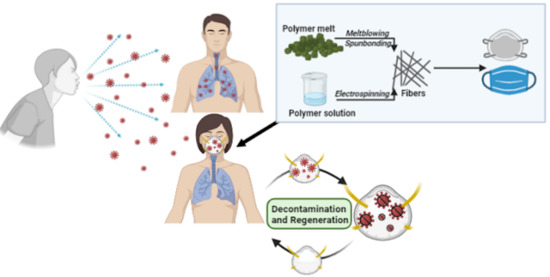
Advanced Research and Development of Face Masks and Respirators Pre and Post the Coronavirus Disease 2019 (COVID-19) Pandemic: A Critical Review
Abstract
:1. Introduction
2. Historical Development of Aerosols Face Protection
2.1. Face Masks
2.2. Respirators
3. Patents in Face Masks and Respirators
4. Filtration Mechanism
4.1. Gravity Sedimentation
4.2. Inertial Impaction
4.3. Interception
4.4. Diffusion
4.5. Electrostatic Interaction
5. Filtration Material
5.1. Common Filter (Nonwoven) Materials
5.1.1. Polyolefins
Polypropylene (PP)
Polyethylene (PE)
5.1.2. Polyesters
5.1.3. Polyamide
5.1.4. Cellulose Acetate (CA)
5.1.5. Polylactic Acid (PLA)
5.1.6. Polytetrafluoroethylene (PTFE) Membranes
5.2. Polymer Composites and Modifications
6. Filtration Experiments and Testing Practices in Academic Research
7. Current Practices of Decontamination and Regeneration of Face Masks and Respirators
7.1. Thermal Disinfection
7.2. Microwave
7.3. Ultraviolet Irradiation (UVI)
7.4. Chemicals
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Ji, D.; He, H.; Ramakrishna, S. Electrospun ultrafine fibers for advanced face masks. Mater. Sci. Eng. R Rep. 2021, 143, 100594. [Google Scholar] [CrossRef] [PubMed]
- Sickbert-Bennett, E.E.; Samet, J.M.; Clapp, P.W.; Chen, H.; Berntsen, J.; Zeman, K.L.; Tong, H.; Weber, D.J.; Bennett, W.D. Filtration efficiency of hospital face mask alternatives available for use during the COVID-19 pandemic. JAMA Intern. Med. 2020, 180, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.H.; Chu, D.K.; Shiu, E.Y.; Chan, K.-H.; McDevitt, J.J.; Hau, B.J.; Yen, H.-L.; Li, Y.; Ip, D.K.; Peiris, J.M. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat. Med. 2020, 26, 676–680. [Google Scholar] [CrossRef] [Green Version]
- Cowling, B.J.; Zhou, Y.; Ip, D.; Leung, G.; Aiello, A.E. Face masks to prevent transmission of influenza virus: A systematic review. Epidemiol. Infect. 2010, 138, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Gardner, P.D.; Eshbaugh, J.P.; Harpest, S.D.; Richardson, A.W.; Hofacre, K.C. Viable viral efficiency of N95 and P100 respirator filters at constant and cyclic flow. J. Occup. Environ. Hyg. 2013, 10, 564–572. [Google Scholar] [CrossRef]
- Maher, B.; Chavez, R.; Tomaz, G.C.; Nguyen, T.; Hassan, Y. A fluid mechanics explanation of the effectiveness of common materials for respiratory masks. Int. J. Infect. Dis. 2020, 99, 505–513. [Google Scholar] [CrossRef]
- Clase, C.M.; Fu, E.L.; Ashur, A.; Beale, R.C.; Clase, I.A.; Dolovich, M.B.; Jardine, M.J.; Joseph, M.; Kansiime, G.; Mann, J.F. Forgotten technology in the COVID-19 pandemic. filtration properties of cloth and cloth masks: A narrative review. Mayo Clin. Proc. 2020, 95, 2204–2224. [Google Scholar] [CrossRef]
- Aydin, O.; Emon, B.; Cheng, S.; Hong, L.; Chamorro, L.P.; Saif, M.T.A. Performance of fabrics for home-made masks against the spread of COVID-19 through droplets: A quantitative mechanistic study. Extreme Mech. Lett. 2020, 40, 100924. [Google Scholar] [CrossRef]
- Beesoon, S.; Behary, N.; Perwuelz, A. Universal masking during COVID-19 pandemic: Can textile engineering help public health? Narrative review of the evidence. Prev. Med. 2020, 139, 106236. [Google Scholar] [CrossRef]
- Goh, Y.; Tan, B.Y.; Bhartendu, C.; Ong, J.J.; Sharma, V.K. The Face Mask How a Real Protection becomes a Psychological Symbol during Covid-19? Brain Behav. Immun. 2020, 88, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.P.; Fischer, M.C.; Grass, D.; Henrion, I.; Warren, W.S.; Westman, E. Low-cost measurement of face mask efficacy for filtering expelled droplets during speech. Sci. Adv. 2020, 6, eabd3083. [Google Scholar] [CrossRef] [PubMed]
- O’Kelly, E.; Pirog, S.; Ward, J.; Clarkson, P.J. Ability of fabric face mask materials to filter ultrafine particles at coughing velocity. BMJ Open 2020, 10, e039424. [Google Scholar] [CrossRef] [PubMed]
- Hill, W.C.; Hull, M.S.; MacCuspie, R.I. Testing of commercial masks and respirators and cotton mask insert materials using SARS-CoV-2 virion-sized particulates: Comparison of ideal aerosol filtration efficiency versus fitted filtration efficiency. Nano Lett. 2020, 20, 7642–7647. [Google Scholar] [CrossRef] [PubMed]
- Zangmeister, C.D.; Radney, J.G.; Vicenzi, E.P.; Weaver, J.L. Filtration efficiencies of nanoscale aerosol by cloth mask materials used to slow the spread of SARS-CoV-2. ACS Nano 2020, 14, 9188–9200. [Google Scholar] [CrossRef] [PubMed]
- Grinshpun, S.A.; Haruta, H.; Eninger, R.M.; Reponen, T.; McKay, R.T.; Lee, S.-A. Performance of an N95 filtering facepiece particulate respirator and a surgical mask during human breathing: Two pathways for particle penetration. J. Occup. Environ. Hyg. 2009, 6, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Times, G. The Evolution of Face Masks. 2020. Available online: https://www.globaltimes.cn/content/1179358.shtml (accessed on 17 January 2021).
- Le Huec, J.; Boué, L.; Bourret, S.; Saffarini, M. Epidemics Over the Centuries. Neurospine 2020, 17, 334–336. [Google Scholar] [CrossRef]
- Blakemore, E. Why Plague Doctors Wore Those Strange Beaked Masks. 2020. Available online: https://www.nationalgeographic.com/history/reference/european-history/plague-doctors-beaked-masks-coronavirus/ (accessed on 18 January 2021).
- Flügge, C. Die Verbreitung der Phthise durch staubförmiges Sputum und durch beim Husten verspritzte Tröpfchen. Z. Hyg. Infekt. 1899, 30, 107–124. [Google Scholar] [CrossRef]
- Matuschek, C.; Moll, F.; Fangerau, H.; Fischer, J.C.; Zänker, K.; Van Griensven, M.; Schneider, M.; Kindgen-Milles, D.; Knoefel, W.T.; Lichtenberg, A. The history and value of face masks. Eur. J. Med. Res. 2020, 25, 1–6. [Google Scholar] [CrossRef]
- Mikulicz, J. Das Operieren in sterilisierten Zwirnhandschuhen und mit Mundbinde. Cent. Chir. 1897, 26, 714–717. [Google Scholar]
- Huebner, W. Ueber die Moeglichkeit der Wundinfektion von Munde aus und ihre Verheutung durch Operationsmasken. Zeirschrift Hyg. 1898, 28, 348. [Google Scholar] [CrossRef]
- Belkin, N.L. The evolution of the surgical mask: Filtering efficiency versus effectiveness. Infect. Control Hosp. Epidemiol. 1997, 18, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A. Dissemination of streptococci through invisible sputum. In relation to scarlet fever and sepsis. J. Am. Med. Assoc. 1905, 44, 1108–1111. [Google Scholar] [CrossRef] [Green Version]
- Chughtai, A.A.; Seale, H.; MacIntyre, C.R. Use of cloth masks in the practice of infection control—Evidence and policy gaps. Int. J. Infect. Control 2013, 9. [Google Scholar] [CrossRef]
- Rockwood, C.A.; O’Donoghue, D.H. The surgical mask: Its development, usage, and efficiency: A review of the literature, and new experimental studies. AMA Arch. Surg. 1960, 80, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.S. Is adequate masking essential for the patient’s protection? Ann. Surg. 1937, 105, 990. [Google Scholar] [CrossRef] [PubMed]
- Mellinger, H.V. A New Mask That Protects Both Physician and Patient. J. Am. Med. Assoc. 1930, 95, 662–663. [Google Scholar] [CrossRef]
- Cohen, H.J.; Birkner, J.S. Respiratory protection. Clin. Chest Med. 2012, 33, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Co, C.M. The History of Respirator. 2016. Available online: https://cambridgemask.com/blog/history-of-respirators/ (accessed on 14 January 2021).
- Pritchard, J.A. A Guide to Industrial Respiratory Protectiom; US Department of Health, Education, and Welfare: Bethesda, MD, USA, 1976.
- Smart, J.K. History of chemical and biological warfare: An American perspective. In Medical Aspects of Chemical and Biological Warfare; Office of the Surgeon General: Washington, DC, USA, 1997; pp. 9–86. [Google Scholar]
- Allgeier, J.R. The B Respirator Respiratory Protection for the Bearded Man; Rochester Institute of Technology: Rochester, NY, USA, 2020. [Google Scholar]
- GGosch, M.E.; Shaffer, R.E.; Eagan, A.E.; Roberge, R.J.; Davey, V.J.; Radonovich, L.J. B95: A new respirator for health care personnel. Am. J. Infect. Control 2013, 41, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- O’Dowd, K.; Nair, K.M.; Forouzandeh, P.; Mathew, S.; Grant, J.; Moran, R.; Bartlett, J.; Bird, J.; Pillai, S.C. Face Masks and Respirators in the Fight against the COVID-19 Pandemic: A Review of Current Materials, Advances and Future Perspectives. Materials 2020, 13, 3363. [Google Scholar] [CrossRef]
- Goldsmith, S.J. Mask. U.S. Patent 2012505A, 5 February 1935. [Google Scholar]
- Rudolph, F. Respirator Mask. U.S. Patent 2376871A, 29 May 1945. [Google Scholar]
- Reese, G.D.; Rich, A.R., Jr.; Brunson, K.K. Face Mask with Enhanced Seal and Method. U.S. Patent 5553608A, 10 September 1996. [Google Scholar]
- Hubbard, V.M.; Brunson, W.K. Surgical Face Mask with Darkened Glare-Reducing Strip and Visor. U.S. Patent 5704349A, 6 June 1998. [Google Scholar]
- Watts, J.H.G. Disposable Surgical Mask. U.S. Patent 3170461A, 23 February 1965. [Google Scholar]
- Aspelin, G.B.; Smith, J.T. Folded Cup-Like Surgical Face Mask and Method of Forming the Same. U.S. Patent 3971369A, 27 June 1976. [Google Scholar]
- Kronzer, J.P.; Stumo, R.J.; Dyrud, J.F.; Berg, H.J. Methods of Forming Fibrous Filtration Face Masks. U.S. Patent 5307796A, 3 May 1994. [Google Scholar]
- Lauer, W. Anti-Fog Surgical Face Mask. U.S. Patent 3888246A, 10 June 1975. [Google Scholar]
- Cox, D. Fog Free Medical Face Mask. U.S. Patent 6988500B1, 24 January 2006. [Google Scholar]
- Facer, J.M.; Wilson, A.A.; Henderson, C.P. Maintenance-Free Anti-Fog Respirator. U.S. Patent 9770611B2, 26 September 2017. [Google Scholar]
- Bora, N.R. Apparatus, System and Method to Prevent Fogging of Eyewear. U.S. Patent 10,357,672B2, 23 July 2019. [Google Scholar]
- Japuntich, D.A.; Mccullough, N.V.; Peterson, J.K.; Baumann, N.R.; Bryant, J.W.; Henderson, C.P.; Penning, B.E. Face Mask that Has a Filtered Exhalation Valve. European Patent 1479413A2, 24 November 2004. [Google Scholar]
- Bell, D.S.; Agarwal, N.; Amante, M.A.; Willis, J.M. Face Mask Having Hook and Loop Type Fastener. U.S. Patent 6928657B2, 16 August 2005. [Google Scholar]
- Gloag, N.J.; Wilson, A.A.; Facer, J.M.; Henderson, C.P. Maintenance-Free Flat-Fold Respirator that Includes a Graspable Table. European Patent 2142261A1, 17 April 2008. [Google Scholar]
- Welchel, D.N.; Kish, T.; Steindorf, E.C.; Gehling, S.C.; Beltz, A.J.; Mccoy, T.M. Vent and Strap Fastening System for a Disposable Respirator Providing Improved Donning. U.S. Patent 20090044812A1, 19 February 2009. [Google Scholar]
- Mckinley, J. Face Mask With Adjustable and Detachable Straps. U.S. Patent 20110180078A1, 28 July 2011. [Google Scholar]
- Steindorf, E.; Welchel, D.N.; Miles, C.; Stephan, S. Collapse Resistant Respirator. U.S. Patent 8267088B2, 18 September 2012. [Google Scholar]
- Tu, M.L.; Lai, Y.Y. Respirator Mask. European Patent 3653266A1, 20 May 2020. [Google Scholar]
- Gordon, R. Respirators and Related Methods. U.S. Patent 10799728B2, 13 October 2020. [Google Scholar]
- Furuya, K.; Shibata, A. Disposable Mask. U.S. Patent 20210022418A1, 28 January 2021. [Google Scholar]
- Mekler, J.; Scott, S.M.; Thornberry, D.; Wilson, C.J. Filtering Face Mask. U.S. Patent 9655392B2, 23 May 2017. [Google Scholar]
- Nagao, S.; Yagi, T. Mask. U.S. Patent 9386813B2, 12 July 2016. [Google Scholar]
- Reese, G.D. Fitted Face Mask. U.S. Patent 20160151650A1, 2 June 2016. [Google Scholar]
- Koehler, R.H. Surgical Face Mask, Including Reusable Masks, with Filtered Inhalation and Exhalation Valves. U.S. Patent 9320923B2, 16 April 2016. [Google Scholar]
- Waterford, S. Facemask with Filter Insert for Protection against Airborne Pathogens. U.S. Patent 9457207B2, 4 October 2016. [Google Scholar]
- Eisenbrey, J.; Daecher, A. Temperature Sensitive Surgical Face Mask for Identifying at Risk Patients and Reducing Viral Infection. U.S. Patent 20190125011A1, 2 May 2019. [Google Scholar]
- Berthon-Jones, M.; Bateman, P.E.; Darkin, D.; Hitchcock, R.G.; Jenkinson, P.J.; Lynch, S.R.; Malouf, G.J.; Mcauliffe, P.J.; Raje, M.C.; Robinson, G.C. Mask and Components Thereof. U.S. Patent 10307554B2, 4 January 2019. [Google Scholar]
- Koehler, R.H. Face Mask Seal for Use with Respirator Devices and Surgical Facemasks, Having an Anatomically Defined Geometry Conforming to Critical Fit Zones of Human Facial Anatomy, and Capable of Being Actively Custom Fitted to the User’s Face. U.S. Patent 10207129B2, 19 February 2019. [Google Scholar]
- Smaller, J. Adjustable Facial Conforming Face Mask. U.S. Patent 20170095681A1, 6 April 2017. [Google Scholar]
- Palomo, J.A.; Carroll, S.C.; Wegener, S.K.; Aguilar, C.M.; Miller, J. Surgical Mask. U.S. Patent 20160235136A1, 18 August 2016. [Google Scholar]
- Tai, J.-C.; Yang, H.-H.; Lin, C.-H.; Su, C.-C. Face Mask and Method for Making the Same. U.S. Patent 9706800B2, 18 July 2017. [Google Scholar]
- Tsuei, A.C. Dispensable Face Mask and Method of Making the Same. U.S. Patent 9616258B2, 11 April 2017. [Google Scholar]
- Mittal, R.; Ni, R.; Seo, J.-H. The flow physics of COVID-19. J. Fluid Mech. 2020, 894. [Google Scholar] [CrossRef]
- Ou, Q.; Pei, C.; Kim, S.C.; Abell, E.; Pui, D.Y. Evaluation of decontamination methods for commercial and alternative respirator and mask materials–view from filtration aspect. J. Aerosol Sci. 2020, 150, 105609. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, B.; Banerjee, S.; Ali, S.B.; Bandyopadhyay, R.; Das, N.; Mandal, D.; Tudu, B. Design of a self-powered triboelectric face mask. Nano Energy 2021, 79, 105387. [Google Scholar] [CrossRef]
- Konda, A.; Prakash, A.; Moss, G.A.; Schmoldt, M.; Grant, G.D.; Guha, S. Aerosol Filtration Efficiency of Common Fabrics Used in Respiratory Cloth Masks. ACS Nano 2020, 14, 6339–6347. [Google Scholar] [CrossRef] [PubMed]
- Tcharkhtchi, A.; Abbasnezhad, N.; Seydani, M.Z.; Zirak, N.; Farzaneh, S.; Shirinbayan, M. An overview of filtration efficiency through the masks: Mechanisms of the aerosols penetration. Bioact. Mater. 2021, 6, 106–122. [Google Scholar] [CrossRef]
- Cook, T. Personal protective equipment during the coronavirus disease (COVID) 2019 pandemic–a narrative review. Anaesthesia 2020, 75, 920–927. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Liao, L.; Xiao, W.; Yu, X.; Wang, H.; Wang, Q.; Lin, Y.L.; Kilinc-Balci, F.S.; Price, A.; Chu, L. Household materials selection for homemade cloth face coverings and their filtration efficiency enhancement with triboelectric charging. Nano Lett. 2020, 20, 5544–5552. [Google Scholar] [CrossRef]
- Abdolghader, P.; Brochot, C.; Haghighat, F.; Bahloul, A. Airborne nanoparticles filtration performance of fibrous media: A review. Sci. Technol. Built Environ. 2018, 24, 648–672. [Google Scholar] [CrossRef]
- Bai, Y.; Han, C.B.; He, C.; Gu, G.Q.; Nie, J.H.; Shao, J.J.; Xiao, T.X.; Deng, C.R.; Wang, Z.L. Washable multilayer triboelectric air filter for efficient particulate matter PM2. 5 removal. Adv. Funct. Mater. 2018, 28, 1706680. [Google Scholar] [CrossRef]
- Drewnick, F.; Pikmann, J.; Fachinger, F.; Moormann, L.; Sprang, F.; Borrmann, S. Aerosol filtration efficiency of household materials for homemade face masks: Influence of material properties, particle size, particle electrical charge, face velocity, and leaks. Aerosol Sci. Technol. 2021, 55, 63–79. [Google Scholar] [CrossRef]
- Tan, N.P.B.; Paclijan, S.S.; Ali, H.N.M.; Hallazgo, C.M.J.S.; Lopez, C.J.F.; Ebora, Y.C. Solution blow spinning (SBS) nanofibers for composite air filter masks. ACS Appl. Nano Mater. 2019, 2, 2475–2483. [Google Scholar] [CrossRef]
- Hao, W.; Parasch, A.; Williams, S.; Li, J.; Ma, H.; Burken, J.; Wang, Y. Filtration performances of non-medical materials as candidates for manufacturing facemasks and respirators. Int. J. Hyg. Environ. Health 2020, 229, 113582. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.W.F.; Sun, Q. Electrostatic charged nanofiber filter for filtering airborne novel coronavirus (COVID-19) and nano-aerosols. Sep. Purif. Technol. 2020, 250, 116886. [Google Scholar] [CrossRef]
- Bandi, M. Electrocharged facepiece respirator fabrics using common materials. Proc. R. Soc. A 2020, 476, 20200469. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Bahl, P.; Chughtai, A.A.; MacIntyre, C.R. Last-resort strategies during mask shortages: Optimal design features of cloth masks and decontamination of disposable masks during the COVID-19 pandemic. BMJ Open Respir. Res. 2020, 7, e000698. [Google Scholar] [CrossRef]
- Tiliket, G.; Le Sage, D.; Moules, V.; Rosa-Calatrava, M.; Lina, B.; Valleton, J.; Nguyen, Q.; Lebrun, L. A new material for airborne virus filtration. Chem. Eng. J. 2011, 173, 341–351. [Google Scholar] [CrossRef]
- Eninger, R.M.; Honda, T.; Adhikari, A.; Heinonen-Tanski, H.; Reponen, T.; Grinshpun, S.A. Filter Performance of N99 and N95 Facepiece Respirators Against Viruses and Ultrafine Particles. Ann. Occup. Hyg. 2008, 52, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Cai, M.; Yang, X.; Yang, Y. Electret nanofibrous membrane with enhanced filtration performance and wearing comfortability for face mask. J. Colloid Interface Sci. 2018, 530, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-M. Home-made masks with filtration efficiency for nano-aerosols for community mitigation of COVID-19 pandemic. Public Health 2020, 188, 42–50. [Google Scholar]
- Wibisono, Y.; Fadila, C.; Saiful, S.; Bilad, M. Facile Approaches of Polymeric Face Masks Reuse and Reinforcements for Micro-Aerosol Droplets and Viruses Filtration: A Review. Polymers 2020, 12, 2516. [Google Scholar] [CrossRef] [PubMed]
- Forouzandeh, P.; O’Dowd, K.; Pillai, S.C. Face masks and respirators in the fight against the COVID-19 pandemic: An overview of the standards and testing methods. Saf. Sci. 2021, 133, 104995. [Google Scholar] [CrossRef]
- Rengasamy, S.; Shaffer, R.; Williams, B.; Smit, S. A comparison of facemask and respirator filtration test methods. J. Occup. Environ. Hyg. 2017, 14, 92–103. [Google Scholar] [CrossRef]
- Smith, P.B.; Agostini, G.; Mitchell, J.C. A scoping review of surgical masks and N95 filtering facepiece respirators: Learning from the past to guide the future of dentistry. Saf. Sci. 2020, 131, 104920. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Nie, J.; Han, C.B.; Jiang, T.; Yang, Z.W.; Pang, Y.K.; Xu, L.; Guo, T.; Bu, T.; Zhang, C.; et al. Self-Powered Electrostatic Adsorption Face Mask Based on a Triboelectric Nanogenerator. ACS Appl. Mater. Interfaces 2018, 10, 7126–7133. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.A. Textiles for Protection; Woodhead Publishing Limited: Cambridge, UK, 2005. [Google Scholar]
- Grafe, T.; Graham, K. Polymeric nanofibers and nanofiber webs: A new class of nonwovens. Int. Nonwovens J. 2003, 12. [Google Scholar] [CrossRef] [Green Version]
- Russell, S.J. Handbook of Nonwovens; Woodhead Publishing Limited: Cambridge, UK, 2006. [Google Scholar]
- Yim, W.; Cheng, D.; Patel, S.; Kui, R.; Meng, Y.S.; Jokerst, J.V. Assessment of N95 and K95 respirator decontamination: Fiber integrity, filtration efficiency, and dipole charge density. medRxiv 2020. [Google Scholar] [CrossRef]
- Mukhopadhyay, A. Composite nonwovens in filters: Applications. In Composite Non-Woven Materials; Elsevier: Cambridge, UK, 2014; pp. 164–210. [Google Scholar]
- Josef, E.; Guterman, R. Designing Solutions for Electrospinning of Poly(ionic liquid)s. Macromolecules 2019, 52, 5223–5230. [Google Scholar] [CrossRef] [Green Version]
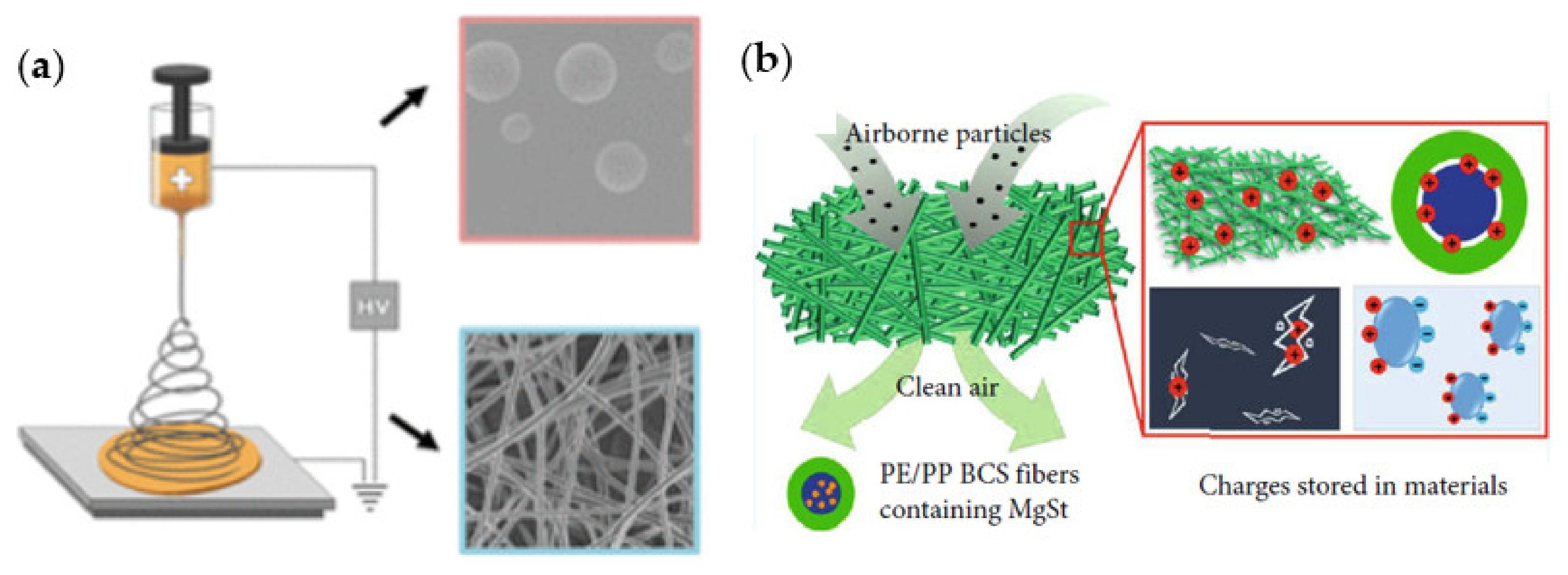
- Liu, J.; Zhang, H.; Gong, R.H.; Zhang, X.; Wang, Y.; Jin, X. Polyethylene/polypropylene bicomponent spunbond air filtration materials containing magnesium stearate for efficient fine particle capture. ACS Appl. Mater. Interfaces 2019, 11, 40592–40601. [Google Scholar] [CrossRef]
- Motyl, E.; Lowkis, B. Effect of air humidity on charge decay and lifetime of PP electret nonwovens. Fibres Text. East. Eur. 2006, 14, 39–42. [Google Scholar]
- Yesil, Y.; Bhat, G.S. Structure and mechanical properties of polyethylene melt blown nonwovens. Int. J. Cloth. Sci. Technol. 2016, 28, 780–793. [Google Scholar] [CrossRef]
- Krcma, R. Manual of Nonwoven Textiles; Textile Trade Press: Manchester, UK, 1972. [Google Scholar]
- Akduman, C. Cellulose acetate and polyvinylidene fluoride nanofiber mats for N95 respirators. J. Ind. Text. 2021, 50, 1239–1261. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Hatton, T.A.; Rutledge, G.C. Aerosol filtration using electrospun cellulose acetate fibers. J. Mater. Sci. 2016, 51, 204–217. [Google Scholar] [CrossRef] [Green Version]
- Vaňková, E.; Kašparová, P.; Khun, J.; Machková, A.; Julák, J.; Sláma, M.; Hodek, J.; Ulrychová, L.; Weber, J.; Obrová, K. Polylactic acid as a suitable material for 3D printing of protective masks in times of COVID-19 pandemic. PeerJ 2020, 8, e10259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, C.; Pan, Z. Porous bead-on-string poly(lactic acid) fibrous membranes for air filtration. J. Colloid Interface Sci. 2015, 441, 121–129. [Google Scholar] [CrossRef]
- Huang, Q.-L.; Xiao, C.-F.; Feng, X.-S.; Hu, X.-Y. Design of super-hydrophobic microporous polytetrafluoroethylene membranes. New J. Chem. 2013, 37, 373–379. [Google Scholar] [CrossRef]
- Shyr, T.-W.; Chung, W.-C.; Lu, W.-L.; Lin, A.-J. Unusually high temperature transition and microporous structure of polytetrafluoroethylene fibre prepared through film fibrillation. Eur. Polym. J. 2015, 72, 50–63. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and perspectives in PTFE membrane: Preparation, modification, and applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Ge, Q.; Yang, L.-L.; Shi, X.-J.; Li, J.-J.; Yang, D.-Q.; Sacher, E. Durable superhydrophobic PTFE films through the introduction of micro- and nanostructured pores. Appl. Surf. Sci. 2015, 339, 151–157. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Liu, L.-Q.; Tang, Z.-Q.; Yan, M.-G.; Gu, J.-S.; Wei, X.-W. Surface modification of polypropylene microporous membrane to improve its antifouling characteristics in an SMBR: Air plasma treatment. J. Membr. Sci. 2008, 311, 216–224. [Google Scholar] [CrossRef]
- Xi, Z.-Y.; Xu, Y.-Y.; Zhu, L.-P.; Zhu, B.-K. Modification of polytetrafluoroethylene porous membranes by electron beam initiated surface grafting of binary monomers. J. Membr. Sci. 2009, 339, 33–38. [Google Scholar] [CrossRef]
- Zhong, Z.; Xu, Z.; Sheng, T.; Yao, J.; Xing, W.; Wang, Y. Unusual Air Filters with Ultrahigh Efficiency and Antibacterial Functionality Enabled by ZnO Nanorods. ACS Appl. Mater. Interfaces 2015, 7, 21538–21544. [Google Scholar] [CrossRef]
- Yang, A.; Cai, L.; Zhang, R.; Wang, J.; Hsu, P.-C.; Wang, H.; Zhou, G.; Xu, J.; Cui, Y. Thermal management in nanofiber-based face mask. Nano Lett. 2017, 17, 3506–3510. [Google Scholar] [CrossRef]
- Amini, G.; Karimi, M.; Ashtiani, F.Z. Hybrid electrospun membrane based on poly(vinylidene fluoride)/poly(acrylic acid)–poly(vinyl alcohol) hydrogel for waterproof and breathable applications. J. Ind. Text. 2020, 1528083720904675. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Yang, T.; Yang, S.; Yang, X.; Zhu, H. Nanofibrous membrane of graphene oxide-in-polyacrylonitrile composite with low filtration resistance for the effective capture of PM2.5. J. Membr. Sci. 2018, 551, 85–92. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Yin, X.; Yu, J.; Ding, B. Anti-deformed Polyacrylonitrile/Polysulfone Composite Membrane with Binary Structures for Effective Air Filtration. ACS Appl. Mater. Interfaces 2016, 8, 8086–8095. [Google Scholar] [CrossRef]
- Gopanna, A.; Rajan, K.P.; Thomas, S.P.; Chavali, M. Polyethylene and polypropylene matrix composites for biomedical applications. In Materials for Biomedical Engineering; Elsevier: Cambridge, UK, 2019; pp. 175–216. [Google Scholar]
- Long, K.D.; Woodburn, E.V.; Berg, I.C.; Chen, V.; Scott, W.S. Measurement of filtration efficiencies of healthcare and consumer materials using modified respirator fit tester setup. PLoS ONE 2020, 15, e0240499. [Google Scholar]
- Kadam, V.; Kyratzis, I.L.; Truong, Y.B.; Schutz, J.; Wang, L.; Padhye, R. Electrospun bilayer nanomembrane with hierarchical placement of bead-on-string and fibers for low resistance respiratory air filtration. Sep. Purif. Technol. 2019, 224, 247–254. [Google Scholar] [CrossRef]
- Lindsley, W.G.; Blachere, F.M.; Law, B.F.; Beezhold, D.H.; Noti, J.D. Efficacy of face masks, neck gaiters and face shields for reducing the expulsion of simulated cough-generated aerosols. Aerosol Sci. Technol. 2021, 55, 449–457. [Google Scholar] [CrossRef]
- Xiao, L.; Sakagami, H.; Miwa, N. A new method for testing filtration efficiency of mask materials under sneeze-like pressure. In Vivo 2020, 34, 1637–1644. [Google Scholar] [CrossRef]
- Shakya, K.M.; Noyes, A.; Kallin, R.; Peltier, R.E. Evaluating the efficacy of cloth facemasks in reducing particulate matter exposure. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 352–357. [Google Scholar] [CrossRef]
- Pacitto, A.; Amato, F.; Salmatonidis, A.; Moreno, T.; Alastuey, A.; Reche, C.; Buonanno, G.; Benito, C.; Querol, X. Effectiveness of commercial face masks to reduce personal PM exposure. Sci. Total Environ. 2019, 650, 1582–1590. [Google Scholar] [CrossRef]
- Cherrie, J.W.; Apsley, A.; Cowie, H.; Steinle, S.; Mueller, W.; Lin, C.; Horwell, C.J.; Sleeuwenhoek, A.; Loh, M. Effectiveness of face masks used to protect Beijing residents against particulate air pollution. Occup. Environ. Med. 2018, 75, 446–452. [Google Scholar] [CrossRef]
- Mueller, W.; Horwell, C.J.; Apsley, A.; Steinle, S.; McPherson, S.; Cherrie, J.W.; Galea, K.S. The effectiveness of respiratory protection worn by communities to protect from volcanic ash inhalation. Part I: Filtration efficiency tests. Int. J. Hyg. Environ. Health 2018, 221, 967–976. [Google Scholar] [CrossRef]
- Verma, S.; Dhanak, M.; Frankenfield, J. Visualizing droplet dispersal for face shields and masks with exhalation valves. Phys. Fluids 2020, 32, 091701. [Google Scholar] [CrossRef]
- Kähler, C.J.; Hain, R. Fundamental protective mechanisms of face masks against droplet infections. J. Aerosol Sci. 2020, 148, 105617. [Google Scholar] [CrossRef]
- Zhou, S.S.; Lukula, S.; Chiossone, C.; Nims, R.W.; Suchmann, D.B.; Ijaz, M.K. Assessment of a respiratory face mask for capturing air pollutants and pathogens including human influenza and rhinoviruses. J. Thorac. Dis. 2018, 10, 2059–2069. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.B.; Ko, H.S.; Seo, S.C.; Jung, J.H. Evaluation of filtration characteristics and microbial recovery rates of commercial filtering facepiece respirators against airborne bacterial particles. Sci. Total Environ. 2019, 682, 729–736. [Google Scholar] [CrossRef]
- Teesing, G.; van Straten, B.; de Man, P.; Horeman-Franse, T. Is there an adequate alternative to commercially manufactured face masks? A comparison of various materials and forms. J. Hosp. Infect. 2020, 106, 246–253. [Google Scholar] [CrossRef]
- Rodriguez-Palacios, A.; Cominelli, F.; Basson, A.R.; Pizarro, T.T.; Ilic, S. Textile masks and surface covers—A spray simulation method and a “universal droplet reduction model” against respiratory pandemics. Front. Med. 2020, 7, 260. [Google Scholar] [CrossRef]
- Bayersdorfer, J.; Giboney, S.; Martin, R.; Moore, A.; Bartles, R. Novel manufacturing of simple masks in response to international shortages: Bacterial and particulate filtration efficiency testing. Am. J. Infect. Control 2020, 48, 1543–1545. [Google Scholar] [CrossRef]
- Ueki, H.; Furusawa, Y.; Iwatsuki-Horimoto, K.; Imai, M.; Kabata, H.; Nishimura, H.; Kawaoka, Y. Effectiveness of face masks in preventing airborne transmission of SARS-CoV-2. mSphere 2020, 5. [Google Scholar] [CrossRef]
- Asadi, S.; Cappa, C.D.; Barreda, S.; Wexler, A.S.; Bouvier, N.M.; Ristenpart, W.D. Efficacy of masks and face coverings in controlling outward aerosol particle emission from expiratory activities. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Patel, R.B.; Skaria, S.D.; Mansour, M.M.; Smaldone, G.C. Respiratory source control using a surgical mask: An in vitro study. J. Occup. Environ. Hyg. 2016, 13, 569–576. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Guo, Y.; Gao, H.; Liu, J.; Yue, Y.; Wang, J. Evaluation of Regeneration Processes for Filtering Facepiece Respirators in Terms of the Bacteria Inactivation Efficiency and Influences on Filtration Performance. ACS Nano 2020, 14, 13161–13171. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.K.; Jin, J.; Rafael, G.H.; Zhao, M.; Liao, L.; Simmons, G.; Chu, S.; Weaver, S.C.; Chiu, W.; Cui, Y. Decontamination of SARS-CoV-2 and other RNA viruses from N95 level meltblown polypropylene fabric using heat under different humidities. ACS Nano 2020, 14, 14017–14025. [Google Scholar] [CrossRef]
- Oh, C.; Araud, E.; Puthussery, J.V.; Bai, H.; Clark, G.; Wang, L.; Verma, V.; Nguyen, T.H. Dry Heat as a Decontamination Method for N95 Respirator Reuse. Environ. Sci. Technol. Lett. 2020, 7, 677–682. [Google Scholar] [CrossRef]
- Suen, C.Y.; Leung, H.H.; Lam, K.W.; Karen, P.H.; Chan, M.Y.; Kwan, J.K.C. Feasibility of reusing surgical mask under different disinfection treatments. medRxiv 2020. [Google Scholar] [CrossRef]
- Kumar, A.; Kasloff, S.B.; Leung, A.; Cutts, T.; Strong, J.E.; Hills, K.; Vazquez-Grande, G.; Rush, B.; Lother, S.; Zarychanski, R. N95 mask decontamination using standard hospital sterilization technologies. medRxiv 2020. [Google Scholar] [CrossRef]
- Ludwig-Begall, L.F.; Wielick, C.; Dams, L.; Nauwynck, H.; Demeuldre, P.-F.; Napp, A.; Laperre, J.; Haubruge, E.; Thiry, E. The use of germicidal ultraviolet light, vaporized hydrogen peroxide and dry heat to decontaminate face masks and filtering respirators contaminated with a SARS-CoV-2 surrogate virus. J. Hosp. Infect. 2020, 106, 577–584. [Google Scholar] [CrossRef]
- Daeschler, S.C.; Manson, N.; Joachim, K.; Chin, A.W.; Chan, K.; Chen, P.Z.; Tajdaran, K.; Mirmoeini, K.; Zhang, J.J.; Maynes, J.T. Effect of moist heat reprocessing of N95 respirators on SARS-CoV-2 inactivation and respirator function. CMAJ 2020, 192, E1189–E1197. [Google Scholar] [CrossRef]
- Bergman, M.S.; Viscusi, D.J.; Heimbuch, B.K.; Wander, J.D.; Sambol, A.R.; Shaffer, R.E. Evaluation of Multiple (3-Cycle) Decontamination Processing for Filtering Facepiece Respirators. J. Eng. Fibers Fabr. 2010, 5, 155892501000500405. [Google Scholar] [CrossRef] [Green Version]
- Viscusi, D.J.; Bergman, M.S.; Eimer, B.C.; Shaffer, R.E. Evaluation of Five Decontamination Methods for Filtering Facepiece Respirators. Ann. Occup. Hyg. 2009, 53, 815–827. [Google Scholar]
- Jung, S.; Hemmatian, T.; Song, E.; Lee, K.; Seo, D.; Yi, J.; Kim, J. Disinfection Treatments of Disposable Respirators Influencing the Bactericidal/Bacteria Removal Efficiency, Filtration Performance, and Structural Integrity. Polymers 2020, 13, 45. [Google Scholar] [CrossRef]
- Lindsley, W.G.; Martin, S.B., Jr.; Thewlis, R.E.; Sarkisian, K.; Nwoko, J.O.; Mead, K.R.; Noti, J.D. Effects of Ultraviolet Germicidal Irradiation (UVGI) on N95 Respirator Filtration Performance and Structural Integrity. J. Occup. Environ. Hyg. 2015, 12, 509–517. [Google Scholar] [CrossRef]
- Jatta, M.; Kiefer, C.; Patolia, H.; Pan, J.; Harb, C.; Marr, L.C.; Baffoe-Bonnie, A. N95 reprocessing by low temperature sterilization with 59% vaporized hydrogen peroxide during the 2020 COVID-19 pandemic. Am. J. Infect. Control 2021, 49, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Leung, P.; Yao, L.; Song, Q.; Newton, E. Antimicrobial effect of surgical masks coated with nanoparticles. J. Hosp. Infect. 2006, 62, 58–63. [Google Scholar] [CrossRef]


| Patent No: Title | Applications | Limitations | Reference |
|---|---|---|---|
| US5553608A: Face mask with enhanced seal and method. | For preventing passage of fluids between mask periphery and wearer | Fogging of glasswear | [38] |
| US5704349A: Surgical face mask with darkened glare-reducing strip and visor | Prevents movement of fluids from mask exterior to wearers face | May not be comfortable to wear | [39] |
| US3888246A: Anti-fog surgical face mask | Reduces fogging of wearer glasses(goggles) | No mention of proper face fitting | [43] |
| US6988500B1: Fog free medical face mask | Facilitates escape of exhaled moist air reducing fogging of wearers glass | No filtration efficiency test | [44] |
| US9770611B2: Maintenance-free anti-fog respirator | Prevents fogging by altering intrinsic structure of the mask sinus region | No filtration efficiency test | [45] |
| US10357672B2: Apparatus, system, and method to prevent fogging of eyewear. | Fogging is decreased by removal of exhaled air through the side vents | May have gaps between mask and wearer’s nose | [46] |
| EP1479413A2: Face mask that has a filtered exhalation valve | Efficiently allows movement of exhaled air | Fogging of wearers glasses may not have been considered | [47] |
| US6928657B2: Face mask having hook and loop type fastener | Better fitted mask to reduce wearers exposure | May not have antifogging abilities | [48] |
| US20090044812A1: Vent and strap fastening system for a disposable respirator providing improved donning | Uses exhalation vent to prevent fogging and has comfortable straps | No filtration efficiency data | [50] |
| US20110180078A1: Face Mask with Adjustable and Detachable Straps | Improved mask fit with detachable and reusable straps | Could have fogging problems | [51] |
| US8267088B2: Collapse resistant respirator | Allows for breathing without mask collapse | No pressure drop data | [52] |
| EP3653266A1: Respirator Mask | Increased surface area for enhanced breathability | No pressure drop test to confirm enhanced breathability | [53] |
| US10799728B2: Respirators and related methods | Improved fit and filtration efficiency | No apparent limitation | [54] |
| US20210022418A1: Disposable mask | Improved fit for individuals of different ages and various facial dimensions | Could have gaps between mask and wearer’s face | [55] |
| US9655392B2: Filtering face mask | Effective facemask with provision for graphical design | Fogging of wearer’s glass | [56] |
| US9386813B2: Mask | Provides airtight seal between face mask and wearer’s face preventing inhalation of contaminated air | Could fog glassware since only the side of the mask is airtight sealed | [57] |
| US20160151650A1: Fitted face mask | Inhibits wearers exposure to unfiltered air | No pressure drop data to show breathability | [58] |
| US9320923B2: Surgical face mask, including reusable masks, with filtered inhalation and exhalation valves | Replaceable valves and reusable straps | No pressure drop test | [59] |
| US9457207B2: Facemask with filter insert for protection against airborne pathogens | Equipped with vents which allows exhalation of heat and CO2 | No apparent limitation | [60] |
| US20190125011A1: Temperature sensitive surgical face mask for identifying at risk patients and reducing viral infection | Made up of a thermo-chromatic material which changes color with temperature | May not prevent fogging of wearers glass | [61] |
| US10307554B2: Mask and components thereof. | Improves mask comfort of the wearer | No pressure drop data to show breathability | [62] |
| US20170095681A1: Adjustable facial conforming face mask | Reduces fogging of wearer’s goggles by channeling exhaled air to the side of the mask | Maybe bulky and uncomfortable to wearer | [63] |
| US20160235136A1: Surgical mask | Eliminates the gap between the wearer and the mask by incorporating a sealing member | No pressure drop test | [65] |
| US9706800B2: Face mask and method for making the same | Developed a method for face mask production for increased production rate | N/A | [66] |
| Purpose of Study | Mask Material | Modeled Aerosol Particles | Experimental Aerosol Particle Generator | Notes on Filtration Efficiency | Ref. |
|---|---|---|---|---|---|
| 48 different sample materials |
|
|
| [77] |
| Evaluated FEs as a function of aerosol particulate sizes in the 10 nm to 10 μm range, which is particularly relevant for respiratory virus transmission | Several common fabrics including N95, surgical masks, cotton, silk, chiffon, flannel, various synthetics, and their combinations |
|
|
| [71] |
| Developed a highly breathable and thermal comfort filter medium consisting of electret polyethersulfone/barium titanate nanofibrous membrane (PES/BaTiO3 NFM) integrated on a nonwoven polypropylene substrate |
| NaCl aerosol particles (PM2.5) | The air permeability was tested according to ISO 9237 (1995) standard test method using a Frazier Air Permeability Tester (YG461E, NBFY Co. Ltd., China) with a fixed testing area of 20 cm2 and the pressure drop of 200 Pa | The electret PES/BaTiO3 NFM1.5 medium with a low basis weight of 4.32 g/m2:
| [85] |
| Presented practical design principles for the fabrication of electrocharged filtration layers employed in N95 FRs using commonly available materials and easily replicable methods |
|
|
| Penetration percentage range values measured from filtration tests:
| [81] |
| FE, differential pressure (ΔP), quality factor (QF), and construction parameters were measured for 32 cloth materials (14 cotton, 1 wool, 9 synthetics, 4 synthetic blends, and 4 synthetic/cotton blends) used in cloth masks intended for protection from the SARS-CoV-2 virus (diameter 100 ± 10 nm) |
|
| Aerosol was generated from a 10 mg/mL aqueous solution of NaCl using a constant output atomizer supplied with dry (dew point < −75 °C), HEPA-filtered air (25 psig) |
| [14] |
| Assessed the fitted filtration efficiencies (FFEs) for face mask alternatives used during the COVID-19 pandemic |
|
| A particle generator 8026 (TSI) was used to supplement ambient particle counts in the chamber | FFE using the Occupational Safety and Health Administration Modified Ambient Aerosol CNC Quantitative Fit Testing Protocol for Filtering Facepiece:
| [2] |
| Comprehensively evaluated the overall and size-dependent filtration performances of non-medical materials | Total number of 43 combinations:
|
| Generated by a constant output atomizer (Model 3076, TSI Inc.) nebulizing a NaCl-water solution with a mass concentration of 0.1% |
| [79] |
| Aimed to investigate the FE of home-made masks that could be used as alternatives for community mitigation of COVID-19 |
|
| Scaled air duct system by using nebulizer |
| [86] |
| Evaluated the filtration properties of natural and synthetic materials using a modified procedure for N95 respirator approval |
|
|
|
| [74] |
| Demonstrated the low-cost (<$300) conversion of standard equipment used to fit-test respirators in hospital and industrial settings into a setup that measures quantitative FEs of materials based on NIOSH N95 guidelines, and subsequently measure FEs of materials found in healthcare and consumer spaces | Sample materials available in the hospital (healthcare-grade) and those accessible to the public (consumer-grade) |
|
| FE values:
| [118] |
| Examined the ability of fabrics that might be used to create home-made face masks to filter out ultrafine (0.02 to 0.1 μm) particles at the velocity of adult human coughing | Several common fabrics and their combinations. |
|
|
| [12] |
| Addressed the development of a novel charged PVDF nanofiber filter technology to effectively capture the fast spreading, deadly airborne coronavirus, especially COVID-19, with targeted aerosol size set at 100 nm (nano-aerosol), and not 300 nm | PVDF nanofiber filter average fiber diameters 84, 191, 349 and 525 nm |
| Sub-micron aerosol generator |
| [80] |
| Purpose of Study | Mask Material | Modeled Aerosol Particles | Experimental Aerosol Particle Generator | Notes on Filtration Efficiency | Reference |
|---|---|---|---|---|---|
| Investigated bilayer, beaded ENMs to prepare efficient lightweight respiratory filter media with less pressure drop. Focused on producing a continuous bead on string nanostructure at small basis weight levels (0.5 to 2 g/m2) | Single layer and Bilayer Electrospun nanofiber membrane (ENM) | KCl particles dp (0.3 to 5 μm) | Atomizer generated aerosols of KCl which flow through the rig at a controlled flow rate | Bilayer ENM with bead-free fibers on top and beads at bottom greatly improved FE (η = 95.7%) and reduced the pressure drop (ΔP = 137 Pa) The bilayer ENM performance (η = 95%; ΔP = 112 Pa) at small basis weight (0.5 g/m2) was on par compared to a commercial disposable respirator mask | [119] |
| To reduce the expulsion of small cough-generated aerosol particles into the environment, the study conducted an efficacy quantitative comparison of: N95 respirator. A medical procedure masks. A commercial 3-ply cloth face mask. A single- and double-layer fabric neck gaiter. A commercial disposable face shield as source control devices. | N95 medical respirator (3M model 1860). Medical grade (ASTM Level 3) procedure mask with ear loops (Kimberly-Clark model 47107). Cloth face mask with 3 layers of cotton fabric and ear loops (Hanes Defender). Fabric neck gaiter (FKGIONG Sun UV Protection Neck Gaiter, 95% polyester, 5% Spandex). Disposable face shield (Fisher Scientific # 19-181-600A). | Cough aerosol particles dp (0 to 7 μm) | Modified Greene and Vesley testing method was used. Cough aerosol simulator with a pliable skin head form to propel small aerosol particles. Aerosol particles were generated by nebulizing a solution of 14% KCl and 0.4% sodium fluorescein using a single-jet Collison nebulizer (BGI, Butler, NJ, USA) at 103 kPa (15 lbs./in2), passing the aerosol through a diffusion drier (Model 3062, TSI, Shoreview, MN, USA), and mixing it with 10 L/min of dry filtered air. | Cloth face mask collection efficiency 28% for the <0.6 μm particles. 76% for the 4.7 to 7 μm particles.Double-layer gaiter blocked 24% of the <0.6 μm particles. 76% of the 4.7 to 7 μm particles. | [120] |
| Purpose of Study | Mask Material | Modeled Aerosol Particles | Experimental Aerosol Particle Generator | Notes on Filtration Efficiency | Reference |
|---|---|---|---|---|---|
| Establish a method to evaluate the FE of mask materials under extreme conditions. Present a simple way to test the filter performance of mask materials using micro-droplets sized particles and microspheres with a challenged velocity of 44.4 m/s created by centrifugation (7500 rpm) | Surgical masks. Washed surgical masks. Cotton gauze masks (containing 4 layers of cotton gauze, 2 layers of nonwoven fabric filter and 2 layers of polypropylene filter, washed). 4 types of cotton from T-shirts. 3 types of silk. 3 types of linen, tissue paper and cotton gauze. Surgical masks and cotton gauze masks were purchased from a Japanese drug store | Micro-particle FE test: Blocking micro-droplet sized (starch particles). dp (0.7 to 70 μm), average 8.2 μm. Microsphere FE test and Microsphere-capturing test: Latex microspheres.dp (0.75 μm) | Micro-particle FE test and Microsphere FE test: Centrifugation (7500 rpm, equaling 44.4 m/sec.) for 20 sec. to mimic the velocity of a sneeze. Microsphere-capturing test: Microsphere solution (50 to 100 μL) added on top of the sample | Four layers of silk blockage efficiency: 93.8% of microspheres. 88.9% of starch particles. Gauze mask blockage efficiency: 78.5% of microspheres. 90.4% of starch particles. Two layers of cotton blockage efficiency: 74.6% of microspheres. 87.5% to 89.0% of particles. Other materials blocked: 53.2% to 66.5% of microspheres. 76.4% to 87.9% of particles except the 8 layers of gauze (36.7%). | [121] |
| Examined the efficiency of commonly worn masks in the developing world: Three types of cloth masks. One type of surgical mask. | Two commercially available N95 masks from two different manufacturers in the United States (Rigid Moldex model (2701) and a 3M model (8200). The Moldex mask (N95 mask2) and one of the cloth masks (cloth mask 1) had a plastic and latex exhalation valve | Lab-generated polystyrene latex (PSL) microsphere. Five monodispersed aerosol sphere size (30, 100, and 500 nm and 1 and 2.5 μm). | PSL were generated by a constant output atomizer (model 3076; TSI, Shoreview, MN, USA). PSL were in a colloidal solution of single-size latex spheres. | FE for cloth mask with an exhaust valve (80% to 90%) for the measured polystyrene latex (PSL) particle sizes. Two styles of commercially available fabric masks were the least effective with a FE (39% to 65%) for PSL particles. Performance increased with increased particle size. FE for cloth masks tested against lab-generated whole diesel particles (30, 100, and 500 nm) ranged from 15% to 57% | [122] |
| Purpose of Study | Mask Material | Modeled Aerosol Particles | Experimental Aerosol Particle Generator | Notes on Filtration Efficiency | Reference |
|---|---|---|---|---|---|
| Developed a custom experimental set-up to measure the effectiveness of nine different respirators under real environmental conditions: Particle mass concentration below 2.5 μm (PM2.5). Particle number concentration (PNC). Lung Deposited Surface Area (LDSA). Black Carbon concentration (BC). | Nine low-cost respirators and commonly used by cyclists and pedestrians. N99 filter layer, carbon filter 2. Combination filter for chemical and particle filtration. Electrostatic filter. Filter FFP3 3. Filter FFP1 3. Respirator with no available data. Active carbon filter. Electrostatic and active carbon filter. Non-woven fabric filter | Ambient ultrafine aerosol particles including black carbon. PM2.5 | Face mask performances were assessed in a typically traffic affected urban background environment in the city of Barcelona under three different breathing patterns in order to investigate the influence of flowrate in face mask effectiveness | Median face mask effectiveness: 48% in a range of 14% to 96% for PM2.5. 19% in a range of 6% to 61% for BC concentration. 19% in a range of 4% to 63% for PNC. 22% in a range of 5% to 65% for LDSA. | [123] |
| Test the FE of a range of masks sold to consumers in Beijing. Assessed mask effectiveness in reducing exposure to diesel exhaust particulates when worn by volunteers | Nine masks were purchased in China 3M8210 3M9001 3M9322 3M9501 3M9502 Green Shield Yi Jie PM2.5 Gucheng Yimeijian | Fine diesel exhaust particulates (PM2.5). Black carbon (BC) concentration (50 μg/m3) | Tests were conducted in a chamber in Edinburgh, UK. Tests were conducted in an exposure chamber supplied with air from a mixing chamber connected to a small diesel engine | Mean penetration (%) for each mask material ranged from 0.26% to 29%, depending on the flow rate and mask material. Average total inward leakage (TIL) of BC: 3% to 68% in the sedentary tests. 7% to 66% in the active tests. FE of a face mask does not necessarily translate into consistent exposure reduction for individuals | [124] |
| Aimed to build the first evidence base on the effectiveness of common materials used to protect communities from ash inhalation in volcanic crises. The respiratory protection (RP) materials were characterized and subjected to FE tests, which were performed with three challenges chosen as a low-toxicity surrogate dust of similar particle size distribution. | 17 forms of RP, covering various types of cloth through to disposable masks: Used in occupational settings Communities are known to wear during volcanic crises | Three type of dusts: Ashes from Sakurajima (Japan) and Soufrière Hills. (Montserrat) volcanoes. Sluminum oxide (Aloxite). Two PM2.5 concentrations (1.5 and 2.5 mg/m3) | The particle-air suspension was generated using a Venturi nozzle, and a rotating table was loaded with the dust | Median FEs against volcanic for: N95-equiv. (>98%). N99-equiv. (>98%). PM2.5 surgical-Japan (>98%). Basic flat-fold-Indonesia (>98%). Two standard surgical masks (89% to 91%). All other materials (23% to 76%). No cloth materials (>44%). Folding a bandana resulted in better FE (40%; 3x folded) than single-layered material (29%). Wetting the bandana and surgical mask material did not improve FE overall | [125] |
| Presented a washable multilayer triboelectric air filter (TAF) for efficiently removing the PMs | Washable high-efficiency uncharged and charged triboelectric air filter (TAF). The TAF consists of five layers of the polytetrafluoroethylene (PTFE) and nylon fabrics | Smoke (<0.3 μm to >10 μm). Most of the particulate matter were <1 μm | The removal efficiency of the uncharged and charged TAF was performed in a 30 m3 lab. PMs were generated by burning cigarettes | After charging, the TAF has a removal efficiency of: 84.7% for PM0.5 (3.22 × times unchangred TAF). 96.0% for PM2.5 (1.39 × times unchangred TAF). The TAF is promising for fabricating a reusable and high-efficiency face mask | [76] |
| Developed a novel self-powered electrostatic adsorption face mask (SEA-FM) based on the poly (vinylidene fluoride) electrospun nanofiber film (PVDFESNF) and a triboelectric nanogenerator (TENG) driven by respiration (R-TENG) | A self-powered electrostatic adsorption face mask (SEA-FM) with a low pressure drop based on the RTENG and electrospun. Three PVDF-ESNFs with different electrospun times (30, 60, and 90 min) | Ultrafine particulates. dp (10 to 1000 nm) | The particulate matters used were generated by burning cigarettes because of the merits such as wide particulates size distribution from 10 nm to 10 μm, low price, and close to the existence of real environment particulates | On the basis of the RTENG, the SEA-FM showed that the removal efficiency of coarse and fine particulates was higher than 99.2 wt%. The removal efficiency of ultrafine particulates was 86.9 wt% after continually wearing for 240 min and a 30-day interval | [91] |
| Purpose of Study | Mask Material | Modeled Aerosol Particles | Experimental Aerosol Particle Generator | Notes on Filtration Efficiency | Reference |
|---|---|---|---|---|---|
| Investigate: Aerosol FE of common household materials. Filters effects on flow characteristics in the surrounding flow regions. Compare results to a commercial surgical mask and an R95 mask | Cotton. Non-woven fabric (fabric 1). Microfiber cloth. HVAC filter. Shower curtain. Vacuum bag. Coffee filter. Material made up as: Single-layer. Two-layers. Three-layers | Liquid aerosol droplets. dp (1 to 4.7 µm). Particle Density (1 kg/m3) | Generated liquid aerosol droplets by six jet atomizer (TSI Model 9306) was used to aerosolize the Di-Ethyl-Hexyl-Sebacat (DEHS) fluid | FE for Shower curtain (74.4%) and HVAC filter (74.7%) had lower efficiency for all aerosol droplets sizes. Averaged FE of the combined multilayer materials increased from 4% to 15% when compared to single-layer materials. FE multilayer materials (>95%) at aerosol dp (2.42 µm). FE (>90%) for three-layer materials (cotton-coffee filter-cotton and cotton-coffee filter-fabric 1) | [6] |
| Demonstrated a simple optical measurement method to evaluate the efficacy of masks to reduce the transmission of respiratory droplets during regular speech. Compared the efficacy of different masks by estimating the total transmitted droplet count | 14 commonly available masks or masks alternatives. One patch of mask material. A professionally fit tested N95 mask. | Water particles from a spray bottle | The front of the box had an 18 cm diameter hole for the speaker, large enough for a person wearing a mask to speak into the box but small enough to prevent the face (or mask) from reaching the light sheet | Some mask types approach the performance of standard surgical masks, while some mask alternatives, such as neck fleece or bandanas, offer very little protection | [11] |
| Used qualitative visualizations to examine the performance of face shields and exhalation valves in impeding the spread of aerosol-sized droplets | A face shield (similar in design to those used by healthcare workers in conjunction with masks and other protective equipment). An N95 mask with an exhalation valve located at the front | Droplets of distilled water and glycerin. dp (<10µm) | The setup consists of a hollow manikin head, where a cough/sneeze was emulated via a pressure impulse applied using a manual pump | The visualizations indicated: Face shields blocked the initial forward motion of the jet. Expelled droplets can move around the visor with relative ease and spread out over a large area depending on light ambient disturbances. Visualizations for a mask equipped with an exhalation port indicated that a large number of droplets pass through the exhale valve unfiltered, which significantly reduces its effectiveness as a means of source control | [126] |
| Showed from a fluid physics point of view and under different circumstances the type of masks can protect against droplet infection. Analyzed the flow blockage caused by surgical masks when coughing and qualified the effectiveness of different filter materials and masks to determine the protection ability against droplets. Attempted to prove the effect of gap flows at the edge of surgical and particle filtrating respiratory masks | Surgical face mask Hygienic mask Toilet paper Paper towel Coffee filter Microfibre cloth Fleece Vacuum cleaner bag FFP3 mask with valve Halyard H600 | DEHS (Di-Ethyl-Hexyl-Sebacat) tracer particles. dp (0.1 to 2 μm) | The droplets were generated from DEHS with an aerosol seeding generator (AGF 2.0, Palas GmbH, Karlsruhe, Germany) | Mechanisms that include preventing a smear infection, applying adequate flow resistance to spreading virus in a room, and preventing inhalation of droplet, can be only achieved with FFP2/N95/KN95 or better particle filtering respirator mask | [127] |
| Purpose of Study | Mask Material | Modeled Aerosol Particles | Experimental Aerosol Particle Generator | Notes on Filtration Efficiency | Reference |
|---|---|---|---|---|---|
| Evaluated the relative contributions of a mask, valve, and Micro Ventilator on aerosol FE of a new N95 respiratory face mask | N95-rated (16) respiratory face mask with Micro Ventilator | Influenza A (H1N1) virus, strain A/PR/8/34 (from Charles River Laboratories (Horsham, MA, USA). Rhinovirus type 14, strain 1059 (ATCC VR-284) (from the American Type Culture Collection (Manassas, FL, USA) | Six-jet Collison nebulizer (Mesa Labs, Butler, USA) was filled with a measured amount of virus suspended in 0.1× Minimum Essential Medium (MEM). Virus was aerosolized and delivered into the upstream chamber using high-pressure air | FE (>99.7%) for each test mask configuration for exclusion of influenza A virus, rhinovirus 14, and S. aureus. FE (>99.3%) for paraffin oil and sodium chloride (surrogates for PM2.5) | [128] |
| Evaluated the FEs and microbial recovery rates of commercial filtering facepiece respirators against bioaerosols | Eight filtering facepiece respirators and one surgical mask were selected | Bioaerosols: Staphylococcus epidermidis. Escherichia coli | Bioaersols were released from a from a 6-jet nebulizer (Collison Nebulizer; BGI, Butler, NJ, USA) at 1.0 psig (Bioaerosol Spraying) | FE of each filtering facepiece respirator ranged from 82% to 99%, depending on the filtration grade | [129] |
| To conclude whether there is an effective mask for the population to wear in public that could easily be made during a medical face mask shortage using readily available materials. Test if the filter material of ePM1 85% (ISO 16890) or F9 (EN 779:2012), similar to the American MERV 16 filter standards, could approach the filter capacity of an FFP2 mask | Two filter material types: ePM1 85% (ISO 16890). F9 (EN 779:2012. | dp (0.3, 0.5, 1.0 and 5.0 µm). Staphylococcus aureu was used for testing EN 14683:2014 (surgical masks) | For the fit test: The face mask was equipped with an inlet to a tube. The flow was created through the tube, and the number of particles in the mask is counted | Fourteen of the 25 (combinations of) materials filtered at least 35% of 0.3-mm particles. Four of the materials proved hydrophobic, all commercially manufactured filters. Two models sealed the face. Twenty-two of the 25 materials were breathable at <0.7 mbar. None of the hydrophobic materials stayed intact after washing | [130] |
| Assessed household textiles to quantify their potential as effective environmental droplet barriers (EDBs). Using a bacterial-suspension spray simulation model of droplet ejection (mimicking a sneeze), the extent was quantified by which widely available clothing fabrics reduce the dispersion of droplets onto surfaces within 1.8 m (COVID-19 recommended minimum social distancing) | Six household textiles: 100% combed cotton (widely available, T-shirt material). 100% polyester microfiber 300-thread count fabric (pillow case). 100% cotton fabrics, two loosely homespun woven: 140GSM, 60 × 60-thread count. 115GSM, 52 × 48-thread count). 100% polyester common in sport jerseys (dry technology) | To simulate a cloud of droplets produced by a sneeze, a household spray bottles filled with an aqueous suspension of 12 probiotic cultured dairy product: Lactobacillus lactis L. rhamnosus L. plantarum L. casei L. acidophilus Leuconostoc cremoris Bifidobacterium longum B. breve B. lactis Streptococcus diacetylactis Saccharomyces florentinus 75 mL; 3 × 106−7 cfu/mL, 25 mL Saliva 106−7) in 1000 mL PBS (Fisher BP-399-1) | Spray bottle nozzles were adjusted to produce cloud and jet-propelled droplets that match a specific visual architecture of droplet formation. A high-volume trigger single-v-orifice nozzle sprayer was used (1.0 mL per stroke) with a 28/400 neck and 9-1/4-inch dip tube fitted with a filter screen (model PA-HDTS-EA, Mfr. Model # 922HL, Delta Industries, Inc.). The spray bottle ejected fluid with pressures that can reach 10 psi to create a short burst of fluid/jet and fan clouds of microdroplets | Spray experiments with “two-layers” (of 100%-combed cotton, common in t-shirts; and 100% polyester, in sports jerseys) Completely prevented the ejection of large macro-droplets (100% EnvDC prevention). Drastically reduced the ejection of micro-droplets by a factor of 5.16Log2, which is equivalent to a 97.2% droplet reduction (p < 0.020 vs. single-layers). The least-effective textile as single-layer (most breathable, 100%-cotton homespun-115 material) achieved a (90% to 99.998%) droplet retention improvement when used as two-layers (95% CI = 3.74–15.39 Log2). Two-layers of household textiles were as effective as medical masks preventing EnvDC, and that more breathable materials in ≥2-layers could be effectively used if individuals deem two-layer, “denser” textiles too air-restrictive | [131] |
| Measured the FEs of “N95 FFRs” including six N95 FFR models, three surgical N95 FFR models, and three SM models using testing methods: NIOSH NaCl aerosol. FDA particulate filtration efficiency (PFE). FDA bacterial filtration efficiency (BFE). Viral filtration efficiency (VFE) adapted by Nelson Laboratories from the ASTM F2101 method | N-series FFRs. Six NIOSH-approved. N95 FFR models. Three surgical N95 FFR models. Three SM models. Purchased from the United States Strategic National Stockpile or from respirator manufacturers known to have significant market share. The manufacturers and models in parentheses are: N95 FFRs-3M (Model 8210), 3M (Model 9210). Moldex(Model 2200), Kimberly-Clark (Model 62126). Sperian-Willson (Model SAF-T-FIT), and US Safety (N95B240). Surgical N95 respirators-3M (Model 1860). 3M (Model 1870) and Kimberly-Clark (Model 46727). SMs-3M (Model 1820), Kimberly-Clark (Model 47107) and Precept (15320) | NIOSH NaCl aerosol (Charge neutralized polydisperse sodium chloride, dp 0.022 to 0.259 μm). PFE (unneutralized 0.1 μm polystyrene latex (PSL) particles). BFE (not charge neutralized ∼ 3.0 μm size water droplet particles containing Staphylococcus aureus bacteria). VFE (unneutralized 3.0 μm size water droplet particles containing bacteriophage phiX 174 as the challenge virus and Escherichia coli as the host) | NIOSH NaCl aerosol 2% (wt/vol) NaCl solution was aerosolized, charge neutralized and then passed through the convex side of a test sample properly sealed and placed into a filter holder), total load 200 mg of NaCl. PSL particles were suspended in water and the aerosol was generated using a particle generator (Model PG-100) (Particle Measuring Systems (PMS), Boulder, CO). BFE (suspension of S. aureus was aerosolized using a nebulizer to give a challenge level of 1700–2700 colony-forming units (CFU) per test as specified by the ASTM F2101 standard). VFE (suspension of phiX174 was aerosolized in a nebulizer and each test was performed with a challenge level of 1700-2700 plaque-forming units (PFU) with a MPS of 3.0 ± 0.3 μm for 2 min | N95 FFRs FE values: NIOSH NaCl method (98.15% to 99.68%) PFE (99.74% to 99.99%) BFE (99.62% to 99.9%) VFE (99.8% to 99.9%) Efficiencies by the NIOSH NaCl method were significantly (p ≤ 0.05) lower than the other methods. SMs showed lower efficiencies (54.72% to 88.40%) than “N95 FFRs” measured by the NIOSH NaCl method, while PFE, BFE, and VFE methods produced no significant difference | [89] |
| Developed a method for generating PPE that can be easily replicated at other sites for use when supplies are critically low, and use of locally manufactured masks with known bacterial filtration efficiency (BFE) ratings is logically superior to alternatives (like cloth masks or scarves) | Four different surgical wraps, all from the Medline GEM Series with a single and a double layer ply. Eight mask prototypes were constructed in a consistent tri-fold design from each type of GEM wrap and single or double material layers | All eight prototypes were sent to an environmental lab for: BFE testing. Latex particle FE testing. Delta P testing. | BFE rates depending on specific material and ply (83.0% to 98.1%): Two ply masks produced with Medline GEM 1, 2, and 3 materials (96.3% to 98.1%). One single ply mask separated prior to mask manufacture (83.0% to 97.7%). Particular FE rates (92.3% to 97.7%) | [132] |
| Purpose of Study | Mask Material | Modeled Aerosol Particles | Experimental Aerosol Particle Generator | Notes on Filtration Efficiency | Reference |
|---|---|---|---|---|---|
| Developed an airborne transmission simulator of infectious SARS-CoV-2 containing droplets/aerosols produced by human respiration and coughs Assessed: The transmissibility of the infectious droplets/aerosols. The ability of various types of face masks to block the transmission. | Cotton masks Surgical masks N95 masks N95 (fit masks) | Droplets/aerosols produced by human. Infectious droplets/aerosol. Virus suspension (5 105 PFU [A to E], 1 108 PFU [F and G], 1 105 PFU [H], and 1 104 PFU [I])dp (5.5 ± 0.2 µm) Particle size percentages: 20% (<3 µm) 40% (3 to 5 µm) 40% (5 to 8 µm) | Airborne transmission simulator. Charged nebulizer with 6 ml of virus suspension at the viral doses in culture medium (without fetal calf serum) or diluted in phosphate-buffered saline to generate droplets/aerosols | Airborne simulation experiments showed that cotton masks, surgical masks, and N95 masks provide some protection from the transmission of infective SARS-CoV-2 droplets/aerosols. Medical masks (surgical masks and N95 masks) could not completely block the transmission of virus droplets/aerosols even when sealed | [133] |
| Measured outward emissions of micron-scale aerosol particles by healthy humans performing various expiratory activities while wearing different types of medical-grade or homemade masks | Surgical mask (ValuMax 5130E SB). Unvented KN95 respirator (GB2626-2006). Homemade single-layer paper towel mask (Kirkland, 2-PLY sheet). Homemade single-layer t-shirt mask (Calvin Klein). Homemade double-layer t-shirt mask. Vented N95 respirator (NIOSH N95, Safety Plus, TC-84A-7448) | Micron-scale aerosol particles by healthy human. dp (0.3 to 20 μm) | Healthy Human Expiratory activities: Breathing (2 min). Talking (100–150 s). Coughing (30 s). Jaw Movement (1 min). | Outward particle emission rate for speaking and coughing: Surgical masks (Reduced by 90%). Unvented KN95 (Reduced by 74%). Outward particle emission rate for all expiratory activities: Homemade cotton masks (remained unchanged). Homemade single-layer t-shirt mask (Increased by 492%). | [134] |
| Explored the importance of respiratory droplet and aerosol routes of transmission with a particular focus on coronaviruses, influenza viruses, and rhinoviruses, by quantifying the amount of respiratory virus in exhaled breath of participants with medically attended ARIs and determining the potential efficacy of surgical face masks to prevent respiratory virus transmission | Surgical face mask (cat. no. 62356, Kimberly-Clark) | Respiratory droplets > 5 μm. Aerosol droplets ≤ 5 μm. | Breathing as normal during the collection, but (natural) coughing was allowed and the number of coughs was recorded by study staff. Participants were then invited to provide a second exhaled breath sample of the alternate type (for example, if the participant was first assigned to wearing a mask, they would then provide a second sample without a mask), but most participants did not agree to stay for a second measurement because of time constraints. | Detected coronavirus in samples collected without face masks: Respiratory droplets in 3 of 10 (30%) Aerosols in 4 of 10 (40%) No detection of any virus in respiratory droplets or aerosols collected from participants wearing face masks. In samples collected without face masks, influenza virus was detected: Respiratory droplet in 6 of 23 (26%). Aerosol in 8 of 23 (35%). There was a significant reduction by wearing face masks to 1 of 27 (4%) in detection of influenza virus in respiratory droplets, but no significant reduction in detection in aerosols. Results indicated that surgical face masks could prevent transmission of human coronaviruses and influenza viruses from symptomatic individuals. Surgical face masks significantly reduced detection of influenza virus RNA in respiratory droplets and coronavirus RNA in aerosols, with a trend toward reduced detection of coronavirus RNA in respiratory droplets | [3] |
| Purpose of Study | Mask Material | Modeled Aerosol Particles | Experimental Aerosol Particle Generator | Notes on Filtration Efficiency | Reference |
|---|---|---|---|---|---|
| Ascertained the performance of 11 common household fabrics at blocking large, high-velocity droplets, using a commercial medical mask as a benchmark. Assessed the breathability (air permeability), texture, fiber composition, and water absorption properties of the fabrics. Developed a method of quantifying the effectiveness of fabrics at blocking large droplets containing 100 nm diameter nanoparticles which serve as a mimic for viruses in terms of size | One medial mask as control 11 common household fabrics: Medical mask (FM-EL-style, polypropylene, non-woven). Fabric (used shirts, under shirts, T-shirt, new bed sheet, new quilt cloth, variable % of cotton and polyester, woven or knit). Fabric (new dish cloth, 80% polyester, 20% polyamide, napped). Fabric (used shirt, silk, woven) | Fluorescent nanoparticles (beads). dp (25 nm, 300 nm) | Used a metered-dose inhaler (HFA-propelled, 210 sprays, GlaxoSmithKline) and loaded its nozzle with 10 μL of distilled water to generate droplets. Droplets were generated using a suspension of 100 nm-diameter red fluorescent beads (ex/em 580/605 nm, Invitrogen, catalog #F8801) diluted in distilled water | Blocking FE Values: Most fabrics (median values > 70%). Two layers of highly permeable fabric (>94%) similar to that of medical masks, while being approximately twice as breathable | [8] |
| Designed an in vitro model using various facepieces to assess their contribution to exposure reduction when worn at the infectious source (Source) relative to facepieces worn for primary (Receiver) protection, and the factors that contribute to each | Fitted (SecureFit™) surgical mask and an N95-class filtering facepiece respirator (commonly known as an ‘N95 respirator’) with and without a Vaseline-seal | Nebulizer and exhaled radioactive aerosols | Aerosol released from the source by tidal breathing or cough. Two manikins were connected to a Harvard ventilation pump (Harvard Apparatus SN No. A52587; Millis, MA, USA) | With cough, source control (mask or respirator on Source) was statistically superior to mask or unsealed respirator protection on the Receiver (Receiver protection) in all environments. To equal source control during coughing, the N95 respirator must be Vaseline-sealed.During tidal breathing, source control was comparable or superior to mask or respirator protection on the receiver | [135] |
| Addressed concerns that publication of only the ideal FE of materials in perfectly sealed settings can give mask wearers a false sense of security when venturing into areas of high exposure risk. Evaluated the FE of respirators, masks, and filter media against the smallest possible virus-carrying particulates | Several commercially available masks and respirators were tested as received without further modification: Cotton 1 Layer Dust Mask #4 Coffee filter Cotton 2 layers Shop towel Filtrete 1500 Surgical wrap N95 1 layer Medical mask ShopVac KN95 N95 2 layers 3M 8511 FTR467 ULPA | Polydisperse silicon dioxide nanoaerosol. dp (60 nm and 125 nm) | Polydisperse silicon dioxide nanoaerosol was generated in an electropolished steel environmental chamber designed according to the specifications of ANSI/CAN/UL 2904, measuring 4′ × 3′ × 3′ with cleanroom air (background total particulate concentration <10 particles/cm3) injected at a rate sufficient to induce one full chamber air exchange per hour | Results demonstrate the importance of fit on FE. Wearing a homemade mask can and does significantly reduce virion-sized particulate exposure (as reported worn filtration efficiencies of 15% to 40%). Homemade masks cannot provide the level of protection measured and more commonly reported in ideal-fit scenarios.For 3M 8511 and KN95: FE (>98%). When fit to the headform, the FE dropped to less than 40%, slightly better than the fitted cotton mask. Insertion of the extra layer to cotton masks: Did not significantly improve the cotton mask performance for most tested materials. In most cases, the cotton mask offered practically equivalent levels of protection without the insertion of the extra layer | [13] |
| Method | Modeled Aerosol | Material | Decontamination Condition(s) | Filtration Performance | Microbial Activity | Reference |
|---|---|---|---|---|---|---|
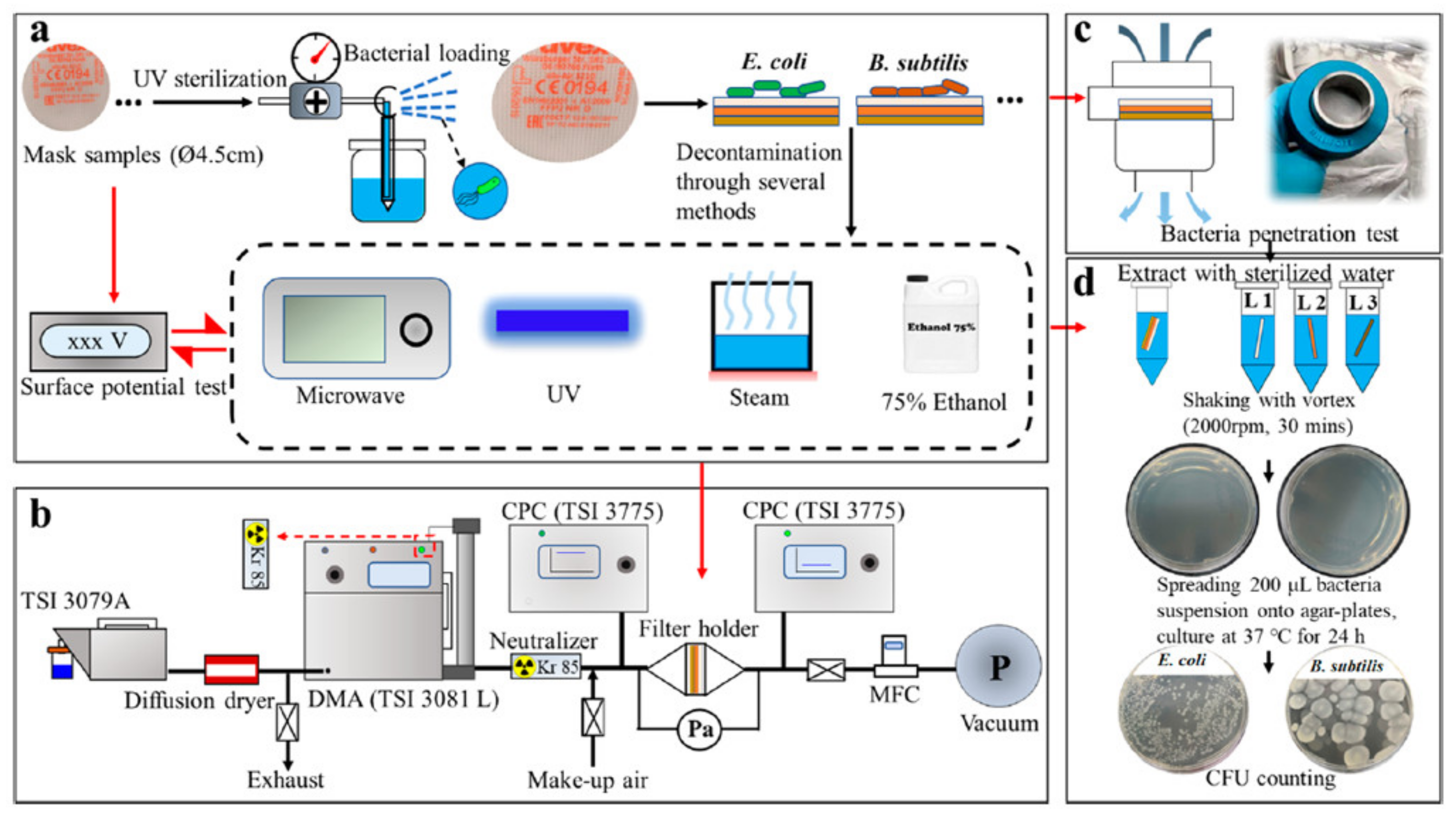
| Thermal Disinfection | SARS-CoV-2; Human coronavirus NL63 (Hcov-NL63) Chikungunya virus | N95 | Heating block T (75–85 °C) Thermal Loading (20–30 min) RH (100%) | Steady FE at: 20 cycles of 75 °C for 30 min/cycle 85 °C for 20 min/cycle at 100% RH. 75 °C for 30 min reduced viral titers by 3.5 log10-fold at 60% RH. Sharp drop in FE (90%) at T (125 °C) at fifth cycle | SARS-CoV-2 virus: ND at T (75 to 95 °C) and RH (100%) CHIKV-181/25: ND at T (85 or 95 °C) HCoV-NL63 titers below LOD at T (85 °C) for 20 minHCoV-NL63 titers: D at T (95 °C) for 5 min | [137] |
| Human Adenovirus Type 2 (AdV; Adenoviridae) Rotavirus OSU (RV; Reoviridae) Tulane virus (TV) Porcine transmissible gastroenteritis virus (TGEV) | N95 (1860, 3M) | Dry heat using electric cooker. T(100 °C) Thermal loading (50 min) RH (5%) | FE (97%) and fit testing did not degrade after 20 cycles and thermal loading (50 min) using dry heat treatment Pressure drop was not significant | Thermal energy conveyed to viruses by dry heat T (100 °C) and thermal loading (50 min) resulting in: >5.2-log10 reduction for TV >6.6-log10 reduction for RV >4.0-log10 reduction for AdV >4.7-log10 reduction for TGEV | [138] | |
| S. aureus | Surgical mask | Dry heat T (100 °C), thermal loading (15 min) Steam-used steamer cooker at T(100 °C), thermal loading (10 min) Water bath at T(100 °C), thermal loading (10 min) Autoclave T(121 °C), thermal loading (20 min) | All treatments showed a decrease in FE Dry heating did not show significant decrease after 3 cycles. Maintained FE (95%) Dry heat did not show significant effect on mask hydrophobicity Boiling, steaming, and autoclaving caused structural changes in mask material | Deactivation of bacterial activity (< 4-log reduction) for all heating methods | [139] | |
| Escherichia coli Bacillus subtilis | Surgical mask FFP1, FFP2, FFP3 | Steam used was less than 100 °C at normal atmospheric pressure | FE decreased (98.86% to 97.58%) at particle size (50 nm) FE was affected by a slight decay in the surface potential Steam changed morphology of mask sample | Deactivated E. coli by 100% at thermal loading (90 min) | [136] | |
| Vesicular stomatitis virus Indiana serotype (VSV) SARS-CoV-2 | N95 (3M VFlex 1804, Aura 1870, 1860, 8210, and 9210) | Autoclave at T(121 °C), thermal loading (15 min) Air removal, exposure, and drying, leading a total of 40 min per cycle | Functional integrity was kept for all masks after 10 cycles besides 3M 1860 and 8210 (molded) models which failed | >6-log 10 reduction of infectious virus | [140] | |
| Porcine respiratory coronavirus (PRCV) | Surgical masks KN95 | Dry heat at T(102 °C), thermal loading (60 min) | N/A | Infectivity of virus reduced by 2 orders of magnitude | [141] | |
| Escherichia coli SARS-CoV-2 | N95 (3M-1860S,8110S,8210S,9105S) | T (70 °C) RH (0% to 70%) Thermal loading (60 min) | Post treated respirators showed >95% FE after 10 cycles Heating did not affect the structural integrity of the mask after 10 cycles at RH (0% and 50%) | Infectious virus ND after dry heating at T(70 °C), thermal loading (60 min) Inactivated E. coli after dry heating at T(70 °C), thermal loading (60 min), RH (50%) | [142] | |
| E.Coli NaCL aerosol was used for FE test | N95 KF94 | Drying oven T (90 °C) Thermal loading (60 min) for N95 and KF94 grade respirators | Treatment did not show any significant effect on FE (<2%) | 82% deactivation of the bacteria | [145] |
| Method | Modeled Aerosol | Material | Decontamination Condition(s) | Filtration Performance | Microbial Activity | Reference |
|---|---|---|---|---|---|---|
| Micro-waving | Escherichia coli Bacillus subtilis | Surgical mask FFP1, FFP2, FFP3 | 400 W Thermal loading (10 min) | MWI did not show any significant effect on surface potential (no significant effect on FE) Morphology was affected over a long period of microwaving | Deactivated E. coli and B. subtilis by 100% (>4-log 10 reduction) in 10 min | [136] |
| Poly-disperse sodium chloride aerosol | Surgical mask N95 | 1100 W (750 W/ft3) Loading 2 min (1 min for each side of the mask) Mask were cooled at ambient temp between trials | No FE drop for 2 min exposure. N95 filter melted after 4 min forming visible holes | N/A | [144] | |
| Polydisperse sodium chloride aerosol | Surgical mask N95 | 1100 W (750 W/ft3) Loading: 2 min total exposure duration at a power setting of 10 | Partial separation of mask foam cushion and melting of head straps in mask samples | N/A | [143] | |
| E. coli NaCl aerosol was used for FE test | N95 KF94 | 750 W Loading: 1 min on both sides after removal of the metal nose clips | Treatment did not show any significant effect on filtration efficiency (<2%) | 82% deactivation of the bacteria | [145] |
| Method | Modeled Aerosol | Material | Decontamination Condition(s) | Filtration Performance | Microbial Activity | Reference |
|---|---|---|---|---|---|---|
| UV irradiation | Escherichia coli Bacillus subtilis | Surgical mask FFP1, FFP2, FFP3 | 254 nm wavelength Loading: 5 min (126 mj/cm2) | No significant effect on FE | 100% deactivation of E. coli UV+MW showed 100% inactivation of E. coli and B. subtilis in 5 min UV + 4/8/12 min MWI | [136] |
| S. aureus | Surgical mask | 254 nm UV irradiation Loading: 5 min | FE (>95%) No significant FE drop after 3 treatment cycles. | Eliminates all bacterial activity on exposure to 450 microW/cm2 for 10 min due to irradiation penetration limitations | [139] | |
| Poly-dispersed sodium chloride | Surgical mask N95 | 40 W (UV light intensity 0.18 to 0.20 mW/cm2) Loading: 5 min exposure on each side. | No particle filtration efficiency drop | N/A | [144] | |
| Porcine respiratory coronavirus (PRCV) | Surgical masks KN95 | 5.5 W (UV lamp) Loading: 2–4 min | N/A | Infectivity of virus reduced by 3 orders of magnitude | [141] | |
| E. coli NaCl aerosol was used for FE test | N95 KF94 | 10 W (UV lamp) Loading: 60 min on both sides of the respirator | No significant effect on FE | 82% deactivation of the bacteria | [145] | |
| NaCl particles were used for FE test | N95 Surgical mask | Variable UGVI dosage | 950 j/cm2 showed a slight decrease in particle penetration FE (up to 1.25%) | N/A | [146] |
| Method | Modeled Aerosol | Material | Decontamination Condition(s) | Filtration Performance | Microbial Activity | Reference |
|---|---|---|---|---|---|---|
| Chemicals | Porcine respiratory coronavirus (PRCV) | Surgical masks FFRs | Vaporized hydrogen peroxide (59% H2O2 with peak VHP concentration of 750 ppm) | N/A | Infectivity of virus reduced by 1 order of magnitude | [141] |
| Escherichia coli Bacillus subtilis | Surgical mask FFP1, FFP2, FFP3 | 75% ethanol Loading: 2 min | Showed significant effect in surface potential. Partial change in morphology | Inactivates bacteria completely | [136] | |
| Vesicular stomatitis virus, Indiana serotype (VSV) or SARS-CoV-2 | N95 (3M VFlex 1804, Aura 1870,1860,8210 and 9210) | Ethylene oxide-(1hr exposure)Low temperature hydrogen peroxide gas plasma-47 min cycle Vaporous hydrogen peroxide (VHP) with peak VHP concentration of 750 ppm Peracetic acid fogging | EtO maintained FE after 3 cycles for all tests. LT-HPGT-treated masks failed after the first cycle.VHP and PAF treatments maintained both functional and structural integrity after 10 cycles. Could not validate the effectiveness against SARS-CoV-2 | > 6 log 10 reduction of infectious virus for all methods. | [140] | |
| E. coli NaCl aerosol was used for FE test | N95 KN94 | sodium hypochlorite (NaClO) (5.5%) Sodium hydroxide (NaOH) (0.3%) and water 5%(v/v) Ethanol solution immersion of 70% (v/v) for 10 min Isopropanol immersion 100% for 10 min | Significant decrease in FE (up to 28%) in IPA and EtOH | 100% deactivation of the bacteria | [145] |
| Method | Modeled Aerosol | Material | Decontamination Condition(s) | Filtration Performance | Microbial Activity | Reference |
|---|---|---|---|---|---|---|
| Detergent-laundering | S. aureus | Surgical mask | Household detergent (Ultra Axion) 0.55 w/v was prepared with DI water. Sample soaked in water for 30 min | Showed significant decrease in FE after first cycle | Did not show any effect on bacterial activity | [139] |
| NaCl aerosol | N95 KN94 | Laundering was done with and without detergent: Without detergent: agitation speed of 90 rpm at 24C for 10 min, with 2 repeats at 3 min With detergent: 1 L of 0.1 wt% aq. detergent solution at 24C and 90 rpm for 10 min | Without detergent: No significant change in FE With detergent: Significant decrease in FE (>23%) | N/A | [145] |
| Surgical | N95 | Others | |
|---|---|---|---|
| Mask Type |  |  |  |
| Common Materials |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogbuoji, E.A.; Zaky, A.M.; Escobar, I.C. Advanced Research and Development of Face Masks and Respirators Pre and Post the Coronavirus Disease 2019 (COVID-19) Pandemic: A Critical Review. Polymers 2021, 13, 1998. https://doi.org/10.3390/polym13121998
Ogbuoji EA, Zaky AM, Escobar IC. Advanced Research and Development of Face Masks and Respirators Pre and Post the Coronavirus Disease 2019 (COVID-19) Pandemic: A Critical Review. Polymers. 2021; 13(12):1998. https://doi.org/10.3390/polym13121998
Chicago/Turabian StyleOgbuoji, Ebuka A., Amr M. Zaky, and Isabel C. Escobar. 2021. "Advanced Research and Development of Face Masks and Respirators Pre and Post the Coronavirus Disease 2019 (COVID-19) Pandemic: A Critical Review" Polymers 13, no. 12: 1998. https://doi.org/10.3390/polym13121998
APA StyleOgbuoji, E. A., Zaky, A. M., & Escobar, I. C. (2021). Advanced Research and Development of Face Masks and Respirators Pre and Post the Coronavirus Disease 2019 (COVID-19) Pandemic: A Critical Review. Polymers, 13(12), 1998. https://doi.org/10.3390/polym13121998