Simple and Fast One-Pot Cellulose Gel Preparation in Aqueous Pyrrolidinium Hydroxide Solution–Cellulose Solvent and Antibacterial Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of [C4mpyr][OH] Aqueous Solution
2.3. Characterization of [C4mpyr][OH] Aqueous Solution
2.4. Dissolution of Microcrystalline Cellulose
2.5. Gelation Test
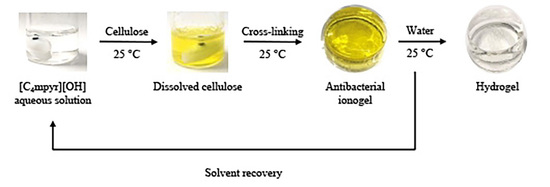
2.6. Preparation of Cellulose Gels (Ionogels and Hydrogels)
2.7. [C4mpyr][OH]-Content of the Cellulose Gels
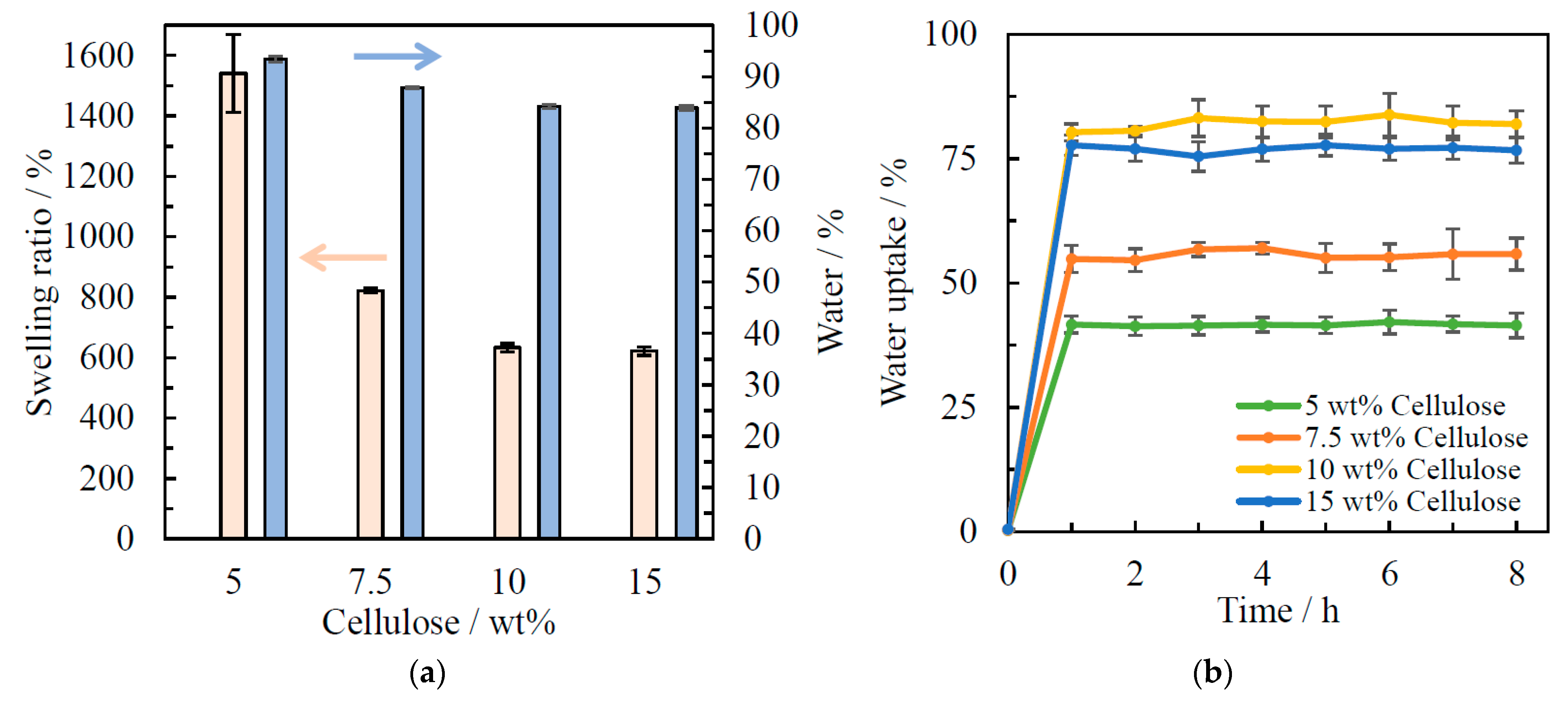
2.8. Swelling Ratio, Water Content, and Water Uptake
2.9. Solvent Recovery
2.10. Compression Test of the Cellulose Gels
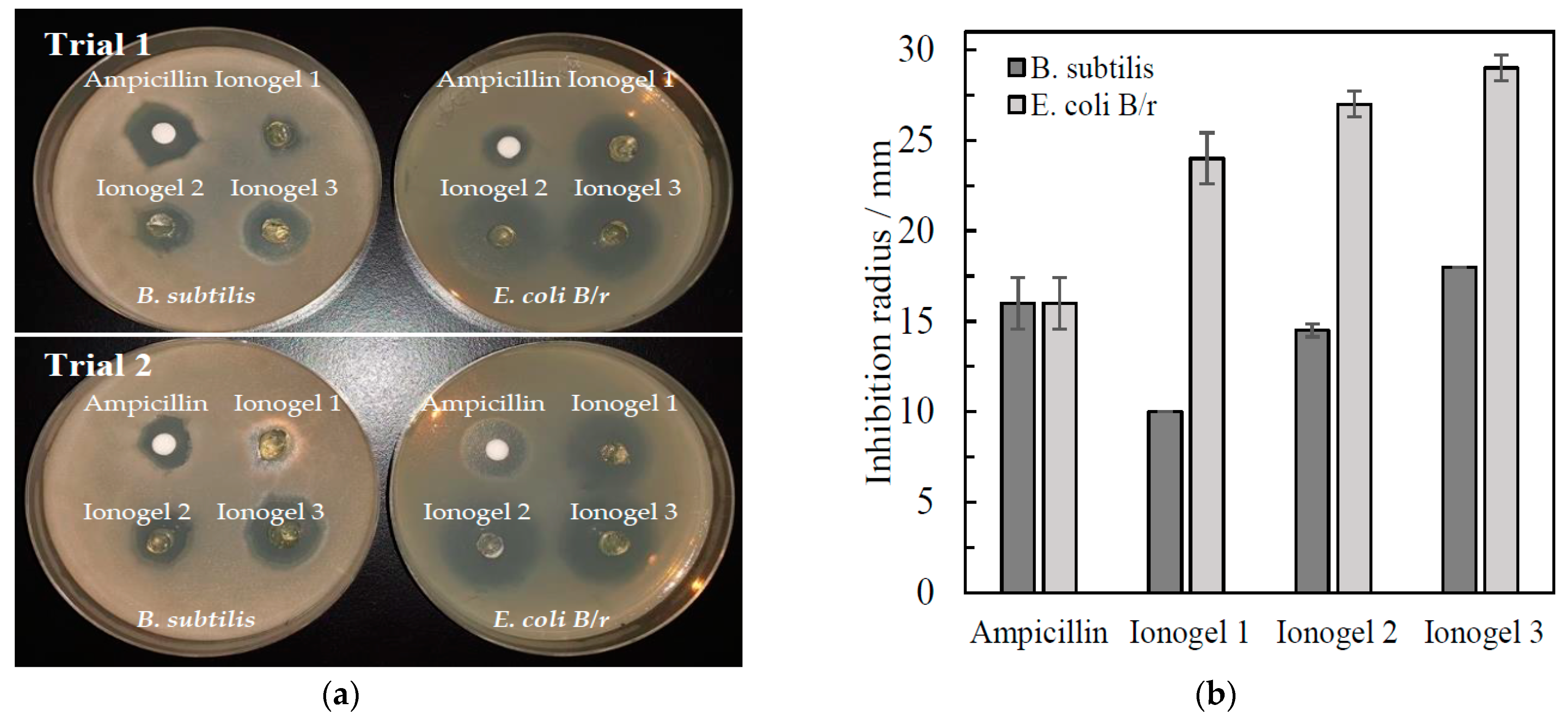
2.11. Antimicrobial Test of Cellulose Gels
3. Results and Discussion
3.1. Gelation Test
3.2. Appearance of the Cellulose Gels
3.3. [C4mpyr][OH]-Content of the Cellulose Gels
3.4. Swelling Ratio, Water Content, and Water Uptake
3.5. Recovery of [C4mpyr][OH] Aqueous Solution
3.6. Compression Test of the Cellulose Gels
3.7. Antimicrobial Test of Cellulose Gels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Filipe, A.; Almeida, I.F.; Costa, P.C.; Rosado, C.; Pascoal, C.; Silvestre, A.J.D.; Freire, C.S.R. Bacterial Cellulose Membranes as Transdermal Delivery Systems for Diclofenac: In Vitro Dissolution and Permeation Studies. Carbohydr. Polym. 2014, 106, 264–269. [Google Scholar] [CrossRef]
- Miyamoto, T.; Takahashi, S.; Ito, H.; Inagaki, H. Tissue Biocompatibility of Cellulose and Its Derivatives. J. Biomed. Mater. Res. 1989, 23, 125–133. [Google Scholar] [CrossRef]
- Beguin, P.; Aubert, J.-P. The Biological Degradation of Cellulose. FEMS Microbiol. Rev. 1994, 13, 25–58. [Google Scholar] [CrossRef]
- Lacin, N.T. Development of Biodegradable Antibacterial Cellulose Based Hydrogel Membranes for Wound Healing. Int. J. Biol. Macromol. 2014, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-Based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Demitri, C.; Sannino, A.; Conversano, F.; Casciaro, S.; Distante, A.; Maffezzoli, A. Hydrogel Based Tissue Mimicking Phantom for In-Vitro Ultrasound Contrast Agents Studies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 338–345. [Google Scholar] [CrossRef]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A.; Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-Responsive Hydrogels in Drug Delivery and Tissue Engineering. Drug Deliv. 2016, 23, 748–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Bri, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of Silver Nanoparticles Using Curcumin-Cyclodextrins Loaded into Bacterial Cellulose-Based Hydrogels for Wound Dressing Applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef]
- Huber, D.; Tegl, G.; Mensah, A.; Beer, B.; Baumann, M.; Borth, N.; Sygmund, C.; Ludwig, R.; Guebitz, G.M. A Dual-Enzyme Hydrogen Peroxide Generation Machinery in Hydrogels Supports Antimicrobial Wound Treatment. ACS Appl. Mater. Interfaces 2017, 9, 15307–15316. [Google Scholar] [CrossRef]
- Mao, C.; Xiang, Y.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wang, X.; Chu, P.K.; Wu, S. Photo-Inspired Antibacterial Activity and Wound Healing Acceleration by Hydrogel Embedded with Ag/Ag@AgCl/ZnO Nanostructures. ACS Nano 2017, 11, 9010–9021. [Google Scholar] [CrossRef]
- Boyer, C.; Figueiredo, L.; Pace, R.; Lesoeur, J.; Rouillon, T.; Visage, C.L.; Tassin, J.F.; Weiss, P.; Guicheux, J.; Rethore, G. Laponite Nanoparticle-Associated Silated Hydroxypropylmethyl Cellulose as an Injectable Reinforced Interpenetrating Network Hydrogel for Cartilage Tissue Engineering. Acta Biomater. 2018, 65, 112–122. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Peppas, N.A. Advances in Biomaterials, Drug Delivery, and Bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of Cellulose-Based Superabsorbent Hydrogels as Water Reservoir in Agriculture. Int. J. Polym. Sci. 2013, 2013, 435073. [Google Scholar] [CrossRef]
- Bashari, A.; Shirvan, A.R.; Shakeri, M. Cellulose—Based Hydrogels for Personal Care Products. Polym. Adv. Technol. 2018, 29, 2853–2867. [Google Scholar] [CrossRef]
- Hynninen, V.; Hietala, S.; Mckee, J.R.; Murtoma, L.; Rojas, O.J.; Ikkala, O.; Nonappa. Inverse Thermoreversible Mechanical Sti Ff Ening and Birefringence in a Methylcellulose/Cellulose Nanocrystal Hydrogel. Biomacromolecules 2018, 19, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, Y.; Nie, W.; Song, L.; Chen, P. Fabrication of Hydrogel of Hydroxypropyl Cellulose (HPC) Composited with Graphene Oxide and Its Application for Methylene Blue Removal. J. Mater. Sci. 2015, 50, 6113–6123. [Google Scholar] [CrossRef]
- Kwon, S.S.; Kong, B.J.; Park, S.N. Physicochemical Properties of PH-Sensitive Hydrogels Based on Hydroxyethyl Cellulose—Hyaluronic Acid and for Applications as Transdermal Delivery Systems for Skin Lesions. Eur. J. Pharm. Biopharm. 2015, 92, 146–154. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Biomaterials Science Advances in Crosslinking Strategies of Biomedical Hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Fink, H.; Weigel, P.; Purz, H.J.; Ganster, J. Structure Formation of Regenerated Cellulose Materials from NMMO-Solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Zhang, L.; Ruan, D.; Gao, S. Dissolution and Regeneration of Cellulose in NaOH/Thiourea Aqueous Solution. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1521–1529. [Google Scholar] [CrossRef]
- Potthast, A.; Rosenau, T.; Sixta, H.; Kosma, P. Degradation of Cellulosic Materials by Heating in DMAc/LiCl. Tetrahedron. Lett. 2002, 43, 7757–7759. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, C.; Zhang, R.; Zhang, L. Hydrogels Prepared from Unsubstituted Cellulose in NaOH/Urea Aqueous Solution. Macromol. Biosci. 2007, 7, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huang, J.; Zhong, Y.; Li, K.; Zhang, L.; Cai, J. High-Strength and High-Toughness Double-Cross-Linked Cellulose Hydrogels: A New Strategy Using Sequential Chemical and Physical Cross-Linking. Adv. Funct. Mater. 2016, 26, 6279–6287. [Google Scholar] [CrossRef]
- Kono, H.; Fujita, S. Biodegradable Superabsorbent Hydrogels Derived from Cellulose by Esterification Crosslinking with 1,2,3,4-Butanetetracarboxylic Dianhydride. Carbohydr. Polym. 2012, 87, 2582–2588. [Google Scholar] [CrossRef]
- Yoshimura, T.; Matsuo, K.; Fujioka, R. Novel Biodegradable Superabsorbent Hydrogels Derived from Cotton Cellulose and Succinic Anhydride: Synthesis and Characterization. J. Appl. Polym. Sci. 2006, 99, 3251–3256. [Google Scholar] [CrossRef]
- Rastin, H.; Ramezanpour, M.; Hassan, K.; Mazinani, A.; Tung, T.T.; Vreugde, S.; Losic, D. 3D Bioprinting of a Cell-Laden Antibacterial Polysaccharide Hydrogel Composite. Carbohydr. Polym. 2021, 264, 117989. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Nourmohammadi, J.; Ghaee, A.; Soleimani, N. Carboxymethyl Cellulose-Human Hair Keratin Hydrogel with Controlled Clindamycin Release as Antibacterial Wound Dressing. Int. J. Biol. Macromol. 2020, 147, 1239–1247. [Google Scholar] [CrossRef]
- Tavakolian, M.; Munguia-lopez, J.G.; Valiei, A.; Islam, S.; Kinsella, J.M.; Tufenkji, N.; Van De Ven, T.G.M. Highly Absorbent Antibacterial and Bio Film-Disrupting Hydrogels from Cellulose for Wound Dressing Applications. ACS Appl. Mater. Interfaces 2020, 12, 39991–40001. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Dai, L.; Si, C.; Zeng, Z. Antibacterial and Hemostatic Hydrogel via Nanocomposite from Cellulose Nanofibers. Carbohydr. Polym. 2018, 195, 63–70. [Google Scholar] [CrossRef]
- El Fawal, G.F.; Abu-serie, M.M.; Hassan, M.A.; Elnouby, M.S. Hydroxyethyl Cellulose Hydrogel for Wound Dressing: Fabrication, Characterization and in Vitro Evaluation. Int. J. Biol. Macromol. 2018, 111, 649–659. [Google Scholar] [CrossRef]
- Savitskaya, I.S.; Shokatayeva, D.H.; Kistaubayeva, A.S.; Ignatova, L.V.; Digel, I.E. Heliyon Antimicrobial and Wound Healing Properties of a Bacterial Cellulose Based Material Containing B. Subtilis Cells. Heliyon 2019, 5, e02592. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zheng, Y.; Wen, X.; Lin, Q.; Chen, X.; Wu, Z. Silver Nanoparticle/Bacterial Cellulose Gel Membranes for Antibacterial Wound Dressing: Investigation in Vitro and in Vivo. Biomed. Mater. 2014, 9, 035005. [Google Scholar] [CrossRef] [PubMed]
- Mohan, Y.M.; Lee, K.; Premkumar, T.; Geckeler, K.E. Hydrogel Networks as Nanoreactors: A Novel Approach to Silver Nanoparticles for Antibacterial Applications. Polymer 2007, 48, 158–164. [Google Scholar] [CrossRef]
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and Characterization of Antibacterial Carboxymethyl Cellulose/ZnO Nanocomposite Hydrogels. Int. J. Biol. Macromol. 2015, 74, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fu, S.; Li, X.; Zhou, Y.; Zhan, H. Preparation of Salt-Sensitive and Antibacterial Hydrogel Based on Quaternized Cellulose. BioResources 2010, 5, 1114–1125. [Google Scholar]
- Seiler, E.R.D.; Takeoka, Y.; Rikukawa, M.; Yoshizawa-fujita, M. RSC Advances. RSC Adv. 2020, 10, 11475–11480. [Google Scholar] [CrossRef] [Green Version]
- Hodyna, D.; Bardeau, J.; Metelytsia, L.; Riabov, S.; Kobrina, L.; Laptiy, S.; Kalashnikova, L.; Parkhomenko, V.; Tarasyuk, O.; Rogalsky, S. Efficient Antimicrobial Activity and Reduced Toxicity of 1-Dodecyl-3-Methylimidazolium Tetrafluoroborate Ionic Liquid/b-Cyclodextrin Complex. Chem. Eng. J. 2016, 284, 1136–1145. [Google Scholar] [CrossRef]
- Trush, M.M.; Semenyuta, I.V.; Vdovenko, S.I.; Rogalsky, S.P.; Lobko, E.O.; Metelytsia, L.O. Synthesis, Spectroscopic and Molecular Docking Studies of Imidazolium and Pyridinium Based Ionic Liquids with HSA as Potential Antimicrobial Agents. J. Mol. Struct. 2017, 1137, 692–699. [Google Scholar] [CrossRef]
- Sun, J.; MacFarlane, D.R.; Forsyth, M. N,N-Dimethylpyrrolidinium Hydroxide: A Highly Conductive Solid Material at Ambient Temperature. J. Mater. Chem. 2001, 11, 2940–2942. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent Hydrogels Based on Cellulose for Smart Swelling and Controllable Delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Ye, D.; Chang, C.; Zhang, L. High-Strength and Tough Cellulose Hydrogels Chemically Dual Cross-Linked by Using Low- and High-Molecular-Weight Cross-Linkers. Biomacromolecules 2019, 20, 1989–1995. [Google Scholar] [CrossRef]
- Vanhatalo, K.; Maximova, N.; Perander, A.-M.; Johansson, L.-S.; Haimi, E.; Dahl, O. Comparison of Conventional and Lignin-Rich Microcrystalline Cellulose. BioResources 2016, 11, 4037–4054. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Zhang, L.; Zhou, J.; Zhang, L.; Kennedy, J.F. Structure and Properties of Hydrogels Prepared from Cellulose in NaOH/Urea Aqueous Solutions. Carbohydr. Polym. 2010, 82, 122–127. [Google Scholar] [CrossRef]
- Chang, C.; Chen, S.; Zhang, L. Novel Hydrogels Prepared via Direct Dissolution of Chitin at Low Temperature: Structure and Biocompatibility. J. Mater. Chem. 2011, 21, 3865–3871. [Google Scholar] [CrossRef]
- Tahara, N.; Tabuchi, M.; Watanabe, K.; Yano, H.; Morinaga, Y.; Yoshinaga, F. Degree of Polymerization of Cellulose from Acetobacter Xylinum BPR2001 Decreased by Cellulase Produced by the Strain. Biosci. Biotechnol. Biochem. 1997, 61, 1862–1865. [Google Scholar] [CrossRef]
- Setu, N.I.; Mia, Y.; Lubna, N.J.; Chowdhury, A.A. Preparation of Microcrystalline Cellulose from Cotton and Its Evaluation as Direct Compressible Excipient in the Formulation of Naproxen Tablets. Dhaka Univ. J. Pharm. Sci. 2014, 13, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Numata, Y.; Sakata, T.; Furukawa, H.; Tajima, K. Bacterial Cellulose Gels with High Mechanical Strength. Mater. Sci. Eng. C 2015, 47, 57–62. [Google Scholar] [CrossRef]
- Mohamed, A.; Shahid, S.M.A.; Alkhamaiseh, S.I.; Ahmed, M.Q.; Albalwi, F.O.; Al-gholaigah, M.H.; Alqhtani, M.M.; Alshammari, M.G. Antibacterial Activity of Commiphora Molmol in Wound Infections. J. Diagnostic Pathol. Oncol. 2017, 2, 32–36. [Google Scholar]
- Tolesa, L.D.; Gupta, B.S.; Tiwikrama, A.H.; Wu, Y.; Lee, M.-J. Alkali Lignin Degradation with Aqueous Ammonium-Based Ionic Liquid Solutions. J. Clean. Prod. 2020, 258, 120724. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an Essential Oil of Clove) Acts as an Antibacterial Agent against Salmonella Typhi by Disrupting the Cellular Membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, W.; Wang, Z. Synthesis and in Vitro Antibacterial Activity of Oxazolidine LBM-415 Analogs as Peptide Deformylase Inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 1541–1544. [Google Scholar] [CrossRef]
- Ye, D.; Zhong, Z.; Xu, H.; Chang, C.; Yang, Z.; Wang, Y.; Ye, Q.; Zhang, L. Construction of Cellulose/Nanosilver Sponge Materials and Their Antibacterial Activities for Infected Wounds Healing. Cellulose 2016, 23, 749–763. [Google Scholar] [CrossRef]
- Bass, L.; Liebert, C.A.; Lee, M.D.; Summers, A.O.; White, D.G.; Thayer, S.G.; Maurer, J.J. Incidence and Characterization of Integrons, Genetic Elements Mediating Multiple-Drug Resistance, in Avian Escherichia coli. Antimicrob. Agents Chemother. 1999, 43, 2925–2929. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seiler, E.R.D.; Koyama, K.; Iijima, T.; Saito, T.; Takeoka, Y.; Rikukawa, M.; Yoshizawa-Fujita, M. Simple and Fast One-Pot Cellulose Gel Preparation in Aqueous Pyrrolidinium Hydroxide Solution–Cellulose Solvent and Antibacterial Agent. Polymers 2021, 13, 1942. https://doi.org/10.3390/polym13121942
Seiler ERD, Koyama K, Iijima T, Saito T, Takeoka Y, Rikukawa M, Yoshizawa-Fujita M. Simple and Fast One-Pot Cellulose Gel Preparation in Aqueous Pyrrolidinium Hydroxide Solution–Cellulose Solvent and Antibacterial Agent. Polymers. 2021; 13(12):1942. https://doi.org/10.3390/polym13121942
Chicago/Turabian StyleSeiler, Elisabeth R. D., Kohei Koyama, Tomoyuki Iijima, Tamao Saito, Yuko Takeoka, Masahiro Rikukawa, and Masahiro Yoshizawa-Fujita. 2021. "Simple and Fast One-Pot Cellulose Gel Preparation in Aqueous Pyrrolidinium Hydroxide Solution–Cellulose Solvent and Antibacterial Agent" Polymers 13, no. 12: 1942. https://doi.org/10.3390/polym13121942