Characterization of Chitosan Extracted from Fish Scales of the Colombian Endemic Species Prochilodus magdalenae as a Novel Source for Antibacterial Starch-Based Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock and Reagents
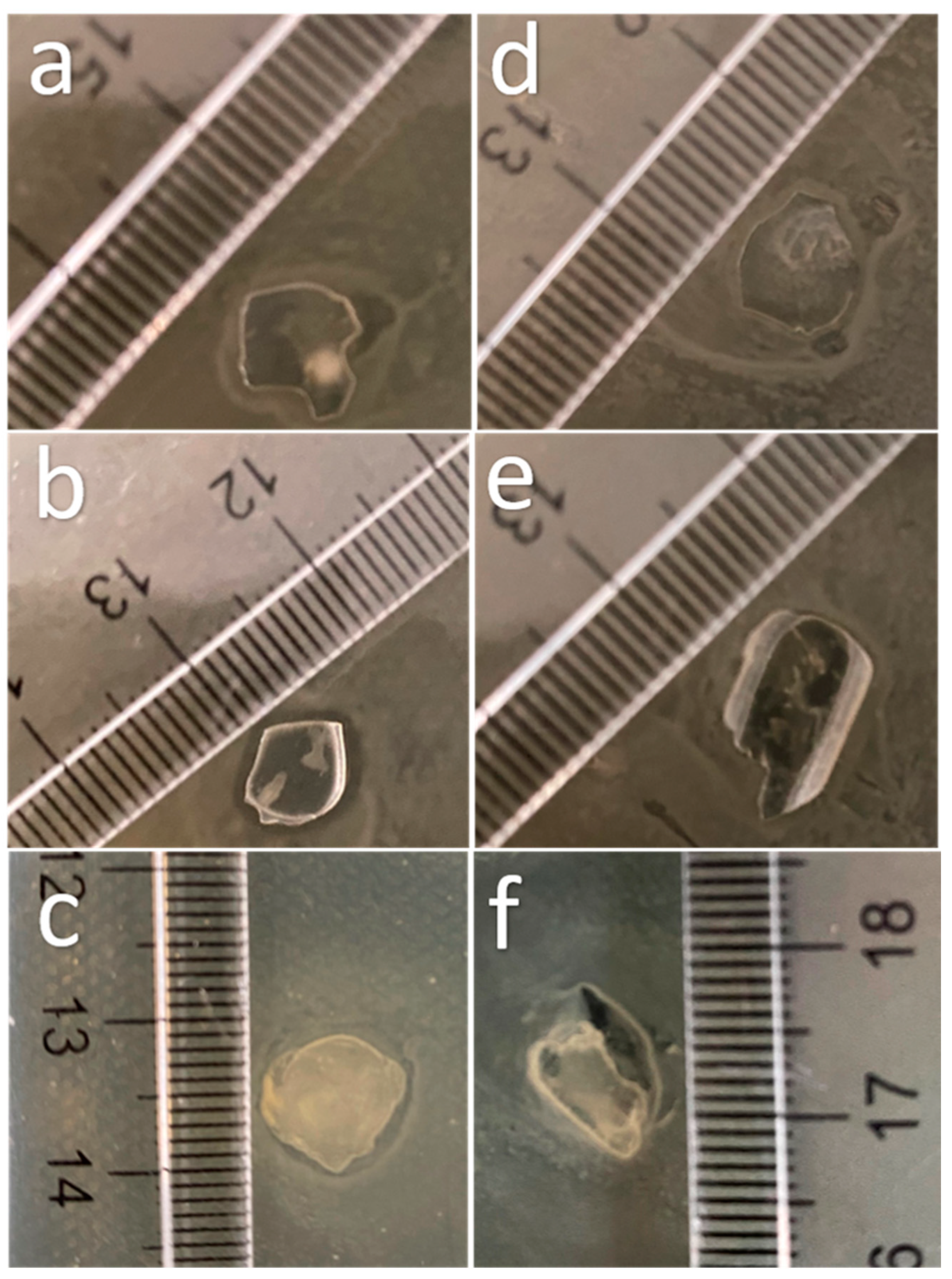
2.2. Sample Preparation
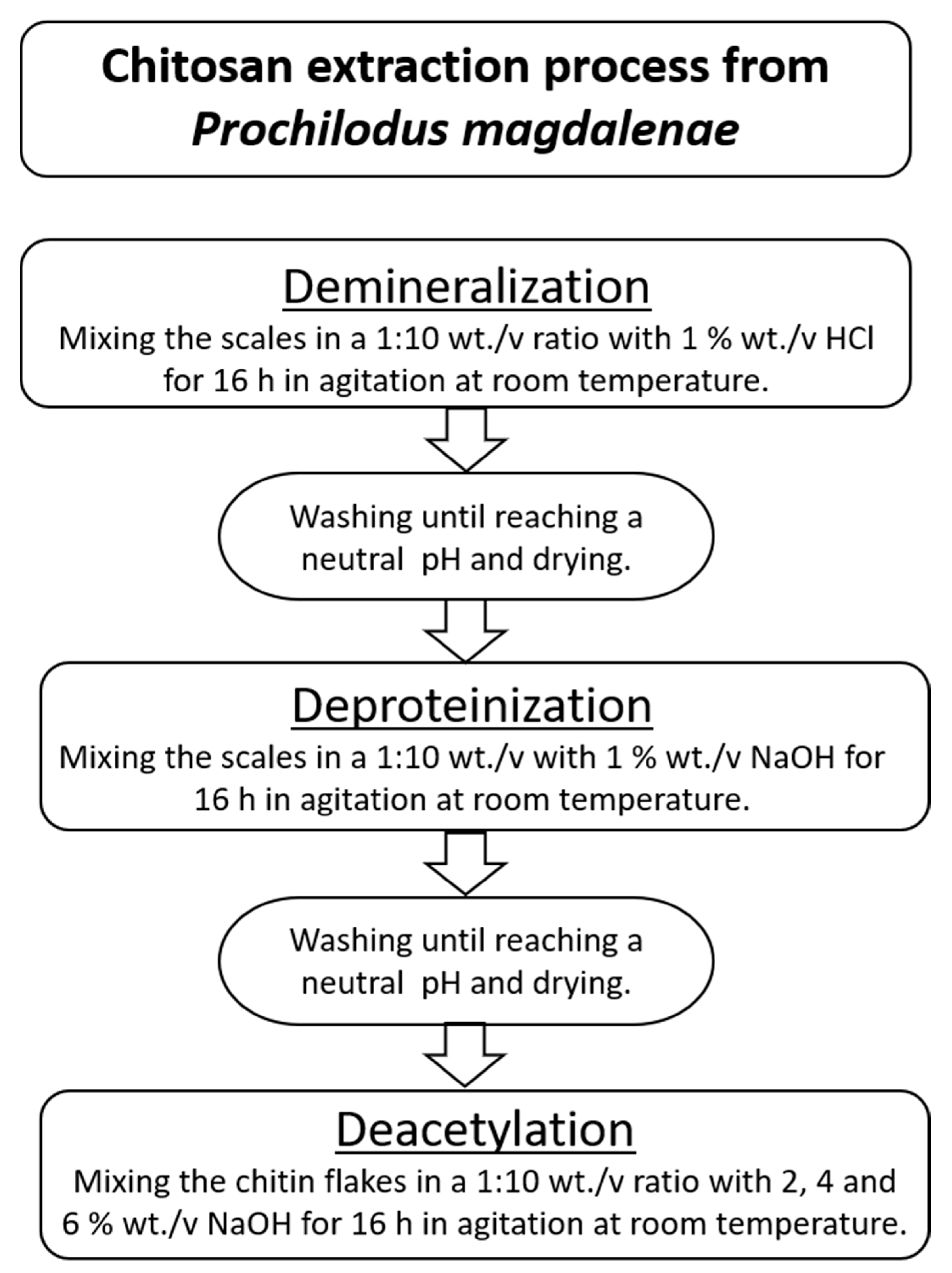
2.3. Chitosan Extraction
2.4. Chitosan Characterization
2.4.1. Protein Determination
2.4.2. Mineral Quantification (Phosphorous and Calcium)
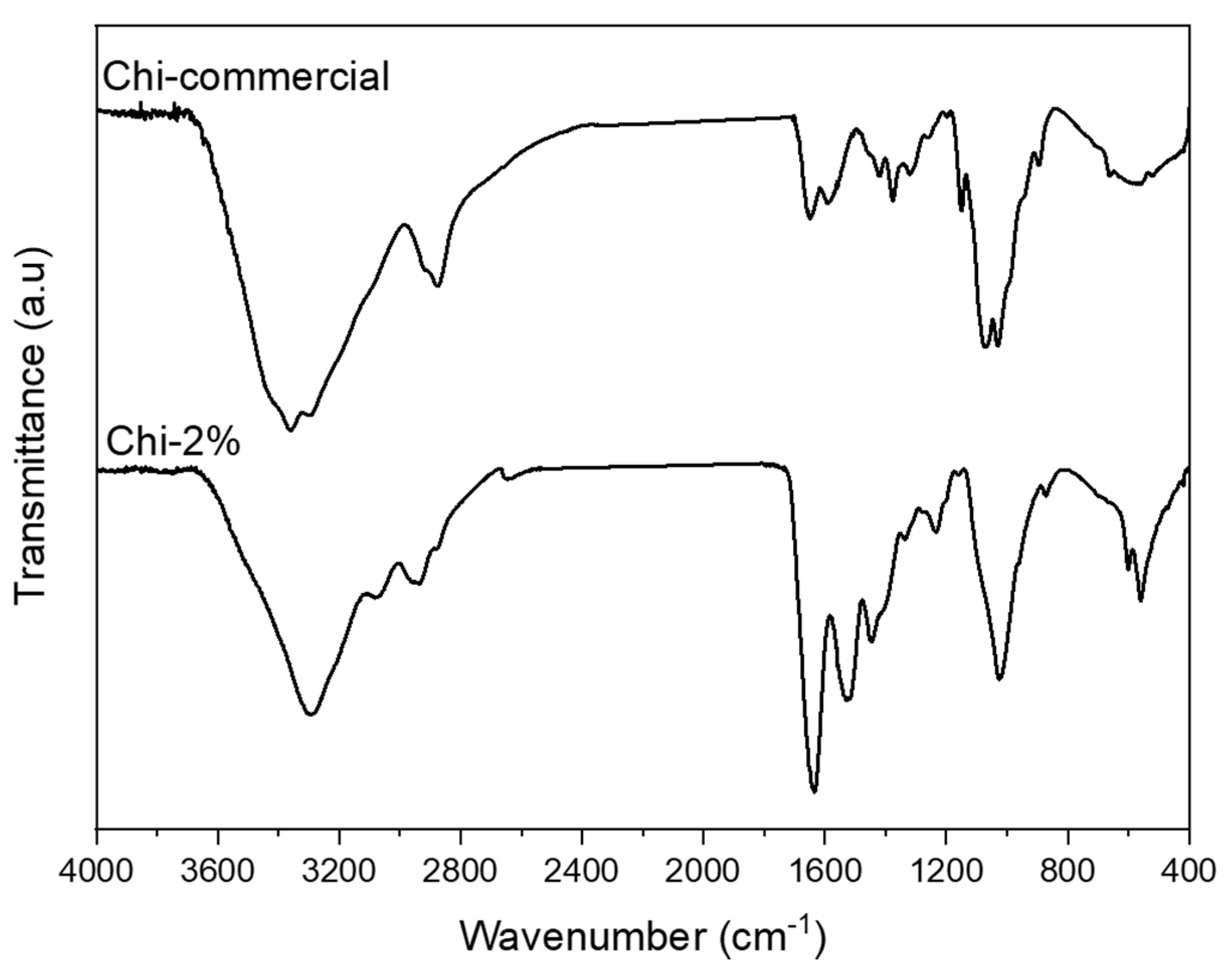
2.4.3. Attenuated Total Reflection Fourier Transform Infrared (FTIR–ATR) Spectroscopy
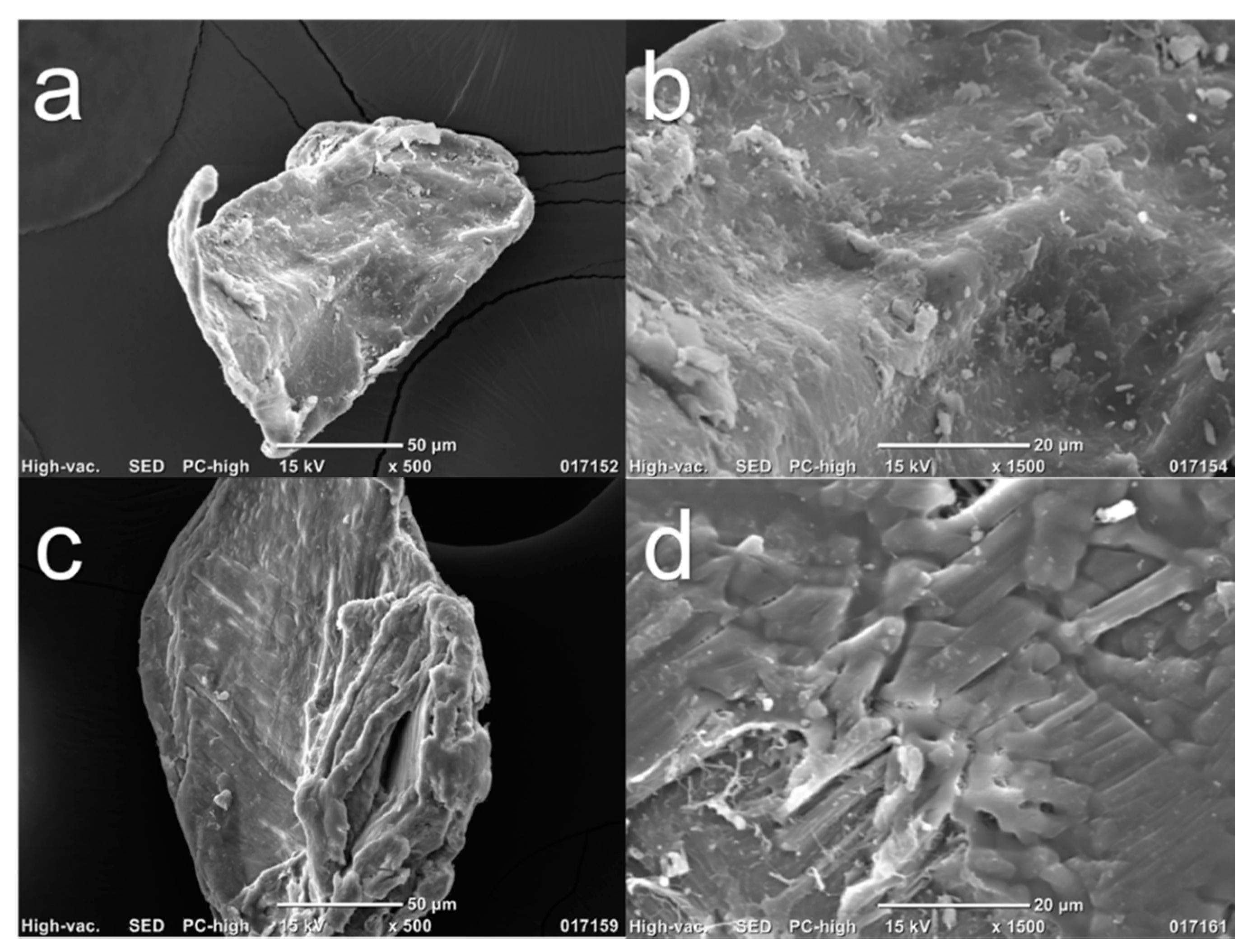
2.4.4. Scanning Electron Microscopy (SEM)
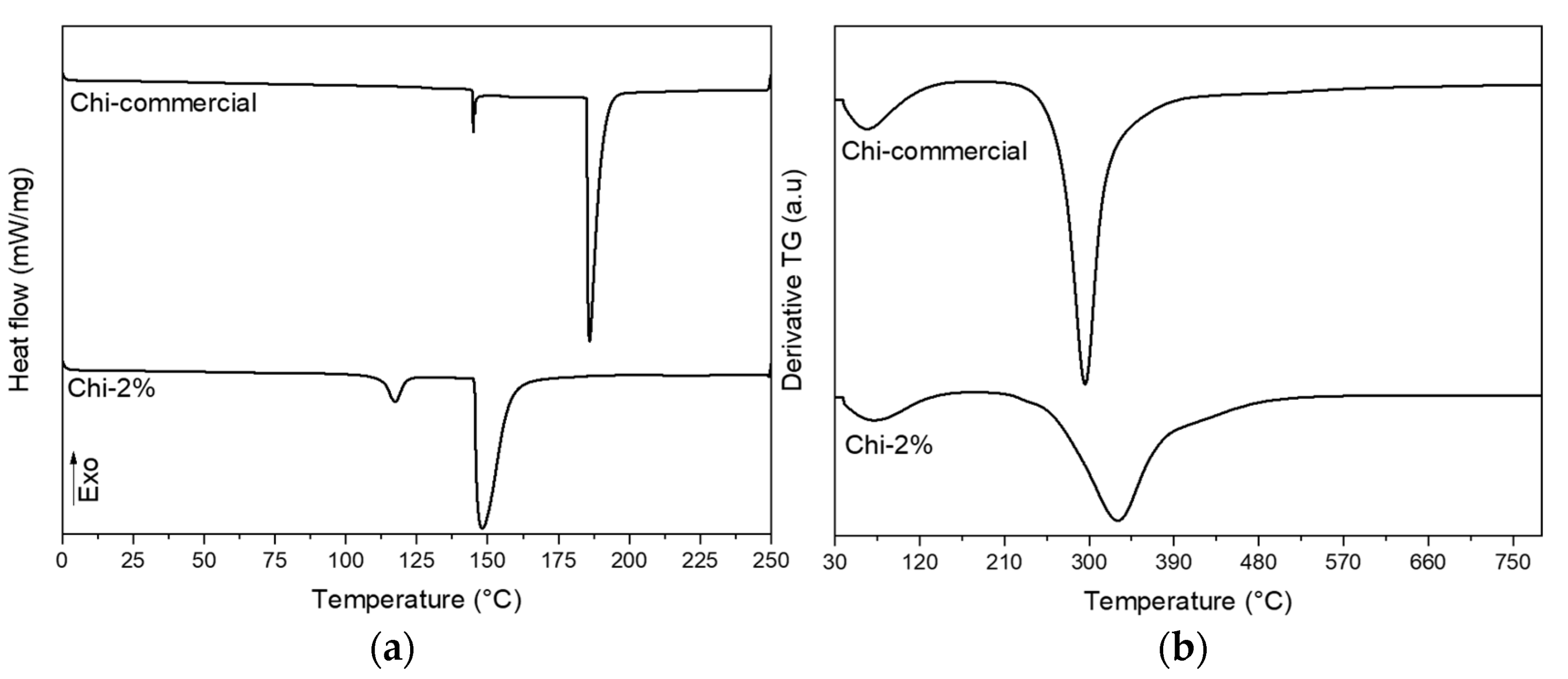
2.4.5. Thermogravimetric Analysis (TGA)
2.4.6. Differential Scanning Calorimetry (DSC)
2.4.7. Viscosity Molecular Weight
2.4.8. Deacetylation Degree
2.4.9. Evaluation of Antibacterial Effect in the Starch-Based Film
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.A.; Elsabee, M.Z. Extraction and Characterization of Chitin and Chitosan from Local Sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.; Reader, S.; Falshaw, A. Chitosan Functional Properties. Glycoconj. J. 1997, 14, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.V.S.; Torquato, L.D.M.; Cruz, G. Potential Application of Fish Scales as Feedstock in Thermochemical Processes for the Clean Energy Generation. Waste Manag. 2019, 100, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Rath, P.K. Extraction and Characterization of Chitin and Chitosan from (Labeo Rohit) Fish Scales. Procedia Mater. Sci. 2014, 6, 482–489. [Google Scholar] [CrossRef] [Green Version]
- Ural, E.; Kandirmaz, E.A. Potential of Fish Scales as a Filling Material in Surface Coating of Cellulosic Paper. J. Appl. Biomater. Funct. Mater. 2018, 16, 23–27. [Google Scholar] [CrossRef]
- Mustafiz, S. The Application of Fish Scales in Removing Heavy, Metals from Energy-Produced Waste Streams: The Role of Microbes. Energy Sources 2003, 25, 905–916. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and Chitosan: Chemistry, Properties and Applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Jaramillo-Villa, U.; Jiménez-Segura, L.F. Algunos aspectos biológicos de la población de Prochilodus magdalenae en las ciénagas de Tumaradó (Río Atrato), Colombia. Actual. Biológicas 2008, 30, 55–66. [Google Scholar]
- Berdugo, G.O.; Narváez Barandica, J.C. Genetic Diversity and Population Structure of Bocachico Prochilodus Magdalenae (Pisces, Prochilodontidae) in the Magdalena River Basin and Its Tributaries, Colombia. Genet. Mol. Biol. 2014, 37, 37–45. [Google Scholar] [CrossRef]
- Sun, K.; Li, Z.H. Preparations, Properties and Applications of Chitosan Based Nanofibers Fabricated by Electrospinning. Express Polym. Lett. 2011, 5, 342–361. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Truong, T.T.T.; Vu, T.N.; Dinh, T.D.; Pham, T.T.; Nguyen, T.A.H.; Nguyen, M.H.; Nguyen, T.D.; Yusa, S.; Pham, T.D. Adsorptive Removal of Cefixime Using a Novel Adsorbent Based on Synthesized Polycation Coated Nanosilica Rice Husk. Prog. Org. Coat. 2021, 158, 106361. [Google Scholar] [CrossRef]
- Ariffin, M.; Hassan, M.; Li, T.; Zainon Noor, Z. Coagulation and Flocculation Treatment of Wastewater in Textile Industry Using Chitosan. J. Chem. Nat. Resour. Eng. 2009, 4, 43–53. [Google Scholar]
- Leonida, M.; Ispas-Szabo, P.; Mateescu, M.A. Self-Stabilized Chitosan and Its Complexes with Carboxymethyl Starch as Excipients in Drug Delivery. Bioact. Mater. 2018, 3, 334–340. [Google Scholar] [CrossRef]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food Applications of Chitin and Chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Avery, S.V.; Singleton, I. Microbes Associated with Fresh Produce: Sources, Types and Methods to Reduce Spoilage and Contamination. Adv. Appl. Microbiol. 2019, 107, 29–82. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of Serum Proteins by Means of the Biuret Reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Rinaudo, M.; Milas, M.; Dung, P.L. Characterization of Chitosan. Influence of Ionic Strength and Degree of Acetylation on Chain Expansion. Int. J. Biol. Macromol. 1993, 15, 281–285. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Jarosińska, D.; Rokita, B.; Ulański, P.; Rosiak, J.M. Determination of Degree of Deacetylation of Chitosan—Comparison of methods. Prog. Chem. Appl. Chitin Its Deriv. 2012, 17, 5–20. [Google Scholar]
- Luchese, C.L.; Pavoni, J.M.F.; dos Santos, N.Z.; Quines, L.K.; Pollo, L.D.; Spada, J.C.; Tessaro, I.C. Effect of Chitosan Addition on the Properties of Films Prepared with Corn and Cassava Starches. J. Food Sci. Technol. 2018, 55, 2963–2973. [Google Scholar] [CrossRef]
- Lavall, R.L.; Assis, O.B.G.; Campana-Filho, S.P. β-Chitin from the Pens of Loligo Sp.: Extraction and Characterization. Bioresour. Technol. 2007, 98, 2465–2472. [Google Scholar] [CrossRef]
- Kurita, K.; Ishii, S.; Tomita, K.; Nishimura, S.-I.; Shimoda, K. Reactivity Characteristics of Squid β-Chitin as Compared with Those of Shrimp Chitin: High Potentials of Squid Chitin as a Starting Material for Facile Chemical Modifications. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 1027–1032. [Google Scholar] [CrossRef]
- Minh, N.C.; Van Hoa, N.; Trung, T.S. Preparation, Properties, and Application of Low-Molecular-Weight Chitosan. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 453–471. [Google Scholar]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan Kills Bacteria through Cell Membrane Damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef]
- Je, J.-Y.; Kim, S.-K.; Byun, H.-G.; Moon, S.-H. Antimicrobial Activity of Hetero-Chitosans and Their Oligosaccharides with Different Molecular Weights. J. Microbiol. Biotechnol. 2004, 14, 317–323. [Google Scholar]
- Varma, R.; Vasudevan, S. Extraction, Characterization, and Antimicrobial Activity of Chitosan from Horse Mussel Modiolus Modiolus. ACS Omega 2020, 5, 20224–20230. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; Analytical Techniques in the Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2004; ISBN 978-0-470-01114-0. [Google Scholar]
- Varun, T.K.; Senani, S.; Jayapal, N.; Chikkerur, J.; Roy, S.; Tekulapally, V.B.; Gautam, M.; Kumar, N. Extraction of Chitosan and Its Oligomers from Shrimp Shell Waste, Their Characterization and Antimicrobial Effect. Vet. World 2017, 10, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR Analysis of Natural and Synthetic Collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar Annamareddy, S.H.; Abanti, S.; Kumar Rath, P. Physicochemical Properties and Characterization of Chitosan Synthesized from Fish Scales, Crab and Shrimp Shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kishor, R. Chapter 1—Chitin and chitosan: Origin, properties, and applications. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. ISBN 978-0-12-817970-3. [Google Scholar]
- Kim, H.-S.; Lee, S.-H.; Eun, C.-J.; Yoo, J.; Seo, Y.-S. Dispersion of Chitosan Nanoparticles Stable over a Wide PH Range by Adsorption of Polyglycerol Monostearate. Nanomater. Nanotechnol. 2020, 10, 184798042091726. [Google Scholar] [CrossRef]
- Pereira, F.S.; Da Silva Agostini, D.L.; Job, A.E.; González, E.R.P. Thermal Studies of Chitin-Chitosan Derivatives. J. Therm. Anal. Calorim. 2013, 114, 321–327. [Google Scholar] [CrossRef]
- Bair, H.E.; Salovey, R. The Effect of Molecular Weight on the Structure and Thermal Properties of Polyethylene. J. Macromol. Sci. Part B 1969, 3, 3–18. [Google Scholar] [CrossRef]
- Kawai, T. Melting Points of Polyethylene Crystals and the Relation between Molecular Length and Chain Folding. Kolloid-Zeitschrift Zeitschrift für Polymere 1965, 201, 15–20. [Google Scholar] [CrossRef]
- Subhapradha, N.; Ramasamy, P.; Shanmugam, V.; Madeswaran, P.; Srinivasan, A.; Shanmugam, A. Physicochemical Characterisation of β-Chitosan from Sepioteuthis Lessoniana Gladius. Food Chem. 2013, 141, 907–913. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Zhu, J.F. Study on Antimicrobial Activity of Chitosan with Different Molecular Weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]

| Component | P. magdalenae Scales (mg/kg) | Chitin (mg/kg) |
|---|---|---|
| Phosphorous | 109.34 × 103 ± 8638.30 | 7.64 ± 0.61 |
| Calcium | 131.79 × 103 ± 4.372 | 1.95 × 103 ± 4.37 |
| Protein | 314.10 × 103 ± 9081.99 | 75.65 × 103 ± 1454.28 |
| Sample | Deacetylation Degree (%) | Molecular Weight (kDa) |
|---|---|---|
| Chi-2% | 94.91 ± 1.35 | 107.18 ± 24.99 |
| Chi-4% | 100.06 ± 4.60 | 134.58 ± 24.39 |
| Chi-6% | 100.99 ± 0.00 | 240.3 ± 134.02 |
| Chi-commercial (control) | 72.00 ± 2.98 | 151.11 ± 4.47 |
| Sample | TO (°C, Onset) | TP (°C, Peak) | TC (°C, Completion) | ∆H (J/g Dry Weight, Enthalpy) |
|---|---|---|---|---|
| Chi-commercial | 182.85 | 186.25 | 199.00 | 195.66 |
| Chi-2% | 144.82 | 148.42 | 170.51 | 143.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Ramírez, C.; Mazo, P.; Zuluaga, R.; Gañán, P.; Álvarez-Caballero, J. Characterization of Chitosan Extracted from Fish Scales of the Colombian Endemic Species Prochilodus magdalenae as a Novel Source for Antibacterial Starch-Based Films. Polymers 2021, 13, 2079. https://doi.org/10.3390/polym13132079
Molina-Ramírez C, Mazo P, Zuluaga R, Gañán P, Álvarez-Caballero J. Characterization of Chitosan Extracted from Fish Scales of the Colombian Endemic Species Prochilodus magdalenae as a Novel Source for Antibacterial Starch-Based Films. Polymers. 2021; 13(13):2079. https://doi.org/10.3390/polym13132079
Chicago/Turabian StyleMolina-Ramírez, Carlos, Paulina Mazo, Robin Zuluaga, Piedad Gañán, and Juan Álvarez-Caballero. 2021. "Characterization of Chitosan Extracted from Fish Scales of the Colombian Endemic Species Prochilodus magdalenae as a Novel Source for Antibacterial Starch-Based Films" Polymers 13, no. 13: 2079. https://doi.org/10.3390/polym13132079
APA StyleMolina-Ramírez, C., Mazo, P., Zuluaga, R., Gañán, P., & Álvarez-Caballero, J. (2021). Characterization of Chitosan Extracted from Fish Scales of the Colombian Endemic Species Prochilodus magdalenae as a Novel Source for Antibacterial Starch-Based Films. Polymers, 13(13), 2079. https://doi.org/10.3390/polym13132079








