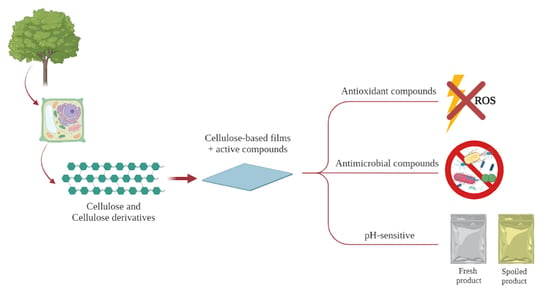
Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging
Abstract
1. Introduction
2. Biopolymers Usage as Food Packaging Alternatives
2.1. Biobased Polymers
2.2. Cellulose Derivatives and Properties
2.2.1. Cellulose Acetate
2.2.2. Cellulose Sulfate
2.2.3. Cellulose Nitrate
2.2.4. Ethyl Cellulose
2.2.5. Methylcellulose
2.2.6. Hydroxypropyl Methylcellulose
2.2.7. Carboxymethyl Cellulose
2.2.8. Hydroxyethyl Cellulose
2.2.9. Hydroxypropyl Cellulose
2.3. Novel Cellulose-Based Films Aiming Food Packaging Applications
2.3.1. Cellulose Films with Antimicrobial Properties
| Active Compounds/Extracts | Cellulose Matrix | Functions | Ref. |
|---|---|---|---|
| Pink pepper EO | CA |
| [119] |
| Rosemary and Aloe Vera EOs | CA |
| [120] |
| Cinnamon EO | CMC |
| [121] |
| Cinnamon and ginger EOs | CMC/chitosan |
| [122] |
| Clove oil | CMC |
| [123] |
| Zinc oxide nanoparticles/Grape seed extract | CMC |
| [124] |
| Oregano and bergamot EOs | HPMC |
| [125] |
| Nisin | HPMC |
| [127] |
| HPMC |
| [128] | |
| Nisin and Lactococcus lactis | CMC/corn starch |
| [129] |
| Lysozyme | CMC |
| [131] |
| Sodium-CMC/Gelatin |
| [132] | |
| Silver nanoparticles | CA |
| [133] |
| [134] | ||
| Silver, zinc oxide, and copper oxide nanoparticles | CMC |
| [113] |
| Titanium | CMC |
| [135] |
| Potassium sorbate | CMC/Pectin |
| [136] |
| Chitosan/Zinc oxide nanoparticles | CMC |
| [137] |
| Chitosan/Zinc oxide nanoparticles | CMC |
| [138] |
| Sodium caseinate | CMC/Sodium caseinate |
| [139] |
2.3.2. Cellulose Films with Antioxidant Properties
| Active Compounds/Extracts | Cellulose Matrix | Functions | Ref. |
|---|---|---|---|
| Carotenoids: norbixin, lycopene, zeaxanthin | CA |
| [142] |
| Murta extract | MC-Glutaraldehyde (GA) |
| [79] |
| Pomegranate seed extract | CMC, HEC, HPMC, MC |
| [143] |
| Antioxidants of bamboo leaves | CMC |
| [1144] |
| Chinese chives root extract | CMC |
| [145] |
| α-tocopherol | CMC-lecithin/CMC-Tween80 |
| [146] |
| Curcumin and Zinc oxide | CMC |
| [147] |
| Epigallocatechin gallate(EGCG) | Sodium alginate (SA)-CMC |
| [148] |
| Maqui extract | MC-Glutaraldehyde (GA) |
| [80] |
| Clove EO | EC |
| [149] |
| Lippia alba extract and Silver nanoparticles | MC |
| [150] |
| Chitosan ascorbate | MC |
| [151] |
2.3.3. Intelligent Cellulose Films
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fay, D.L. Foodborne Diseases; Holban, A.M., Grumezescu, A.M., Eds.; Andre G. Wolff: Cambridge, MA, USA, 1967; Volume 6, pp. 951–952. [Google Scholar]
- CDC. Food Safety-Foodborne Germs and Illnesses. Available online: https://www.cdc.gov/foodsafety/foodborne-germs.html. (accessed on 30 June 2022).
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Hansen, M.F.; Roeder, H.L.; Wang, N.; Burmølle, M.; He, G. Mixed-species biofilms in the food industry: Current knowledge and novel control strategies. Crit. Rev. Food Sci. Nutr. 2019, 60, 2277–2293. [Google Scholar] [CrossRef] [PubMed]
- Galiè, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New Weapons to Fight Old Enemies: Novel Strategies for the (Bio)control of Bacterial Biofilms in the Food Industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food Loss and Food Waste. Available online: http://www.fao.org/food-loss-and-food-waste/flw-data (accessed on 30 June 2022).
- Raak, N.; Symmank, C.; Zahn, S.; Aschemann-Witzel, J.; Rohm, H. Processing- and product-related causes for food waste and implications for the food supply chain. Waste Manag. 2017, 61, 461–472. [Google Scholar] [CrossRef]
- Nations United. World Population Prospects 2022 Summary of Results; Nations United: New York, NY, USA, 2022; p. 46. [Google Scholar]
- Alamar, M.D.C.; Falagan, N.; Aktas, E.; A Terry, L. Minimising food waste: A call for multidisciplinary research. J. Sci. Food Agric. 2017, 98, 8–11. [Google Scholar] [CrossRef]
- Kibler, K.M.; Reinhart, D.; Hawkins, C.; Motlagh, A.M.; Wright, J. Food waste and the food-energy-water nexus: A review of food waste management alternatives. Waste Manag. 2018, 74, 52–62. [Google Scholar] [CrossRef]
- Müller, P.; Schmid, M. Intelligent Packaging in the Food Sector: A Brief Overview. Foods 2019, 8, 16. [Google Scholar] [CrossRef]
- Youssef, A.M.; Assem, F.M.; Abdel-Aziz, M.E.; Elaaser, M.; Ibrahim, O.A.; Mahmoud, M.; Abd El-Salam, M.H. Development of bionanocomposite materials and its use in coating of Ras cheese. Food Chem. 2019, 270, 467–475. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R. Antimicrobial food packaging based on sustainable Bio-based materials for reducing foodborne Pathogens: A review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef]
- PlasticEurope-Association of Plastics Manufactures. Plastics-the Facts 2021. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 22 September 2022).
- Lam, C.-S.; Ramanathan, S.; Carbery, M.; Gray, K.; Vanka, K.S.; Maurin, C.; Bush, R.; Palanisami, T. A Comprehensive Analysis of Plastics and Microplastic Legislation Worldwide. Water Air Soil Pollut. 2018, 229, 345. [Google Scholar] [CrossRef]
- Katiyar, V. Bio-Based Plastics for Food Packaging Applications; Smithers Pira: Shropshire, UK, 2017; p. 276. [Google Scholar]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging: Trends and Perspectives. Foods 2020, 9, 1438. [Google Scholar] [CrossRef] [PubMed]
- Jariyasakoolroj, P.; Leelaphiwat, P.; Harnkarnsujarit, N. Advances in research and development of bioplastic for food packaging. J. Sci. Food Agric. 2018, 100, 5032–5045. [Google Scholar] [CrossRef] [PubMed]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Bioplastics. Guide-Knowledge Zone. Available online: http://www.bioplastics.guide/ref (accessed on 1 July 2022).
- Vianna, T.C.; Marinho, C.O.; Júnior, L.M.; Ibrahim, S.A.; Vieira, R.P. Essential oils as additives in active starch-based food packaging films: A review. Int. J. Biol. Macromol. 2021, 182, 1803–1819. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Emerging Chitosan-Based Films for Food Packaging Applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, Y.; Chen, M.; Xiao, N.; Zhang, J.; Liu, C. Development of functional chitosan-based composite films incorporated with hemicelluloses: Effect on physicochemical properties. Carbohydr. Polym. 2020, 246, 116489. [Google Scholar] [CrossRef]
- Sedayu, B.B.; Cran, M.J.; Bigger, S.W. A Review of Property Enhancement Techniques for Carrageenan-based Films and Coatings. Carbohydr. Polym. 2019, 216, 287–302. [Google Scholar] [CrossRef]
- Singh, P.; Magalhães, S.; Alves, L.; Antunes, F.; Miguel, M.; Lindman, B.; Medronho, B. Cellulose-based edible films for probiotic entrapment. Food Hydrocoll. 2018, 88, 68–74. [Google Scholar] [CrossRef]
- He, X.; Lu, W.; Sun, C.; Khalesi, H.; Mata, A.; Andaleeb, R.; Fang, Y. Cellulose and cellulose derivatives: Different colloidal states and food-related applications. Carbohydr. Polym. 2020, 255, 117334. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- David, G.; Gontard, N.; Angellier-Coussy, H. Mitigating the Impact of Cellulose Particles on the Performance of Biopolyester-Based Composites by Gas-Phase Esterification. Polymers 2019, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Cheng, T.; Duan, C.; Zhao, W.; Zhang, W.; Zou, X.; Aspler, J.; Ni, Y. 3D printing using plant-derived cellulose and its derivatives: A review. Carbohydr. Polym. 2018, 203, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Harini, K.; Mohan, C.C. Isolation and characterization of micro and nanocrystalline cellulose fibers from the walnut shell, corncob and sugarcane bagasse. Int. J. Biol. Macromol. 2020, 163, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Gao, R.; Gao, L.; Li, J. Poly(vinyl alcohol) films reinforced with nanofibrillated cellulose (NFC) isolated from corn husk by high intensity ultrasonication. Carbohydr. Polym. 2016, 136, 1027–1034. [Google Scholar] [CrossRef]
- Saiful; Hasima, S.; Kamila, N.; Rahmi. Cellulose acetate from palm oil bunch waste for forward osmosis membrane in desalination of brackish water. Results Eng. 2022, 15, 100611. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Chen, M.; Goff, H.D.; Zhong, F.; Sharif, H.R.; Li, Y. Functionality and nutritional aspects of microcrystalline cellulose in food. Carbohydr. Polym. 2017, 172, 159–174. [Google Scholar] [CrossRef]
- Mu, R.; Hong, X.; Ni, Y.; Li, Y.; Pang, J.; Wang, Q.; Xiao, J.; Zheng, Y. Recent trends and applications of cellulose nanocrystals in food industry. Trends Food Sci. Technol. 2019, 93, 136–144. [Google Scholar] [CrossRef]
- Fischer, S.; Thümmler, K.; Volkert, B.; Hettrich, K.; Schmidt, I.; Fischer, K. Properties and Applications of Cellulose Acetate. Macromol. Symp. 2008, 262, 89–96. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, E.; Shockravi, A.; Vatanpour, V. High performance compatible thiazole-based polymeric blend cellulose acetate membrane as selective CO2 absorbent and molecular sieve. Carbohydr. Polym. 2020, 252, 117215. [Google Scholar] [CrossRef] [PubMed]
- Nimir, W.; Al-Othman, A.; Tawalbeh, M.; Al Makky, A.; Ali, A.; Karimi-Maleh, H.; Karimi, F.; Karaman, C. Approaches towards the development of heteropolyacid-based high temperature membranes for PEM fuel cells. Int. J. Hydrogen Energy 2021, in press. [Google Scholar] [CrossRef]
- Jain, H.; Garg, M.C. Fabrication of polymeric nanocomposite forward osmosis membranes for water desalination—A review. Environ. Technol. Innov. 2021, 23, 101561. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Kahya, N.; Adımcılar, V.; Kaygusuz, H.; Torlak, E.; Akın-Evingür, G.; Erim, F.B. Antibacterial nano cerium oxide/chitosan/cellulose acetate composite films as potential wound dressing. Eur. Polym. J. 2020, 133, 109777. [Google Scholar] [CrossRef]
- David, M.E.; Ion, R.M.; Grigorescu, R.M.; Iancu, L.; Holban, A.M.; Iordache, F.; Nicoara, A.I.; Alexandrescu, E.; Somoghi, R.; Teodorescu, S.; et al. Biocompatible and Antimicrobial Cellulose Acetate-Collagen Films Containing MWCNTs Decorated with TiO2 Nanoparticles for Potential Biomedical Applications. Nanomaterials 2022, 12, 239. [Google Scholar] [CrossRef]
- Pereira, N.R.L.; Lopes, B.; Fagundes, I.V.; de Moraes, F.M.; Morisso, F.D.P.; Parma, G.O.C.; Zepon, K.M.; Magnago, R.F. Bio-packaging based on cellulose acetate from banana pseudostem and containing Butia catarinensis extracts. Int. J. Biol. Macromol. 2021, 194, 32–41. [Google Scholar] [CrossRef]
- Said Mahmoud, A.; Peters, R.W. Application of Entrapped Nano Zero Valent Iron into Cellulose Acetate Membranes for Domestic Wastewater Treatment. In Proceedings of the Annual AIChE Meeting, Minneapolis, MN, USA, 29 October–3 November 2017. [Google Scholar]
- Agarwal, A.; Jeengar, A. Performance Characteristics of Electrospun Cellulose Acetate Nanofiber Mat Embedded with Nano-Zno/Vitamins. IJNA 2016, 6, 1–12. [Google Scholar]
- Liang, L.; Chen, H. Development and characterization of biodegradable ultraviolet protective and antibacterial polylactic acid-cellulose acetate film modified by phenyl salicylate. Int. J. Biol. Macromol. 2022, 211, 85–93. [Google Scholar] [CrossRef]
- Celuppi, L.C.M.; Capelezzo, A.P.; Cima, L.B.; Zeferino, R.C.F.; Zanetti, M.; Riella, H.G.; Fiori, M.A. Antimicrobial cellulose acetate films by incorporation of geranyl acetate for active food packaging application. Res. Soc. Dev. 2022, 11, e40111125141. [Google Scholar] [CrossRef]
- Laroque, D.A.; de Aragão, G.M.F.; de Araújo, P.H.H.; Carciofi, B.A.M. Active cellulose acetate-carvacrol films: Antibacterial, physical and thermal properties. Packag. Technol. Sci. 2021, 34, 463–474. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, D.; Yao, S. Review on biomedical and bioengineering applications of cellulose sulfate. Carbohydr. Polym. 2015, 132, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Scott, Y.; Dezzutti, C.S. Non-Antiretroviral Microbicides for HIV Prevention. Aids Rev. 2016, 18, 145–150. [Google Scholar] [PubMed]
- Song, S.; Liu, X.; Ding, L.; Abubaker, M.A.; Zhang, J.; Huang, Y.; Yang, S.; Fan, Z. Conformational and rheological properties of bacterial cellulose sulfate. Int. J. Biol. Macromol. 2021, 183, 2326–2336. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Wu, Q.-X.; Chen, Y.; Zhao, J.; Cheng, X.-D.; Chen, J. Fabrication of the polyphosphates patched cellulose sulfate-chitosan hydrochloride microcapsules and as vehicles for sustained drug release. Int. J. Pharm. 2018, 555, 291–302. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, B.; Zhao, J.; Chen, H. Development and characterization of food packaging film from cellulose sulfate. Food Hydrocoll. 2013, 35, 476–483. [Google Scholar] [CrossRef]
- Chen, G.; Liu, B. Cellulose sulfate based film with slow-release antimicrobial properties prepared by incorporation of mustard essential oil and β-cyclodextrin. Food Hydrocoll. 2016, 55, 100–107. [Google Scholar] [CrossRef]
- Neves, A.; Angelin, E.M.; Roldão, É.; Melo, M.J. New insights into the degradation mechanism of cellulose nitrate in cinematographic films by Raman microscopy. J. Raman Spectrosc. 2018, 50, 202–212. [Google Scholar] [CrossRef]
- Berthumeyrie, S.; Collin, S.; Bussiere, P.-O.; Therias, S. Photooxidation of cellulose nitrate: New insights into degradation mechanisms. J. Hazard. Mater. 2014, 272, 137–147. [Google Scholar] [CrossRef]
- Kim, B.B.; Im, W.J.; Byun, J.Y.; Kim, H.M.; Kim, M.-G.; Shin, Y.-B. Label-free CRP detection using optical biosensor with one-step immobilization of antibody on nitrocellulose membrane. Sens. Actuators B Chem. 2014, 190, 243–248. [Google Scholar] [CrossRef]
- Fernández, J.G.; Almeida, C.A.; Fernández-Baldo, M.A.; Felici, E.; Raba, J.; Sanz, M.I. Development of nitrocellulose membrane filters impregnated with different biosynthesized silver nanoparticles applied to water purification. Talanta 2016, 146, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, J.; Xu, F.; Gong, S. Crepe cellulose paper and nitrocellulose membrane-based triboelectric nanogenerators for energy harvesting and self-powered human-machine interaction. Nano Energy 2019, 61, 69–77. [Google Scholar] [CrossRef]
- Tian, Y.; Guo, K.; Bian, X.; Wang, T.; Chen, S.; Sun, J. Durable and room-temperature curable superhydrophobic composite coating on nitrocellulose lacquer. Surf. Coat. Technol. 2017, 328, 444–450. [Google Scholar] [CrossRef]
- Licciardello, F.; Wittenauer, J.; Saengerlaub, S.; Reinelt, M.; Stramm, C. Rapid assessment of the effectiveness of antioxidant active packaging—Study with grape pomace and olive leaf extracts. Food Packag. Shelf Life 2015, 6, 1–6. [Google Scholar] [CrossRef]
- HSE-Health and Safety Executive. The Dangers of Cellulose Nitrate Film. 2013. Available online: https://www.hse.gov.uk/pubns/indg469.htm (accessed on 14 September 2022).
- Yang, D.; Peng, X.; Zhong, L.; Cao, X.; Chen, W.; Zhang, X.; Liu, S.; Sun, R. “Green” films from renewable resources: Properties of epoxidized soybean oil plasticized ethyl cellulose films. Carbohydr. Polym. 2014, 103, 198–206. [Google Scholar] [CrossRef]
- Gilbert, M. Cellulose Plastics. In Brydson’s Plastics Materials, 8th ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 617–630. [Google Scholar]
- Yang, Y.; Wang, H.; Li, H.; Ou, Z.; Yang, G. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release. Eur. J. Pharm. Sci. 2018, 115, 11–18. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Godakanda, V.U.; Chiu, Y.-J.; Angkawinitwong, U.; Patel, K.; Stapleton, P.G.; de Silva, R.M.; de Silva, K.N.; Zhu, L.-M.; et al. The effect of collection substrate on electrospun ciprofloxacin-loaded poly(vinylpyrrolidone) and ethyl cellulose nanofibers as potential wound dressing materials. Mater. Sci. Eng. C 2019, 104, 109917. [Google Scholar] [CrossRef]
- Hokmabad, V.R.; Davaran, S.; Aghazadeh, M.; Rahbarghazi, R.; Salehi, R.; Ramazani, A. Fabrication and characterization of novel ethyl cellulose-grafted-poly (ɛ-caprolactone)/alginate nanofibrous/macroporous scaffolds incorporated with nano-hydroxyapatite for bone tissue engineering. J. Biomater. Appl. 2019, 33, 1128–1144. [Google Scholar] [CrossRef]
- Li, X.; Jiang, F.; Ni, X.; Yan, W.; Fang, Y.; Corke, H.; Xiao, M. Preparation and characterization of konjac glucomannan and ethyl cellulose blend films. Food Hydrocoll. 2015, 44, 229–236. [Google Scholar] [CrossRef]
- Su, X.; Yang, Z.; Tan, K.B.; Chen, J.; Huang, J.; Li, Q. Preparation and characterization of ethyl cellulose film modified with capsaicin. Carbohydr. Polym. 2020, 241, 116259. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, Y.; Cao, X.; Liu, Q.; Wang, H.; Kong, B. Preparation and functional properties of poly(vinyl alcohol)/ethyl cellulose/tea polyphenol electrospun nanofibrous films for active packaging material. Food Control 2021, 130, 108331. [Google Scholar] [CrossRef]
- US Food and Drug Administration GRAS Substances (SCOGS) Database. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-substances-scogs-database (accessed on 2 September 2022).
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical Applications of Cellulose Ethers and Cellulose Ether Esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef] [PubMed]
- Oprea, M.; Voicu, S.I. Recent advances in composites based on cellulose derivatives for biomedical applications. Carbohydr. Polym. 2020, 247, 116683. [Google Scholar] [CrossRef]
- Sahoo, G.P.; Bhui, D.K.; Bar, H.; Sarkar, P.; Samanta, S.; Pyne, S.; Misra, A. Synthesis and characterization of gold nanoparticles adsorbed in methyl cellulose micro fibrils. J. Mol. Liq. 2010, 155, 91–95. [Google Scholar] [CrossRef]
- Ho, M.T.; Teal, C.J.; Shoichet, M.S. A hyaluronan/methylcellulose-based hydrogel for local cell and biomolecule delivery to the central nervous system. Brain Res. Bull. 2019, 148, 46–54. [Google Scholar] [CrossRef]
- CDC|NIOSH. Glutaraldehyde. Available online: https://www.cdc.gov/niosh/topics/glutaraldehyde/default.html (accessed on 23 September 2022).
- de Dicastillo, C.L.; Bustos, F.; Guarda, A.; Galotto, M.J. Cross-linked methyl cellulose films with murta fruit extract for antioxidant and antimicrobial active food packaging. Food Hydrocoll. 2016, 60, 335–344. [Google Scholar] [CrossRef]
- de Dicastillo, C.L.; Rodríguez, F.; Guarda, A.; Galotto, M.J. Antioxidant films based on cross-linked methyl cellulose and native Chilean berry for food packaging applications. Carbohydr. Polym. 2016, 136, 1052–1060. [Google Scholar] [CrossRef]
- Patil, V.A.; Baswal, A.K.; Parab, A.; Patil, V.; Jakhar, V. Effect of hydroxypropyl methylcellulose and methylcellulose-based edible coatings on storage life and quality of horticultural crops: Review. Pharma Innov. J. 2022, 11, 2746–2752. [Google Scholar]
- Ghadermazi, R.; Hamdipour, S.; Sadeghi, K.; Ghadermazi, R.; Asl, A.K. Effect of various additives on the properties of the films and coatings derived from hydroxypropyl methylcellulose—A review. Food Sci. Nutr. 2019, 7, 3363–3377. [Google Scholar] [CrossRef]
- Tundisi, L.; Mostaço, G.; Carricondo, P.; Petri, D. Hydroxypropyl methylcellulose: Physicochemical properties and ocular drug delivery formulations. Eur. J. Pharm. Sci. 2021, 159, 105736. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.N.; Fonseca, J.D.M.; Feldhaus, H.K.; Soares, L.S.; Valencia, G.A.; de Campos, C.E.M.; Di Luccio, M.; Monteiro, A.R. Physical and morphological properties of hydroxypropyl methylcellulose films with curcumin polymorphs. Food Hydrocoll. 2019, 97, 105217. [Google Scholar] [CrossRef]
- Lee, J.Y.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Antibacterial and antioxidant properties of hydroxypropyl methylcellulose-based active composite films incorporating oregano essential oil nanoemulsions. LWT 2019, 106, 164–171. [Google Scholar] [CrossRef]
- Vieira, A.C.F.; Fonseca, J.D.M.; Menezes, N.M.C.; Monteiro, A.R.; Valencia, G.A. Active coatings based on hydroxypropyl methylcellulose and silver nanoparticles to extend the papaya (Carica papaya L.) shelf life. Int. J. Biol. Macromol. 2020, 164, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Feng, H.; Wu, M.; Chen, L.; Xia, W.; Zhang, W. Preparation and characterization of arginine-modified chitosan/hydroxypropyl methylcellose antibacterial film. Int. J. Biol. Macromol. 2019, 145, 750–758. [Google Scholar] [CrossRef]
- Mohan, T.; Štiglic, A.D.; Beaumont, M.; Konnerth, J.; Gürer, F.; Makuc, D.; Maver, U.; Gradišnik, L.; Plavec, J.; Kargl, R.; et al. Generic Method for Designing Self-Standing and Dual Porous 3D Bioscaffolds from Cellulosic Nanomaterials for Tissue Engineering Applications. ACS Appl. Bio Mater. 2020, 3, 1197–1209. [Google Scholar] [CrossRef]
- Verma, N.; Pramanik, K.; Singh, A.K.; Biswas, A. Design of magnesium oxide nanoparticle incorporated carboxy methyl cellu-lose/poly vinyl alcohol composite film with novel composition for skin tissue engineering. Mater. Technol. 2021, 37, 706–716. [Google Scholar] [CrossRef]
- Sharmila, G.; Muthukumaran, C.; Kirthika, S.; Keerthana, S.; Kumar, N.M.; Jeyanthi, J. Fabrication and characterization of Spinacia oleracea extract incorporated alginate/carboxymethyl cellulose microporous scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 156, 430–437. [Google Scholar] [CrossRef]
- Matinfar, M.; Mesgar, A.S.; Mohammadi, Z. Evaluation of physicochemical, mechanical and biological properties of chitosan/carboxymethyl cellulose reinforced with multiphasic calcium phosphate whisker-like fibers for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 341–353. [Google Scholar] [CrossRef]
- Sadeghi, S.; Nourmohammadi, J.; Ghaee, A.; Soleimani, N. Carboxymethyl cellulose-human hair keratin hydrogel with controlled clindamycin release as antibacterial wound dressing. Int. J. Biol. Macromol. 2019, 147, 1239–1247. [Google Scholar] [CrossRef]
- Saladino, M.L.; Markowska, M.; Carmone, C.; Cancemi, P.; Alduina, R.; Presentato, A.; Scaffaro, R.; Biały, D.; Hasiak, M.; Hreniak, D.; et al. Graphene Oxide Carboxymethylcellulose Nanocomposite for Dressing Materials. Materials 2020, 13, 1980. [Google Scholar] [CrossRef] [PubMed]
- Maver, U.; Xhanari, K.; Žižek, M.; Gradišnik, L.; Repnik, K.; Potočnik, U.; Finšgar, M. Carboxymethyl cellulose/diclofenac bioactive coatings on AISI 316LVM for controlled drug delivery, and improved osteogenic potential. Carbohydr. Polym. 2019, 230, 115612. [Google Scholar] [CrossRef] [PubMed]
- Koneru, A.; Dharmalingam, K.; Anandalakshmi, R. Cellulose based nanocomposite hydrogel films consisting of sodium carboxymethylcellulose–grapefruit seed extract nanoparticles for potential wound healing applications. Int. J. Biol. Macromol. 2020, 148, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Atta, O.M.; Manan, S.; Ul-Islam, M.; Ahmed, A.A.Q.; Ullah, M.W.; Yang, G. Development and characterization of plant oil-incorporated carboxymethyl cellulose/bacterial cellulose/glycerol-based antimicrobial edible films for food packaging applications. Adv. Compos. Hybrid Mater. 2022, 5, 973–990. [Google Scholar] [CrossRef]
- Riahi, Z.; Rhim, J.-W.; Bagheri, R.; Pircheraghi, G.; Lotfali, E. Carboxymethyl cellulose-based functional film integrated with chitosan-based carbon quantum dots for active food packaging applications. Prog. Org. Coat. 2022, 166, 106794. [Google Scholar] [CrossRef]
- You, P.; Wang, L.; Zhou, N.; Yang, Y.; Pang, J. A pH-intelligent response fish packaging film: Konjac glucomannan/carboxymethyl cellulose/blackcurrant anthocyanin antibacterial composite film. Int. J. Biol. Macromol. 2022, 204, 386–396. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Tabasum, S.; Khalid, S.; Shareef, R. A review on grafting of hydroxyethylcellulose for versatile applications. Int. J. Biol. Macromol. 2020, 150, 289–303. [Google Scholar] [CrossRef]
- Abdel-Halim, E. Chemical modification of cellulose extracted from sugarcane bagasse: Preparation of hydroxyethyl cellulose. Arab. J. Chem. 2014, 7, 362–371. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, Y.; Zhang, X.; He, X.; Zhang, C. Cellulose-based polymers. Phys. Sci. Rev. 2021, 000010151520200067. [Google Scholar] [CrossRef]
- Ayouch, I.; Kassem, I.; Kassab, Z.; Barrak, I.; Barhoun, A.; Jacquemin, J.; Draoui, K.; El Achaby, M. Crosslinked carboxymethyl cellulose-hydroxyethyl cellulose hydrogel films for adsorption of cadmium and methylene blue from aqueous solutions. Surfaces Interfaces 2021, 24, 101124. [Google Scholar] [CrossRef]
- El Fawal, G.F.; Abu-Serie, M.M.; Hassan, M.A.; Elnouby, M.S. Hydroxyethyl cellulose hydrogel for wound dressing: Fabrication, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018, 111, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Jin, T.; Liu, W.; Hao, W.; Yan, L.; Zheng, L. Effects of hydroxyethyl cellulose and sodium alginate edible coating containing asparagus waste extract on postharvest quality of strawberry fruit. LWT 2021, 148, 111770. [Google Scholar] [CrossRef]
- El Fawal, G.; Hong, H.; Song, X.; Wu, J.; Sun, M.; He, C.; Mo, X.; Jiang, Y.; Wang, H. Fabrication of antimicrobial films based on hydroxyethylcellulose and ZnO for food packaging application. Food Packag. Shelf Life 2020, 23, 100462. [Google Scholar] [CrossRef]
- Rukmanikrishnan, B.; Ramalingam, S.; Kim, S.S.; Lee, J. Rheological and anti-microbial study of silica and silver nanoparticles-reinforced k-carrageenan/hydroxyethyl cellulose composites for food packaging applications. Cellulose 2021, 28, 5577–5590. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; E El-Naggar, M.; Alotaiby, S.; El-Hamshary, H.; Moydeen, M.; Al-Deyab, S. Green Electrospining of Hydroxypropyl Cellulose Nanofibres for Drug Delivery Applications. J. Nanosci. Nanotechnol. 2018, 18, 805–814. [Google Scholar] [CrossRef]
- Tanaka, A.; Furubayashi, T.; Tomisaki, M.; Kawakami, M.; Kimura, S.; Inoue, D.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Nasal drug absorption from powder formulations: The effect of three types of hydroxypropyl cellulose (HPC). Eur. J. Pharm. Sci. 2017, 96, 284–289. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Umemura, K.; Tahara, K.; Takeuchi, H. Formulation design of hydroxypropyl cellulose films for use as orally disintegrating dosage forms. J. Drug Deliv. Sci. Technol. 2018, 46, 93–100. [Google Scholar] [CrossRef]
- Barty-King, C.H.; Chan, C.L.C.; Parker, R.M.; Bay, M.M.; Vadrucci, R.; De Volder, M.; Vignolini, S. Mechanochromic, Structurally Colored, and Edible Hydrogels Prepared from Hydroxypropyl Cellulose and Gelatin. Adv. Mater. 2021, 33, e2102112. [Google Scholar] [CrossRef]
- El-Wakil, N.A.; Kassem, N.F.; Hassan, M.L. Hydroxypropyl cellulose/rice straw oxidized cellulose nanocrystals nanocomposites and their use in paper coating. Ind. Crop. Prod. 2016, 93, 186–192. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Ebrahimi, Y.; Peighambardoust, S.J.; Karkaj, S.Z. Development of Antibacterial Carboxymethyl Cellulose-Based Nanobiocomposite Films Containing Various Metallic Nanoparticles for Food Packaging Applications. J. Food Sci. 2019, 84, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Ludwicka, K.; Kaczmarek, M.; Białkowska, A. Bacterial Nanocellulose—A Biobased Polymer for Active and Intelligent Food Packaging Applications: Recent Advances and Developments. Polymers 2020, 12, 2209. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, S. Active packaging for food biopreservation. In Protective Cultures, Antimicrobial Metabolites and Bacteriophages for Food and Beverage Biopreservation, 1st ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 460–489. [Google Scholar]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric Antimicrobial Food Packaging and Its Applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Arruda, T.R.; Bernardes, P.C.; e Moraes, A.R.F.; Soares, N.D.F.F. Natural bioactives in perspective: The future of active packaging based on essential oils and plant extracts themselves and those complexed by cyclodextrins. Food Res. Int. 2022, 156, 111160. [Google Scholar] [CrossRef]
- Da Silva Dannenberg, G.; Funck, G.D.; dos Santos Cruxen, C.; Marques, J.D.L.; da Silva, W.P.; Fiorentini, M. Essential oil from pink pepper as an antimicrobial component in cellulose acetate film: Potential for application as active packaging for sliced cheese. LWT Food Sci. Technol. 2017, 81, 314–318. [Google Scholar] [CrossRef]
- El Fawal, G.F.; Omer, A.M.; Tamer, T.M. Evaluation of antimicrobial and antioxidant activities for cellulose acetate films incorporated with Rosemary and Aloe Vera essential oils. J. Food Sci. Technol. 2019, 56, 1510–1518. [Google Scholar] [CrossRef]
- Han, Y.; Yu, M.; Wang, L. Physical and antimicrobial properties of sodium alginate/carboxymethyl cellulose films incorporated with cinnamon essential oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Gardrat, C.; Rezaei, M.R.; Hashemi, M.; Le Coz, C.; Coma, V. Cinnamon and ginger essential oils to improve antifungal, physical and mechanical properties of chitosan-carboxymethyl cellulose films. Food Hydrocoll. 2017, 70, 36–45. [Google Scholar] [CrossRef]
- Muppalla, S.R.; Kanatt, S.R.; Chawla, S.; Sharma, A. Carboxymethyl cellulose–polyvinyl alcohol films with clove oil for active packaging of ground chicken meat. Food Packag. Shelf Life 2014, 2, 51–58. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, S.-M.; Rhim, J.-W. Carboxymethyl cellulose-based multifunctional film combined with zinc oxide nanoparticles and grape seed extract for the preservation of high-fat meat products. Sustain. Mater. Technol. 2021, 29, e00325. [Google Scholar] [CrossRef]
- Choi, W.S.; Singh, S.; Lee, Y.S. Characterization of edible film containing essential oils in hydroxypropyl methylcellulose and its effect on quality attributes of ‘Formosa’ plum (Prunus salicina L.). LWT-Food Sci. Technol. 2016, 70, 213–222. [Google Scholar] [CrossRef]
- Barros-Velázquez, J. Antimicrobial Food Packaging, 1st ed; Academic Press: London, UK, 2016. [Google Scholar]
- Hassan, E.A.; Fadel, S.M.; Hassan, M.L. Influence of TEMPO-oxidized NFC on the mechanical, barrier properties and nisin release of hydroxypropyl methylcellulose bioactive films. Int. J. Biol. Macromol. 2018, 113, 616–622. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Silva, R.R.A.; De Oliveira, T.V.; Soares, R.R.A.; Soares, N.F.F. Biodegradable film development by nisin Z addition into hydroxypropylmethylcellulose matrix for mozzarella cheese preservation. Int. J. Food Stud. 2020, 9, 360–372. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, R.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Dai, J.; He, L.; Liu, Y. Improving nisin production by encapsulated Lactococcus lactis with starch/carboxymethyl cellulose edible films. Carbohydr. Polym. 2020, 251, 117062. [Google Scholar] [CrossRef]
- Sheng, L.; Su, P.; Han, K.; Chen, J.; Cao, A.; Zhang, Z.; Jin, Y.; Ma, M. Synthesis and structural characterization of lysozyme–pullulan conjugates obtained by the Maillard reaction. Food Hydrocoll. 2017, 71, 1–7. [Google Scholar] [CrossRef]
- Hu, X.; Liu, Y.; Zhu, D.; Jin, Y.; Jin, H.; Sheng, L. Preparation and characterization of edible carboxymethyl cellulose films containing natural antibacterial agents: Lysozyme. Food Chem. 2022, 385, 132708. [Google Scholar] [CrossRef]
- Dekina, S.; Romanovska, I.; Ovsepyan, A.; Tkach, V.; Muratov, E. Gelatin/carboxymethyl cellulose mucoadhesive films with lysozyme: Development and characterization. Carbohydr. Polym. 2016, 147, 208–215. [Google Scholar] [CrossRef]
- Marrez, D.A.; Abdelhamid, A.E.; Darwesh, O.M. Eco-friendly cellulose acetate green synthesized silver nano-composite as antibacterial packaging system for food safety. Food Packag. Shelf Life 2019, 20, 100302. [Google Scholar] [CrossRef]
- Dairi, N.; Ferfera-Harrar, H.; Ramos, M.; Garrigós, M.C. Cellulose acetate/AgNPs-organoclay and/or thymol nano-biocomposite films with combined antimicrobial/antioxidant properties for active food packaging use. Int. J. Biol. Macromol. 2018, 121, 508–523. [Google Scholar] [CrossRef]
- Salama, H.E.; Aziz, M.S.A. Optimized carboxymethyl cellulose and guanidinylated chitosan enriched with titanium oxide nanoparticles of improved UV-barrier properties for the active packaging of green bell pepper. Int. J. Biol. Macromol. 2020, 165, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.-X.; Hu, C.-Y.; Wang, Z.-W. Release of Potassium SORBATE from Pectin- Carboxymethyl Cellulose Films into Food Simulant. J. Food Process. Preserv. 2016, 41, e12860. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Mokarram, R.R.; Hashemi, M. Novel active packaging based on carboxymethyl cellulose-chitosan-ZnO NPs nanocomposite for increasing the shelf life of bread. Food Packag. Shelf Life 2017, 11, 106–114. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M.; El-Sayed, H.S.; Salama, H.H.; Dufresne, A. Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film. Carbohydr. Polym. 2016, 151, 9–19. [Google Scholar] [CrossRef]
- Mozaffarzogh, M.; Misaghi, A.; Shahbazi, Y.; Kamkar, A. Evaluation of probiotic carboxymethyl cellulose-sodium caseinate films and their application in extending shelf life quality of fresh trout fillets. LWT 2020, 126, 109305. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the use of natural antioxidants in active food packaging: A review. Food Addit. Contam. Part A 2014, 31, 374–395. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernandez-Munoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Assis, R.Q.; Rios, P.D.; Rios, A.D.O.; Olivera, F.C. Biodegradable packaging of cellulose acetate incorporated with norbixin, lycopene or zeaxanthin. Ind. Crop. Prod. 2020, 147, 112212. [Google Scholar] [CrossRef]
- Nemazifard, M.; Kavoosi, G.; Marzban, Z.; Ezedi, N. Physical, mechanical, water binding, and antioxidant properties of cellulose dispersions and cellulose film incorporated with pomegranate seed extract. Int. J. Food Prop. 2016, 20, 1–14. [Google Scholar] [CrossRef]
- He, B.; Wang, W.; Song, Y.; Ou, Y.; Zhu, J. Structural and physical properties of carboxymethyl cellulose/gelatin films functionalized with antioxidant of bamboo leaves. Int. J. Biol. Macromol. 2020, 164, 1649–1656. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Luo, H.; Nie, M.; Dai, Z.; Liu, C.; Abdin, M.; Hashim, M.M.; Li, D.; Song, J. Effect of Chinese chives (Allium tuberosum) addition to carboxymethyl cellulose based food packaging films. Carbohydr. Polym. 2020, 235, 115944. [Google Scholar] [CrossRef] [PubMed]
- Martelli, S.M.; Motta, C.; Caon, T.; Alberton, J.; Bellettini, I.C.; Prado, A.C.P.D.; Barreto, P.L.M.; Soldi, V. Edible carboxymethyl cellulose films containing natural antioxidant and surfactants: α-tocopherol stability, in vitro release and film properties. LWT 2017, 77, 21–29. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Carboxymethyl cellulose-based antioxidant and antimicrobial active packaging film incorporated with curcumin and zinc oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Zhang, Y.; Wang, J.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Preparation and antioxidant activity of sodium alginate and carboxymethyl cellulose edible films with epigallocatechin gallate. Int. J. Biol. Macromol. 2019, 134, 1038–1044. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Ceseracciu, L.; Guzman-Puyol, S.; Paul, U.C.; Alfaro-Pulido, A.; Grande, C.; Vezzulli, L.; Bandiera, T.; Bertorelli, R.; Russo, D.; et al. Antimicrobial, antioxidant, and waterproof RTV silicone-ethyl cellulose composites containing clove essential oil. Carbohydr. Polym. 2018, 192, 150–158. [Google Scholar] [CrossRef]
- Nunes, M.R.; de Souza Maguerroski Castilho, M.D.S.M.; de Lima Veeck, A.P.D.L.; da Rosa, C.G.; Noronha, C.M.; Maciel, M.V.O.B.; Barreto, P.M. Antioxidant and antimicrobial methylcellulose films containing Lippia alba extract and silver nanoparticles. Carbohydr. Polym. 2018, 192, 37–43. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Zhao, X.; Li, Q.; Dong, F.; Guo, Z. Preparation and physicochemical properties of antioxidant chitosan ascorbate/methylcellulose composite films. Int. J. Biol. Macromol. 2019, 146, 53–61. [Google Scholar] [CrossRef]
- EFSA. Active and Intelligent Packaging Substances. Available online: https://www.efsa.europa.eu/en/topics/topic/active-and-intelligent-packaging-substances (accessed on 24 October 2022).
- Salgado, P.R.; Di Giorgio, L.; Musso, Y.S.; Mauri, A.N. Recent Developments in Smart Food Packaging Focused on Biobased and Biodegradable Polymers. Front. Sustain. Food Syst. 2021, 5, 630393. [Google Scholar] [CrossRef]
- Kalpana, S.; Priyadarshini, S.R.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Intelligent packaging: Trends and applications in food systems. Trends Food Sci. Technol. 2019, 93, 145–157. [Google Scholar] [CrossRef]
- Han, J.; Ho, C.; Rodrigues, E. Intelligent Packaging. In Innovations in Food Packaging, 2nd ed; Academic Press: Oxford, UK, 2005; pp. 138–155. [Google Scholar]
- Cheng, H.; Xu, H.; McClements, D.J.; Chen, L.; Jiao, A.; Tian, Y.; Miao, M.; Jin, Z. Recent advances in intelligent food packaging materials: Principles, preparation and applications. Food Chem. 2021, 375, 131738. [Google Scholar] [CrossRef]
- Sohail, M.; Sun, D.-W.; Zhu, Z. Recent developments in intelligent packaging for enhancing food quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662. [Google Scholar] [CrossRef] [PubMed]
- Prietto, L.; Mirapalhete, T.C.; Pinto, V.Z.; Hoffmann, J.F.; Vanier, N.L.; Lim, L.-T.; Dias, A.R.G.; Zavareze, E.D.R. pH-sensitive films containing anthocyanins extracted from black bean seed coat and red cabbage. LWT 2017, 80, 492–500. [Google Scholar] [CrossRef]
- Chen, H.-Z.; Zhang, M.; Bhandari, B.; Yang, C.-H. Novel pH-sensitive films containing curcumin and anthocyanins to monitor fish freshness. Food Hydrocoll. 2019, 100, 105438. [Google Scholar] [CrossRef]
- Freitas, P.A.; Silva, R.R.; de Oliveira, T.V.; Soares, R.R.; Junior, N.S.; Moraes, A.R.; Pires, A.C.D.S.; Soares, N.F. Development and characterization of intelligent cellulose acetate-based films using red cabbage extract for visual detection of volatile bases. LWT 2020, 132, 109780. [Google Scholar] [CrossRef]
- Liang, T.; Sun, G.; Cao, L.; Li, J.; Wang, L. A pH and NH3 sensing intelligent film based on Artemisia sphaerocephala Krasch. gum and red cabbage anthocyanins anchored by carboxymethyl cellulose sodium added as a host complex. Food Hydrocoll. 2018, 87, 858–868. [Google Scholar] [CrossRef]
- Freitas, P.A.; de Oliveira, T.V.; Silva, R.R.; e Moraes, A.R.F.; Pires, A.C.D.S.; Soares, R.R.; Junior, N.S.; Soares, N.F. Effect of pH on the intelligent film-forming solutions produced with red cabbage extract and hydroxypropylmethylcellulose. Food Packag. Shelf Life 2020, 26, 100604. [Google Scholar] [CrossRef]
- Gomes, V.; Pires, A.S.; Mateus, N.; de Freitas, V.; Cruz, L. Pyranoflavylium-cellulose acetate films and the glycerol effect towards the development of pH-freshness smart label for food packaging. Food Hydrocoll. 2022, 127, 107501. [Google Scholar] [CrossRef]
- Zhou, N.; Wang, L.; You, P.; Wang, L.; Mu, R.; Pang, J. Preparation of pH-sensitive food packaging film based on konjac glucomannan and hydroxypropyl methyl cellulose incorporated with mulberry extract. Int. J. Biol. Macromol. 2021, 172, 515–523. [Google Scholar] [CrossRef]
- Sani, M.A.; Tavassoli, M.; Hamishehkar, H.; McClements, D.J. Carbohydrate-based films containing pH-sensitive red barberry anthocyanins: Application as biodegradable smart food packaging materials. Carbohydr. Polym. 2020, 255, 117488. [Google Scholar] [CrossRef]
- Filipini, G.D.S.; Romani, V.P.; Martins, V.G. Biodegradable and active-intelligent films based on methylcellulose and jambolão (Syzygium cumini) skins extract for food packaging. Food Hydrocoll. 2020, 109, 106139. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Liu, Y.; Zhang, J.; Hossen, A.; Sameen, D.E.; Dai, J.; Li, S.; Qin, W. Fabrication and characterization of pH-responsive intelligent films based on carboxymethyl cellulose and gelatin/curcumin/chitosan hybrid microcapsules for pork quality monitoring. Food Hydrocoll. 2021, 124, 107224. [Google Scholar] [CrossRef]
- Boonsiriwit, A.; Lee, M.; Kim, M.; Inthamat, P.; Siripatrawan, U.; Lee, Y.S. Hydroxypropyl methylcellulose/microcrystalline cellulose biocomposite film incorporated with butterfly pea anthocyanin as a sustainable pH-responsive indicator for intelligent food-packaging applications. Food Biosci. 2021, 44, 101392. [Google Scholar] [CrossRef]
- Jiang, G.; Hou, X.; Zeng, X.; Zhang, C.; Wu, H.; Shen, G.; Li, S.; Luo, Q.; Li, M.; Liu, X.; et al. Preparation and characterization of indicator films from carboxymethyl-cellulose/starch and purple sweet potato (Ipomoea batatas (L.) lam) anthocyanins for monitoring fish freshness. Int. J. Biol. Macromol. 2019, 143, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, W.; Zheng, L.; Yu, J.; Sun, P.; Shao, P. Intelligent packaging films incorporated with anthocyanins-loaded ovalbumin-carboxymethyl cellulose nanocomplexes for food freshness monitoring. Food Chem. 2022, 387, 132908. [Google Scholar] [CrossRef] [PubMed]





| Sensors | Cellulose Matrix | Functions | Ref. |
|---|---|---|---|
| Red cabbage extract | CA |
| [160] |
| Sodium-CMC/Artemisia sphaerocephala Krasch gum (ASKG) |
| [161] | |
| HPMC |
| [162] | |
| Pyranoflavylium salt | CA/Glycerol |
| [163] |
| Mulberry extract | HPMC/konjac glucomannan |
| [164] |
| Barberry extract | MC/Chitin nanofibers |
| [165] |
| Jambolão skins extract | MC |
| [166] |
| Blackcurrant anthocyanins | CMC/konjac glucomannan |
| [98] |
| Curcumin | CMC |
| [167] |
| Butterfly pea anthocyanin | HPMC/microcrystalline cellulose |
| [168] |
| Purple sweet potato anthocyanins | CMC |
| [169] |
| Blueberry anthocyanins | CMC/ovalbumin |
| [170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romão, S.; Bettencourt, A.; Ribeiro, I.A.C. Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging. Polymers 2022, 14, 4968. https://doi.org/10.3390/polym14224968
Romão S, Bettencourt A, Ribeiro IAC. Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging. Polymers. 2022; 14(22):4968. https://doi.org/10.3390/polym14224968
Chicago/Turabian StyleRomão, Sofia, Ana Bettencourt, and Isabel A. C. Ribeiro. 2022. "Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging" Polymers 14, no. 22: 4968. https://doi.org/10.3390/polym14224968
APA StyleRomão, S., Bettencourt, A., & Ribeiro, I. A. C. (2022). Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging. Polymers, 14(22), 4968. https://doi.org/10.3390/polym14224968