar] [CrossRef]
- Grau-Bové, J.; Budič, B.; Cigić, I.K.; Thickett, D.; Signorello, S.; Strlič, M. The effect of particulate matter on paper degradation. Herit. Sci. 2016, 4, 79. [Google Scholar] [CrossRef] [Green Version]
- Gaylarde, C.; Morton, L.; Loh, K.; Shirakawa, M. Biodeterioration of external architectural paint films—A review. Int. Biodeterior. Biodegrad. 2011, 65, 1189–1198. [Google Scholar] [CrossRef]
- Shirakawa, M.A.; Tavares, R.G.; Gaylarde, C.; Taqueda, M.E.S.; Loh, K.; John, V.M. Climate as the most important factor determining anti-fungal biocide performance in paint films. Sci. Total Environ. 2010, 408, 5878–5886. [Google Scholar] [CrossRef] [PubMed]
- Berni, A.; Mennig, M.S.H. Doctor blades. In Sol-Gel Technologies for Glass Producers and Users; Springer: Boston, MA, USA, 2004; pp. 89–92. [Google Scholar]
- European Environment Agency. Available online: http://www.eea.europa.eu/ (accessed on 16 December 2020).
- Ormsby, B.; Kampasakali, E.; Miliani, C.; Learner, T. An FTIR-Based Exploration of the Effects of Wet Cleaning Treatments on Artists. E-Preserv. Sci. 2009, 405, 186–195. [Google Scholar]
- Cremonesi, P. L’ambiente Acquoso per il Trattamento di Manufatti Artistici; il Prato, Collana I Talenti: Verona, Italy, 2019. [Google Scholar]
- Baglioni, P.; Carretti, E.; Chelazzi, D. Nanomaterials in art conservation. Nat. Nanotechnol. 2015, 10, 287–290. [Google Scholar] [CrossRef]
- Domingues, J.A.L.; Bonelli, N.; Giorgi, R.; Fratini, E.; Gorel, F.; Baglioni, P. Innovative Hydrogels Based on Semi-Interpenetrating p(HEMA)/PVP Networks for the Cleaning of Water-Sensitive Cultural Heritage Artifacts. Langmuir 2013, 29, 2746–2755. [Google Scholar] [CrossRef]
- Eriksson, H.; Wedberg, I.; Nesson, J.; Bronmark-Thourlund, M. The use of nanorestore gels in the conservation of lime-based wall-paintings. In Gels Conserv. Art; Archetype Publications Ltd.: London, UK, 2017; pp. 270–273. [Google Scholar]
- CSGI. Nanorestore Gel® Medium Water Retention—MWR; CSGI: Florence, Italy, 2015. [Google Scholar]
- ImageJ Software. 2020. Available online: https://imagej.nih.gov/ij/ (accessed on 16 December 2020).
- Wiesinger, R.; Pagnin, L.; Anghelone, M.; Moretto, L.M.; Orsega, E.F.; Schreiner, M. Pigment and Binder Concentrations in Modern Paint Samples Determined by IR and Raman Spectroscopy. Angew. Chem. Int. Ed. 2018, 57, 7401–7407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, R. Problems of dirt accumulation and its removal from unvarnished paintings: A practical review. In Dirt Pict. Separated; UKIC: London, UK, 1990; pp. 3–6. [Google Scholar]
- Giordano, A.; Barresi, G.; Rotolo, V.; Schiavone, S.; Palla, F. The Conservation of Contemporary Paintings: From Dry Cleaning to Microemulsions. Nanotechnol. Nanomater. Diagn. Conserv. Restor. Cult. Herit. 2019, 277–298. [Google Scholar] [CrossRef]
- Moncmanová, A. Environmental Deterioration of Materials. In Environmental Deterioration of Materials; Press, W., Ed.; Southampton: Boston, MA, USA, 2007; pp. 1–25. [Google Scholar]
- Kientz, E.; Dobler, F.; Holl, Y. Desorption of the surfactant from the particle surface during latex film formation. Polym. Int. 1994, 34, 125–134. [Google Scholar] [CrossRef]
- Tan, S.P.; Piri, M. Modeling the Solubility of Nitrogen Dioxide in Water Using Perturbed-Chain Statistical Associating Fluid Theory. Ind. Eng. Chem. Res. 2013, 52, 16032–16043. [Google Scholar] [CrossRef]
- Anghelone, M.; Jembrih-Simbürger, D.; Schreiner, M. Identification of copper phthalocyanine blue polymorphs in unaged and aged paint systems by means of micro-Raman spectroscopy and Random Forest. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 419–425. [Google Scholar] [CrossRef]
- Elashmawi, I.; Gaabour, L.H. Raman, morphology and electrical behavior of nanocomposites based on PEO/PVDF with multi-walled carbon nanotubes. Results Phys. 2015, 5, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Kampasakali, E.; Ormsby, B.; Cosentino, A.; Miliani, C.; Learner, T. A Preliminary Evaluation of the Surfaces of Acrylic Emulsion Paint Films and the Effects of Wet-Cleaning Treatment by Atomic Force Microscopy (AFM). Stud. Conserv. 2011, 56, 216–230. [Google Scholar] [CrossRef]
- Learner, T.J.S. Analysis of Modern Paints; The Getty Conservation Institute: Los Angeles, CA, USA, 2005. [Google Scholar]
- Anghelone, M.; Stoytschew, V.; Jembrih-Simbürger, D.; Schreiner, M. Spectroscopic methods for the identification and photostability study of red synthetic organic pigments in alkyd and acrylic paints. Microchem. J. 2018, 139, 155–163. [Google Scholar] [CrossRef]
- Burdick, C.L.; Freed, E.S. The Equilibrium between Nitric Oxide, Nitrogen Peroxide and Aqueous Solution of Nitric Acid. J. Am. Chem. Soc. 1921, 43, 518–530. [Google Scholar] [CrossRef]
- Schwartz, S.E.; White, W.H. Solubility Equilibria of the Nitrogen Oxides and Oxyacids in Dilute Aqueous Solution. In Advances in Environmental Science and Engineering; Gordon and Breach Science: New York, NY, USA, 1981; pp. 1–45. [Google Scholar]
- Xiao, G.; Huang, A.; Su, H.; Tan, T. The activity of acrylic-silicon/nano-TiO2 films for the visible-light degradation of formaldehyde and NO2. Build. Environ. 2013, 65, 215–221. [Google Scholar] [CrossRef]
- Peleg, M. The chemistry of ozone in the treatment of water. Water Res. 1976, 10, 361–365. [Google Scholar] [CrossRef]
- Chiantore, O.; Trossarelli, L.; Lazzari, M. Photooxidative degradation of acrylic and methacrylic polymers. Polymer 2000, 41, 1657–1668. [Google Scholar] [CrossRef]
- Murray, A.; de Berenfeld, C.C.; Chang, S.S.; Jablonski, E.; Klein, T.; Riggs, M.C.; Robertson, E.C.; Tse, W.A. The Condition and Cleaning of Acrylic Emulsion Paintings. MRS Proc. 2002, 712, 83–90. [Google Scholar] [CrossRef]
- Ormsby, B.; Learner, T.; Schilling, M.; Druzik, J.; Khanjian, H.; Foster, G.; Sloan, M. The effects of surface cleaning on acrylic emulsion paintings: A preliminary investigation. Tate Pap. 2006, 6, 1–14. [Google Scholar]
- Stulik, D.; Miller, D.; Khanjian, H.; Khandekar, N.; Wolbers, R.; Carlson, J.; Petersen, W.C. Solvent Gels for the Cleaning of Works of Art: The Residue Question; The Getty Conservation Institute: Los Angeles, CA, USA, 2004; Volume 1. [Google Scholar]
- Mohamed, M.; Ahmed, N.; Mohamed, W.; Mabrouk, M. Novel water-based coatings of acrylic-polyurethane reinforced with mixed metal pigment for oil and gas pipelines protection. Prog. Org. Coat. 2020, 149, 105941. [Google Scholar] [CrossRef]
- Mahi, O.; Khaldi, K.; Belardja, M.S.; Belmokhtar, A.; Benyoucef, A. Development of a New Hybrid Adsorbent from Opuntia Ficus Indica NaOH-Activated with PANI-Reinforced and Its Potential Use in Orange-G Dye Removal. J. Inorg. Organomet. Polym. 2021, 31, 2095–2104. [Google Scholar] [CrossRef]
- Yang, J.; Lee, S.; Choi, I.; Shin, J.; Han, W.-H.; Hong, M.-H.; Kang, H.-C.; Kim, Y.-W. Effect of fatty acid-based anionic surfactants on the emulsion properties of self-emulsifying poly(ethylene-co-acrylic acid) waxes. J. Ind. Eng. Chem. 2019, 71, 393–401. [Google Scholar] [CrossRef]
- Canosa, E.; Norrehed, S. Strategies for Pollutant Monitoring in Museum Environments; Riksantikvarieämbetet: Stockholm, Sweden, 2019. [Google Scholar] [CrossRef]






| Gas Pollutant | Concentration (ppm) | Relative Humidity (RH%) |
|---|---|---|
| H2S | 0.25 | 80 |
| SO2 | 15 | 80 |
| NOx | 15 | 80 |
| O3 | 2500 | 80 |
| Samples | Weathering Conditions | Average Particle Size (μ) | Standard Deviation (σ) | Correlation Coefficient (R) |
|---|---|---|---|---|
| Acrylic emulsion films | H2S + RH80% | 8.35 µm | ±2.09 µm | 0.92 |
| SO2 + RH80% | 6.84 µm | ±2.93 µm | 0.91 | |
| O3 + RH80% | 8.38 µm | ±3.28 µm | 0.98 | |
| NOx + RH80% | 10.56 µm | ±6.5 µm | 0.99 |
| Samples | Weathering Conditions | Sa [nm] | Average Particle Size [µm] |
|---|---|---|---|
| Pure acrylic film | Unaged | 76.3 | - |
| RH80%, 168 h | 95.2 | - | |
| H2S, RH80%, 168 h | 207.5 | 8.74 ± 1.24 | |
| SO2, RH80%, 168 h | 231.6 | 5.44 ± 2.1 | |
| O3, RH80%, 168 h | 333.5 | 7.84 ± 4.5 | |
| NOx, RH80%, 168 h | 407.8 | 10.8 ± 6.7 |
| Weathering Conditions | Sa [nm] | ||
|---|---|---|---|
| After Aging | After Swab Rolled | After Hydrogel | |
| RH 80% | 95.1 | 85.2 | 123.5 |
| H2S + RH 80% | 207.4 | 75.9 | 145.7 |
| SO2 + RH 80% | 231.6 | 75 | 136 |
| O3 + RH 80% | 333.4 | 62.5 | 146.6 |
| NOx + RH 80% | 407.8 | 174.1 | 385.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagnin, L.; Wiesinger, R.; Koyun, A.N.; Schreiner, M. The Effect of Pollutant Gases on Surfactant Migration in Acrylic Emulsion Films: A Comparative Study and Preliminary Evaluation of Surface Cleaning. Polymers 2021, 13, 1941. https://doi.org/10.3390/polym13121941
Pagnin L, Wiesinger R, Koyun AN, Schreiner M. The Effect of Pollutant Gases on Surfactant Migration in Acrylic Emulsion Films: A Comparative Study and Preliminary Evaluation of Surface Cleaning. Polymers. 2021; 13(12):1941. https://doi.org/10.3390/polym13121941
Chicago/Turabian StylePagnin, Laura, Rita Wiesinger, Ayse Nur Koyun, and Manfred Schreiner. 2021. "The Effect of Pollutant Gases on Surfactant Migration in Acrylic Emulsion Films: A Comparative Study and Preliminary Evaluation of Surface Cleaning" Polymers 13, no. 12: 1941. https://doi.org/10.3390/polym13121941
APA StylePagnin, L., Wiesinger, R., Koyun, A. N., & Schreiner, M. (2021). The Effect of Pollutant Gases on Surfactant Migration in Acrylic Emulsion Films: A Comparative Study and Preliminary Evaluation of Surface Cleaning. Polymers, 13(12), 1941. https://doi.org/10.3390/polym13121941