Linear/Ladder-Like Polysiloxane Block Copolymers with Methyl-, Trifluoropropyl- and Phenyl- Siloxane Units for Surface Modification
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Synthetic Procedure
2.3. Analytical Methods
2.3.1. Contact Angle Measurements
2.3.2. Liquid Chromatography
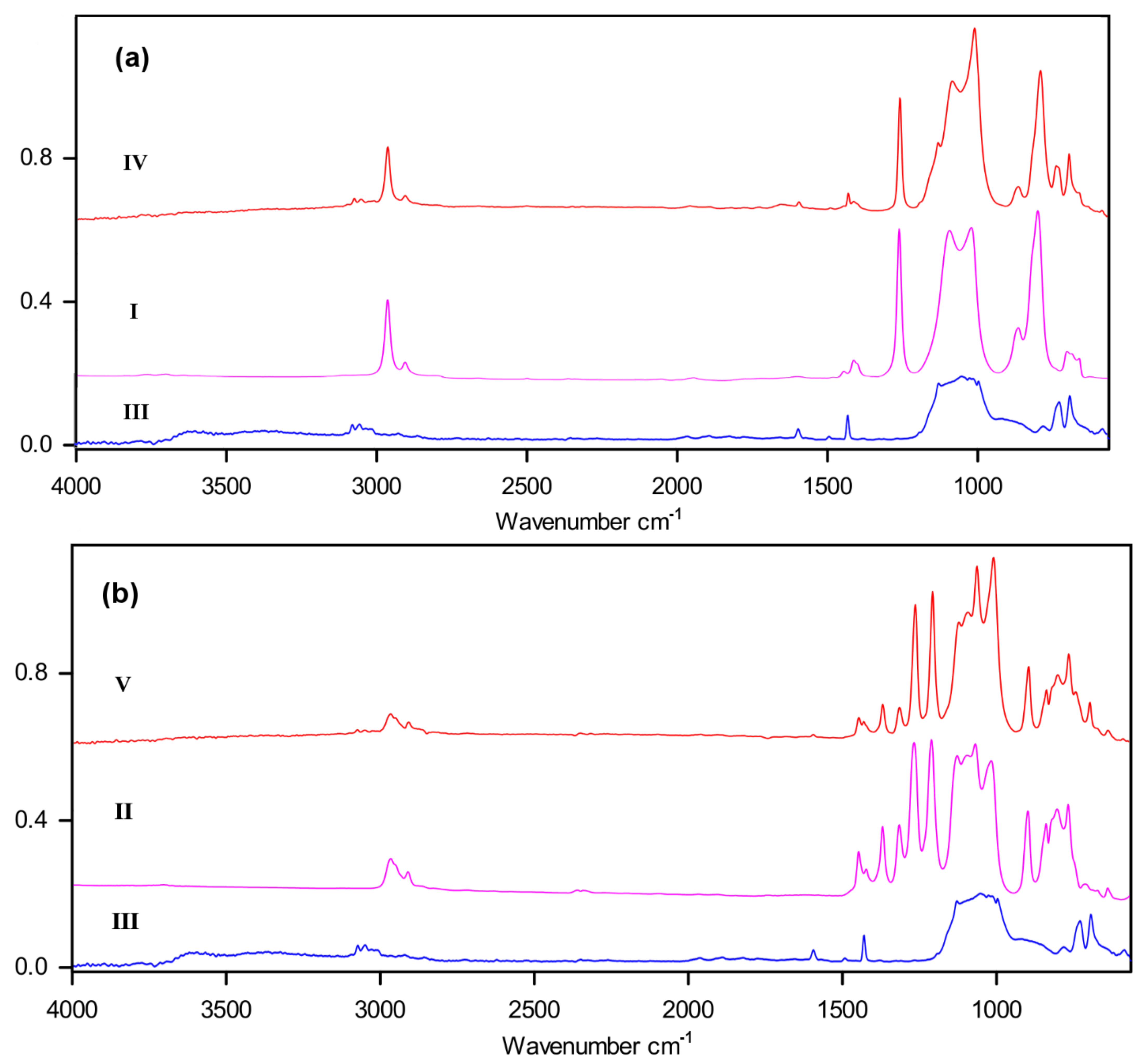
2.3.3. FTIR Spectroscopy
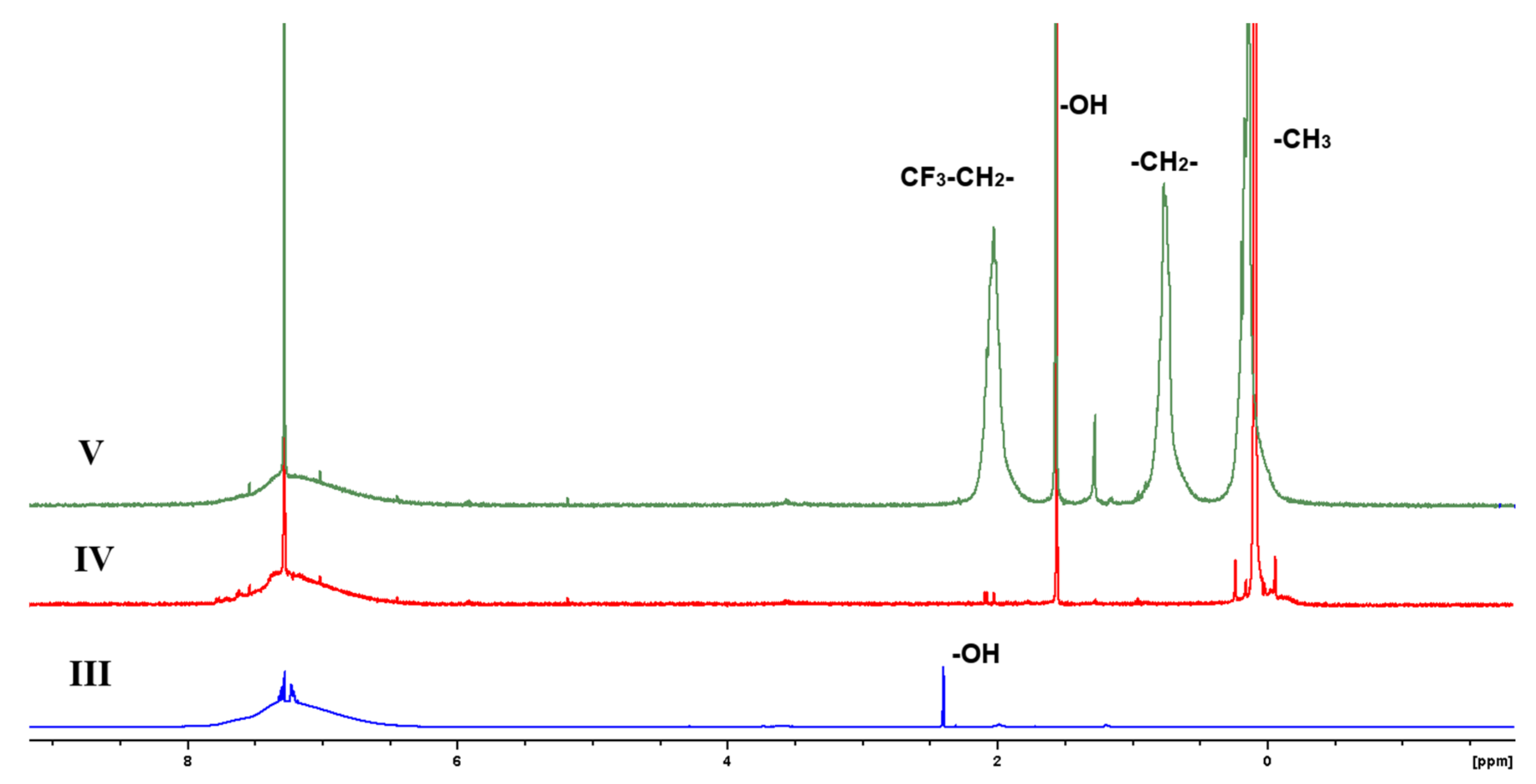
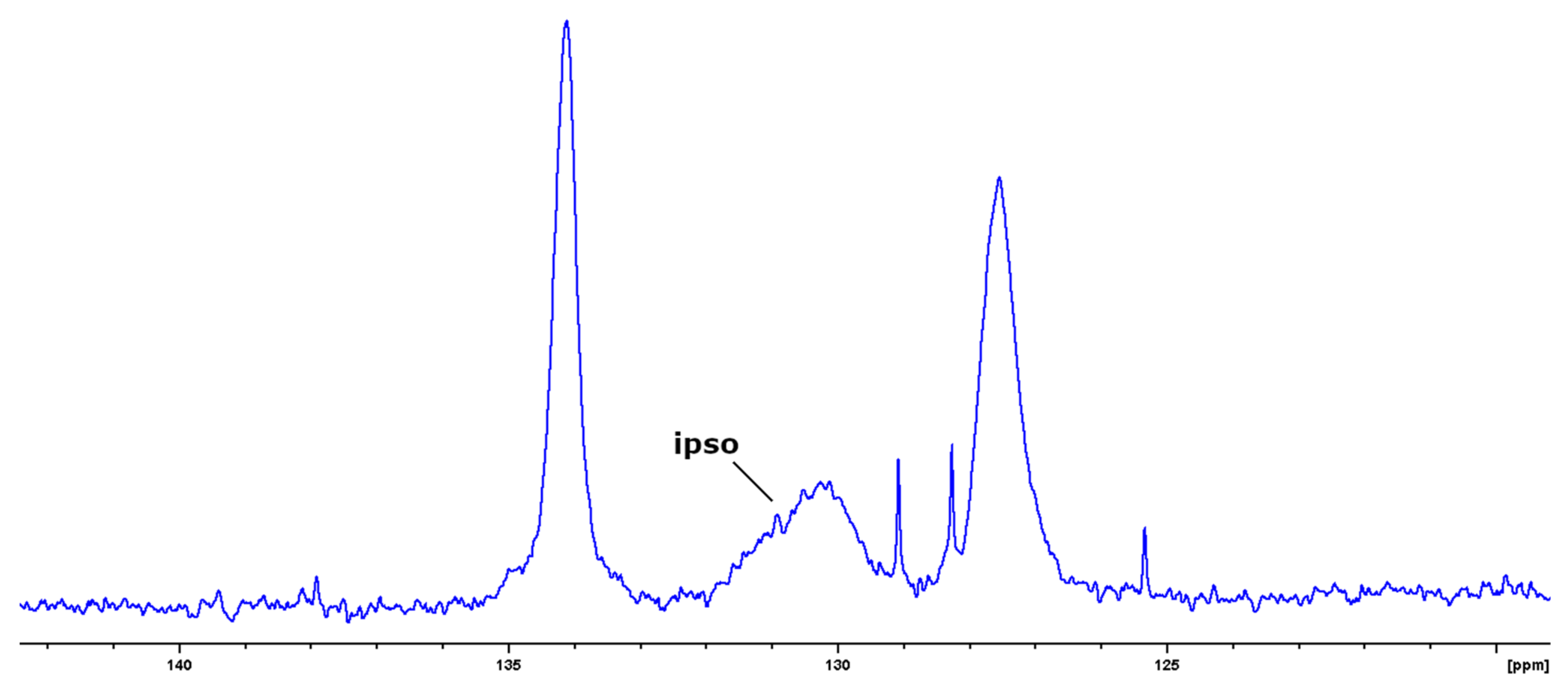
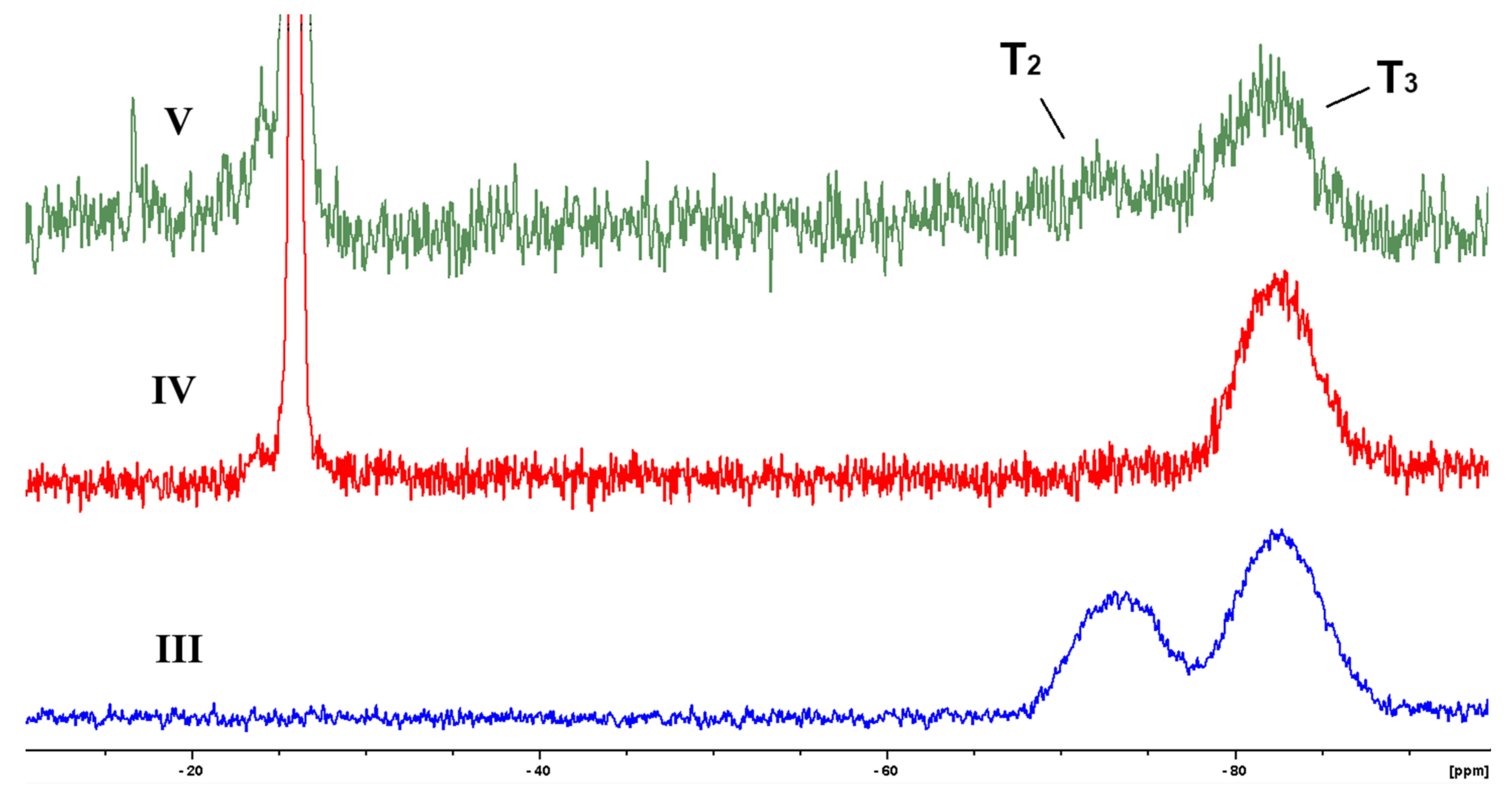
2.3.4. NMR Spectroscopy
2.3.5. Thermal Analysis
2.3.6. Mechanical Properties
2.3.7. Adhesion Strength Testing
2.3.8. Microscopy
3. Results and Discussion
3.1. Synthesis of Block-Copolymers
3.2. Thermal Properties
3.3. The Surface Properties of the Films of Copolymers IV-V
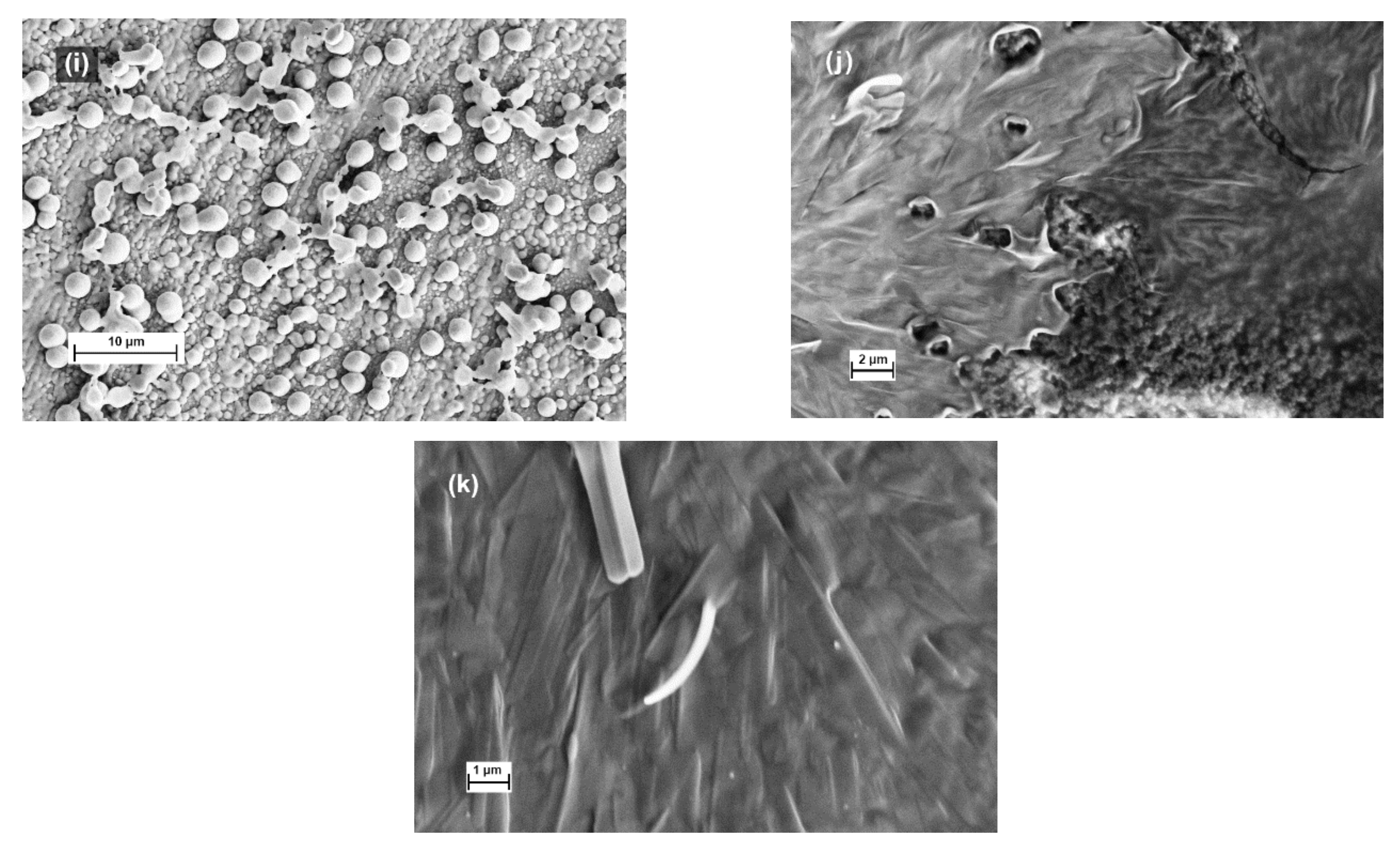
3.4. Solubility and Morphology of Block-Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Directed Self-Assembly of Block Co-Polymers for Nano-Manufacturing Processing, Modeling, Characterization and Applications; Gronheid, R.; Nealey, P. (Eds.) Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Yilgor, E.; Yilgor, I. Silicone containing copolymers: Synthesis, properties andapplications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef] [Green Version]
- Lo, T.-Y.; Krishnan, M.R.; Lu, K.-Y.; Ho, R.-M. Silicon-containing block copolymers for lithographic applications. Prog. Polym. Sci. 2018, 77, 19–68. [Google Scholar] [CrossRef]
- Myshkin, N.; Kovalev, A. Adhesion and surface forces in polymer tribology: A review. Friction 2018, 6, 143–155. [Google Scholar] [CrossRef]
- Govers, S.P.W.; Alexander, N.; Al-Masri, M.; Omeis, J.; van der Ven, L.G.J.; de With, G.; Esteves, A.C.C. Surface segregation of polydimethylsiloxane-polyether block copolymers in coatings driven by molecular architecture. Prog. Org. Coat. 2021, 150, 105991. [Google Scholar] [CrossRef]
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar]
- Rao, Y.Q.; Fenton, J.; Jenkins, R.; Evans, J. Structure and its thickness dependence of thin films of phenyl and methyl silsesquioxane polymers. J. Non-Cryst. Solids 2018, 502, 128–135. [Google Scholar] [CrossRef]
- Frye, C.L.; Klosowski, J.M. So-called ladder-structure of equilibrated phenylsilsesquioxane. J. Am. Chem. Soc. 1971, 93, 4599–4601. [Google Scholar] [CrossRef]
- Wang, R.; Rong, T.; Cao, G.S.; Jia, X.W.; Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Synthesis and synergetic effects of ladder-like silsesquioxane/epoxy compositional gradient hybrid coating. Prog. Organ. Coat. 2019, 130, 58–65. [Google Scholar] [CrossRef]
- Ren, Z.; Xie, P.; Jiang, S.; Yan, S.; Zhang, R. Study of the supramolecular architecture-Directed synthesis of a well-defined triple-chain ladder polyphenylsiloxane. Macromolecules 2010, 43, 2130–2136. [Google Scholar] [CrossRef]
- Shu, J.; Li, P.; Chen, Q.; Zhang, S. Quantitative measurement of polymer compositions by NMR spectroscopy: Targeting polymers with marked difference in phase mobility. Macromolecules 2010, 43, 8993–8996. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Ni, H.; Pittman, C.U. Polyhedral oligomeric silsesquioxane (POSS) polymers and copolymers: A review. J. Inorg. Organomet. Polym. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Siesler, H.W. Vibrational Spectroscopy in Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; Volume 2, pp. 255–300. [Google Scholar]
- Uhlig, F. 29Si NMR Spectroscopy, in Organosilicon Compounds; Chapter 2; Elsevier: Amsterdam, The Netherlands, 2017; pp. 59–77. [Google Scholar]
- Rahimi, A.; Shokrolahi, P. Application of inorganic polymeric materials. I. Polysiloxanes. Int. J. Inorg. Mater. 2001, 3, 843–847. [Google Scholar] [CrossRef]
- Rudolph, T.; Schacher, F.H. Selective crosslinking or addressing of individual domains within block copolymer nanostructures. Eur. Polym. J. 2016, 80, 317–331. [Google Scholar]
- Chang, G.; He, L.; Liang, J.; Wang, N.; Cao, R.; Zhao, X. Polysiloxane/poly(fluorinated acrylate) core–shell latexes and surface wettability of films. J. Fluor. Chem. 2014, 158, 21–28. [Google Scholar] [CrossRef]








| Solvent | IV | V |
|---|---|---|
| THF | ++ | ++ |
| methylethylketone | ++ | ++ |
| Ethyl acetate | ++ | ++ |
| Butyl acetate | ++ | ++ |
| toluene | +++ | + (at heating) |
| chloroform | +++ | + (at heating) |
| chlorobenzene | ++ | + |
| hexafluorobenzene | ++ | +++ |
| carbon tetrachloride | ++ | +- (emulsion) |
| trichloroethylene | ++ | +- (emulsion) |
| hexane | − (swelling) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostanin, S.A.; Kalinin, A.V.; Bratsyhin, Y.Y.; Saprykina, N.N.; Zuev, V.V. Linear/Ladder-Like Polysiloxane Block Copolymers with Methyl-, Trifluoropropyl- and Phenyl- Siloxane Units for Surface Modification. Polymers 2021, 13, 2063. https://doi.org/10.3390/polym13132063
Ostanin SA, Kalinin AV, Bratsyhin YY, Saprykina NN, Zuev VV. Linear/Ladder-Like Polysiloxane Block Copolymers with Methyl-, Trifluoropropyl- and Phenyl- Siloxane Units for Surface Modification. Polymers. 2021; 13(13):2063. https://doi.org/10.3390/polym13132063
Chicago/Turabian StyleOstanin, Stepan A., Alexei V. Kalinin, Yurij Yu. Bratsyhin, Natalia N. Saprykina, and Vjacheslav V. Zuev. 2021. "Linear/Ladder-Like Polysiloxane Block Copolymers with Methyl-, Trifluoropropyl- and Phenyl- Siloxane Units for Surface Modification" Polymers 13, no. 13: 2063. https://doi.org/10.3390/polym13132063
APA StyleOstanin, S. A., Kalinin, A. V., Bratsyhin, Y. Y., Saprykina, N. N., & Zuev, V. V. (2021). Linear/Ladder-Like Polysiloxane Block Copolymers with Methyl-, Trifluoropropyl- and Phenyl- Siloxane Units for Surface Modification. Polymers, 13(13), 2063. https://doi.org/10.3390/polym13132063







