Nanotechnological Manipulation of Nutraceuticals and Phytochemicals for Healthy Purposes: Established Advantages vs. Still Undefined Risks
Abstract
1. Introduction
2. Nutraceuticals, Phytochemicals, Food, and Edible Plants: A Plethora of Benefits
3. Solubility of BACs
3.1. Factors Affecting the Solubility of A Substance
3.2. Techniques and Approaches for Enhancing Solubility of BACs
- Particle Engineering Techniques (PETs)
- Formulation Approaches (FAs)
4. Nanotechnology, Nano-Formulations, and Nanomaterials
4.1. NPs-Mediated Controlled Release
4.2. Nano-Formulations Techniques Based on Nanosuspension and Nanoemulsion Approaches
4.2.1. Nanosuspension-Based Conventional Techniques
4.2.2. Nanosuspension-Based Combined Techniques
Nanoedge™ Technique
H 69 Technology
H 42 Technology
H 96 Technology
Combination Technology (CT)
4.2.3. Emulsion-Based Techniques
Nano-Emulsions (NEs) Preparation Methods
Self-Emulsifying Drug Delivery Systems (SEDDSs)
| Methods | Components | Surfactants | Crucial Factors | Stirring | High Energy Mechanical Devices | Assembling |
|---|---|---|---|---|---|---|
| Co-Surfactants | ||||||
| Co-Solvents | ||||||
| Low energy | Oil phase Water phase Additives | Yes | BAC type Emulsion properties | Mild | No | Self |
| Yes | ||||||
| Optional | ||||||
| High energy | Oil phase Water phase Additives | Yes | BAC type Emulsion properties | High | Micro-fluidizers Ultra-sonicators High-Pressure Homogenizers | By energy devices |
| Yes | ||||||
| Optional | ||||||
| UHPH [77,78] | Oil phase Water phase Additives | Yes | BAC type Emulsion properties | High | Ultra-High-Pressure Homogenizers | By energy devices |
| Yes | ||||||
| Optional |
Application of NE-Based Techniques in the Food Sector
4.3. Nanomaterials for Formulating BACs
4.3.1. The Main Types of Organic Biocompatible and Biodegradable Solid NPs (SNPs)
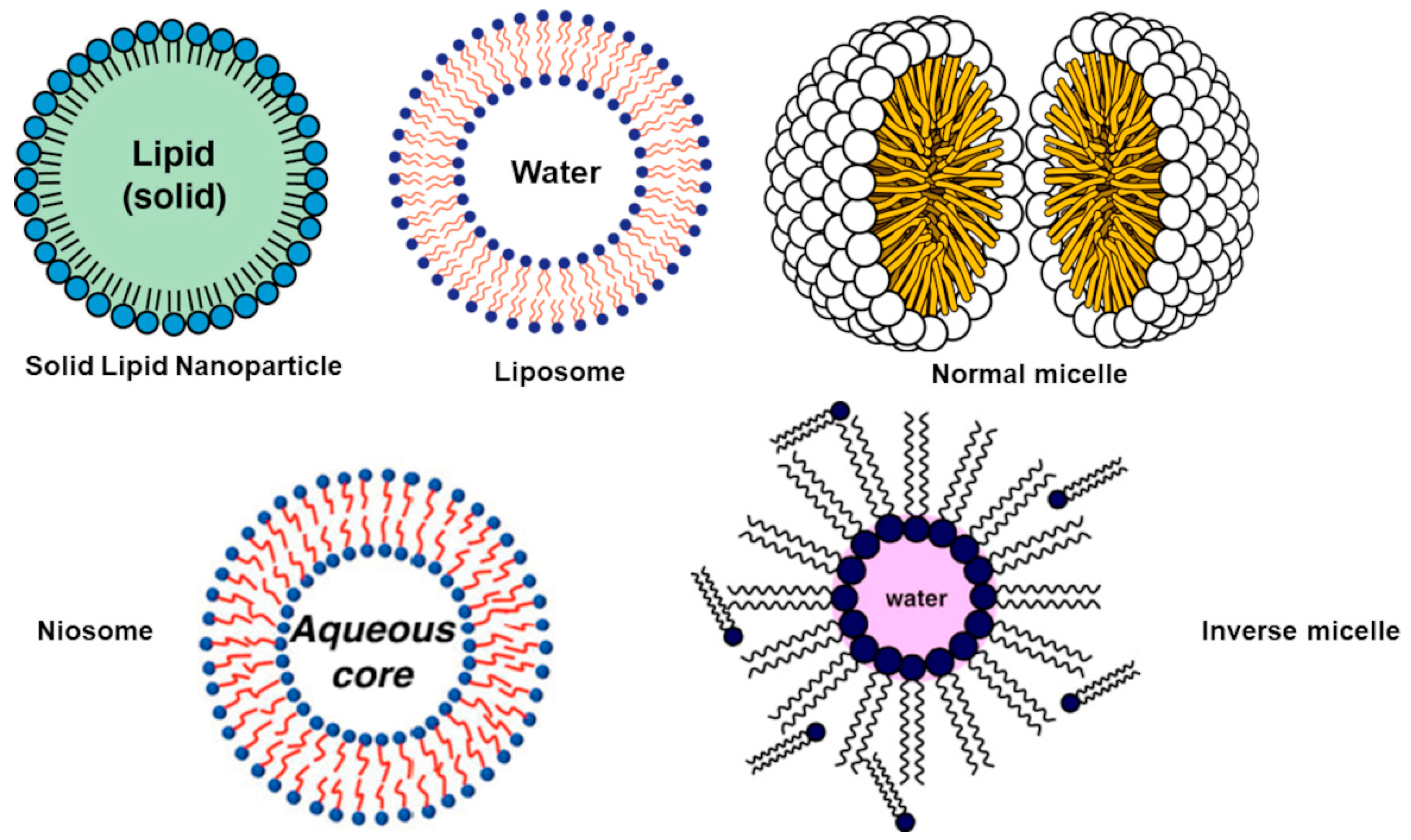
Lipid-Based Nanoparticles (LNPs)
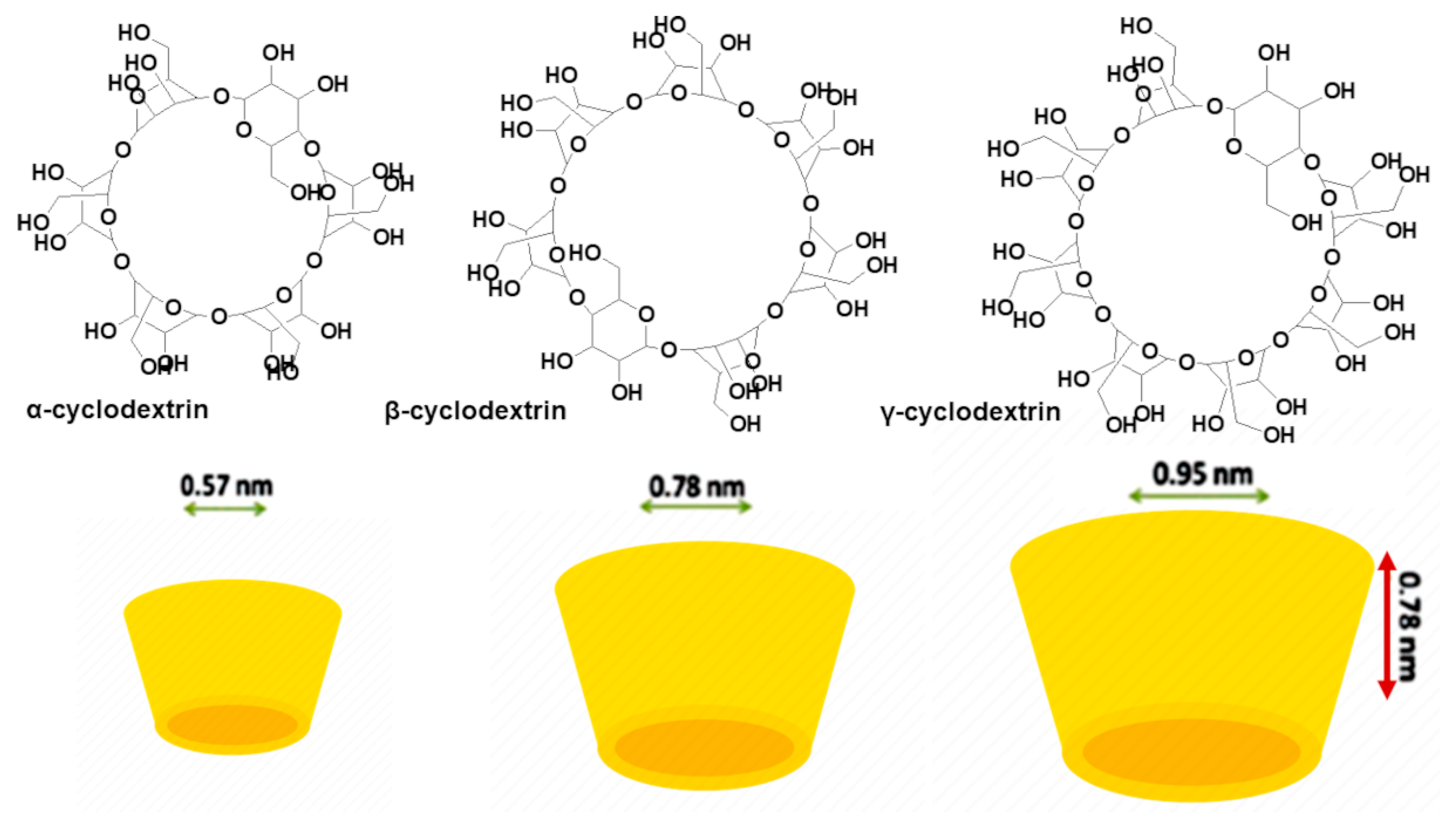
Oligosaccharide-Based NPs (ONPs): Cyclodextrins
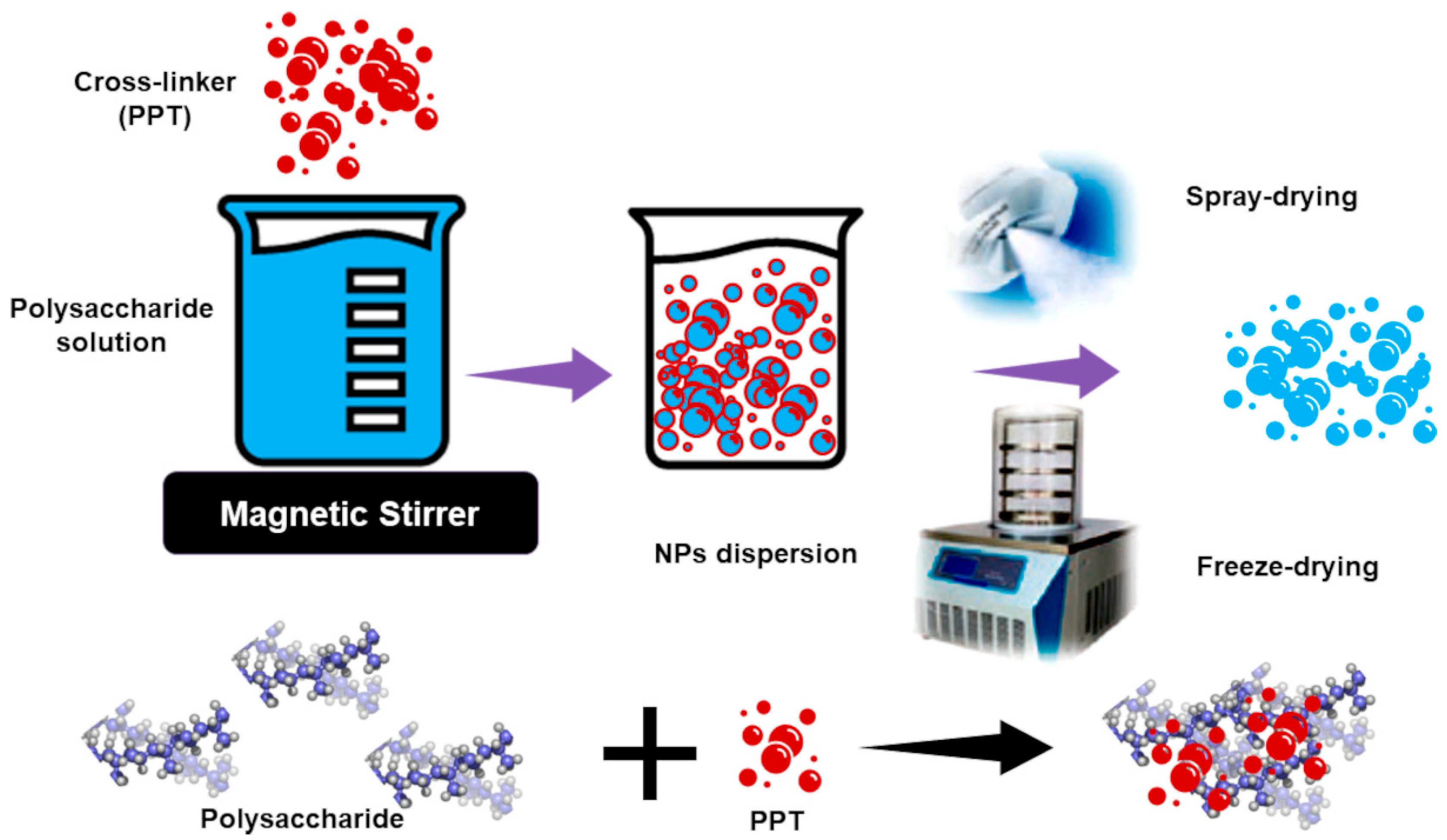
Polysaccharide-Based Nanoparticles (PNPs)
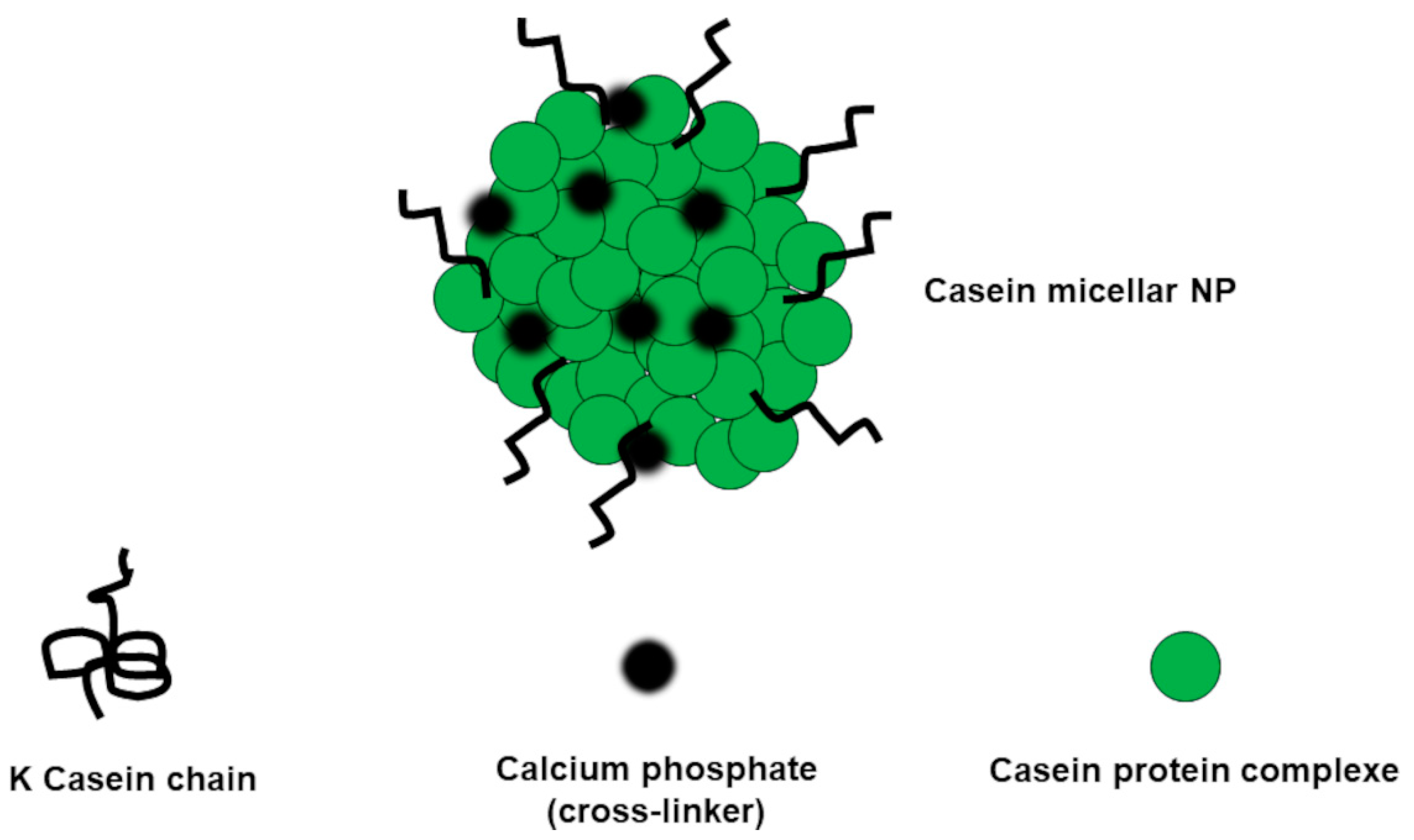
Protein-Based Nanoparticles (ProNPs)
- Simple manufacturing;
- Absence of the requirement of emulsification performance;
- Compatibility with the high-pressure emulsification processes;
- High freeze–thaw stability [152]
- Loading capacity trackable by ultraviolet (UV)-spectrophotometry, fluorescence spectrophotometry, or high-performance liquid chromatography (HPLC);
- Abundance of proteins in nature;
- Suitability to being transformed;
- Absence of strong deleterious effects on the biological systems in which they are applied;
- Susceptibility to modifications, due to the occurrence of functional groups;
- Possibility to achieve the desired biodistribution;
- Biocompatibility;
- Capacity of carrying several molecules;
- Stability.
4.3.2. Organo-Synthetic Biodegradable Polymer Nanoparticles (OBP-NPs)
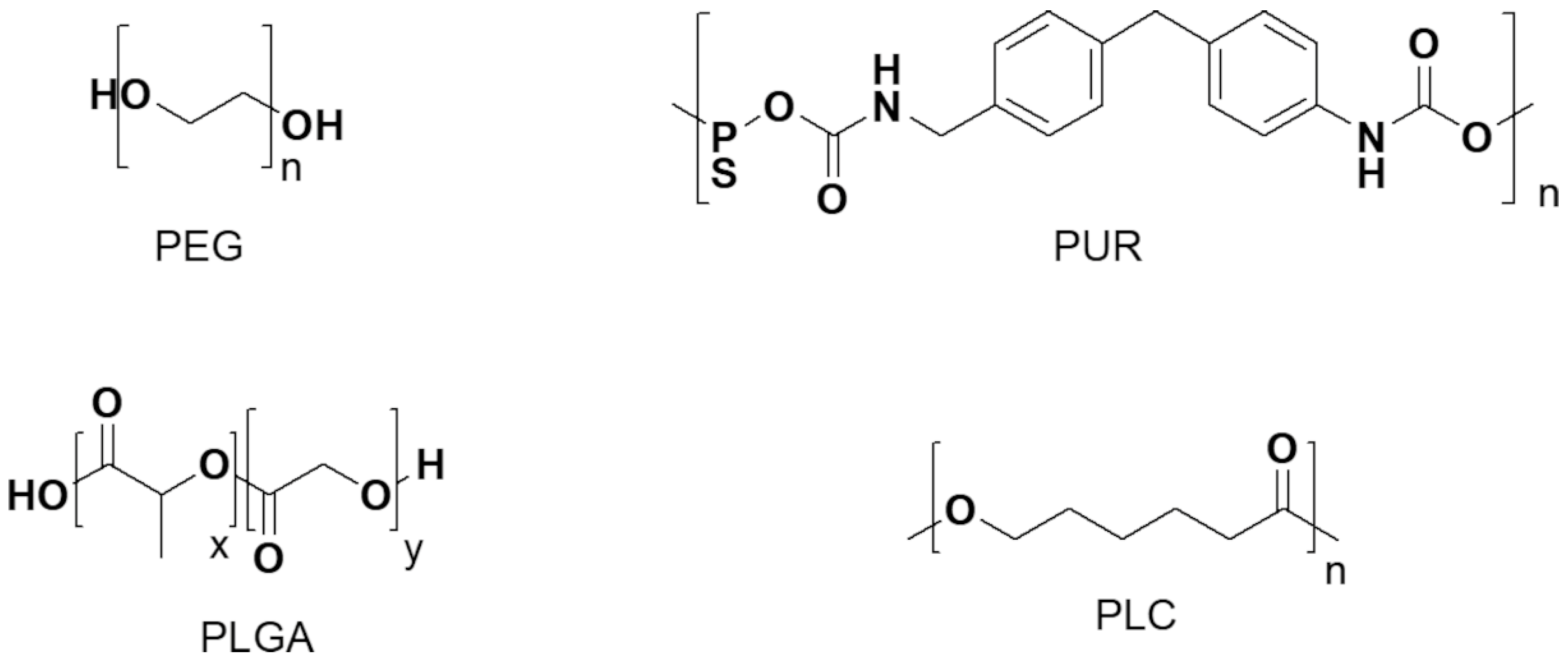
Polyethylene Glycol (PEG)
Polyurethane (PUR)
Poly-(Lactic-co-Glycolic Acid) (PLGA)
Polycaprolactone (PCL)
Applications of OBP-NPs in the Food Industry
4.3.3. Solid NPs to Exploit the Antimicrobial Properties of Essential Oils (EOs)
5. NPs Applications in Food: A Plethora of Advantages vs. Possible Risks and Limited Knowledge
5.1. About NPs Application in FP: The Possible Migration of NPs to Food
5.2. About NPs Application in Food: The Possible Toxicity of Ingesting NPs
5.3. Authors’ Considerations
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bensaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Hsieh, Y.-S.; Yang, S.-F.; Sethi, G.; Shun-Fa, Y. Natural Bioactives in Cancer Treatment and Prevention. BioMed Res. Int. 2015, 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Imm, B.-Y.; Kim, C.H.; Imm, J.-Y. Effects of Partial Substitution of Lean Meat with Pork Backfat or Canola Oil on Sensory Properties of Korean Traditional Meat Patties (Tteokgalbi). Food Sci. Anim. Resour. 2014, 34, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Bisio, A.; Milella, L.; Parricchi, A.; Alfei, S.; Vignola, L.; De Mieri, M.; Schito, A.; Hamburger, M.; De Tommasi, N. Biological activity of constituents of Salvia chamaedryoides. Plant. Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Bisio, A.; De Mieri, M.; Milella, L.; Schito, A.M.; Parricchi, A.; Russo, D.; Alfei, S.; Lapillo, M.; Tuccinardi, T.; Hamburger, M.; et al. Antibacterial and Hypoglycemic Diterpenoids from Salvia chamaedryoides. J. Nat. Prod. 2017, 80, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Jiang, Y.; Ho, C.-T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Bongoni, R.; Steenbekkers, L.; Verkerk, R.; Van Boekel, M.; Dekker, M. Studying consumer behaviour related to the quality of food: A case on vegetable preparation affecting sensory and health attributes. Trends Food Sci. Technol. 2013, 33, 139–145. [Google Scholar] [CrossRef]
- Bongoni, R.; Verkerk, R.; Steenbekkers, B.; Dekker, M.; Stieger, M. Evaluation of Different Cooking Conditions on Broccoli (Brassica oleracea var. italica) to Improve the Nutritional Value and Consumer Acceptance. Plant Foods Hum. Nutr. 2014, 69, 228–234. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef]
- Lee, H.J.; Jayasena, D.D.; Kim, S.H.; Kim, H.J.; Heo, K.N.; Song, J.E.; Jo, C. Comparison of Bioactive Compounds and Quality Traits of Breast Meat from Korean Native Ducks and Commercial Ducks. Food Sci. Anim. Resour. 2015, 35, 114–120. [Google Scholar] [CrossRef]
- Teleki, A.; Hitzfeld, A.; Eggersdorfer, M. 100 Years of Vitamins: The Science of Formulation is the Key to Functionality. KONA Powder Part. J. 2013, 30, 144–163. [Google Scholar] [CrossRef]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health Benefits of Anthocyanins and Their Encapsulation for Potential Use in Food Systems: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef]
- Xiao, J.; Cao, Y.; Huang, Q. Edible Nanoencapsulation Vehicles for Oral Delivery of Phytochemicals: A Perspective Paper. J. Agric. Food Chem. 2017, 65, 6727–6735. [Google Scholar] [CrossRef]
- Zuccari, G.; Baldassari, S.; Ailuno, G.; Turrini, F.; Alfei, S.; Caviglioli, G. Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid. Appl. Sci. 2020, 10, 3353. [Google Scholar] [CrossRef]
- Alfei, S. Nanotechnology Applications to Improve Solubility of Bioactive Constituents of Foods for Health-Promoting Purposes. In Nanofood Engineering, 1st ed.; Hebbar, U., Ranjan, S., Dasgupta, N., Mishra, R.K., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; Volume 1, pp. 189–258. [Google Scholar]
- Turrini, F.; Zunin, P.; Catena, S.; Villa, C.; Alfei, S.; Boggia, R. Traditional or hydro-diffusion and gravity microwave coupled with ultrasound as green technologies for the valorization of pomegranate external peels. Food Bioprod. Process. 2019, 117, 30–37. [Google Scholar] [CrossRef]
- Turrini, F.; Boggia, R.; Donno, D.; Parodi, B.; Beccaro, G.L.; Baldassari, S.; Signorello, M.G.; Catena, S.; Alfei, S.; Zunin, P. From pomegranate marcs to a potential bioactive ingredient: A recycling proposal for pomegranate-squeezed marcs. Eur. Food Res. Technol. 2020, 246, 273–285. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Domenicotti, C. Development of a Fast, Low-Cost, Conservative and Ecological Method for Quantifying Gallic Acid in Polymeric Formulations by FTIR Spectroscopy in Solution. Chemistryselect 2020, 5, 4381–4388. [Google Scholar] [CrossRef]
- Hamri, S.Z.; Zeghichi, M.; Chibane, M.; Kallithraka, S.; Benhalima, A. What is so special about the mediterranean diet in the maghreb? The role of economics in eating choices and chronic diseases outcomes. Ann. Nutr. Metab. 2011, 58, 191–216. [Google Scholar]
- Jeong, J.Y.; Na Park, M.; Cho, E.S.; Jang, H.-J.; Park, S.; Lee, H.-J. Epigallocatechin-3-gallate-induced free-radical production upon adipogenic differentiation in bovine bone-marrow mesenchymal stem cells. Cell Tissue Res. 2015, 362, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Jeong, S.; Lee, J.; Park, S.; Lee, J.; Lee, S. Effect of shortening replacement with oleogels on the rheological and tomographic characteristics of aerated baked goods. J. Sci. Food Agric. 2017, 97, 3727–3732. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chou, C.-W.; Kumar, K.S.; Fu, K.-T.; Wang, H.-M.; Hsu, L.-S.; Kuo, Y.-H.; Wu, C.-R.; Chen, S.-C.; Yang, H.-L. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem. Toxicol. 2012, 50, 1245–1255. [Google Scholar] [CrossRef]
- V, A.; Kp, M. Ellagic Acid as a Potential Anti-Cancer Drug. Int. J. Radiol. Radiat. Ther. 2017, 3, 1–3. [Google Scholar] [CrossRef][Green Version]
- Larrosa, M.; Conesa, M.T.G.; Espín, J.C.; Tomás-Barberán, F.A. Ellagitannins, ellagic acid and vascular health. Mol. Asp. Med. 2010, 31, 513–539. [Google Scholar] [CrossRef]
- Nejad, K.H.; Dianat, M.; Sarkaki, A. Ellagic acid improves electrocardiogram waves and blood pressure against global cerebral ischemia rat experimental models. Electron. Phys. 2015, 7, 1153–1162. [Google Scholar] [CrossRef]
- Hoseinynejad, K.; Gharib-Naseri, M.K.; Sarkaki, A.; Dianat, M.; Badavi, M.; Farbood, Y. Effects of ellagic acid pretreatment on renal functions disturbances induced by global cerebral ischemic-reperfusion in rat. Iran J. Basic Med. Sci. 2017, 20, 75–82. [Google Scholar] [CrossRef]
- Firdaus, F.; Zafeer, M.F.; Waseem, M.; Anis, E.; Hossain, M.M.; Afzal, M. Ellagic acid mitigates arsenic-trioxide-induced mitochondrial dysfunction and cytotoxicity in SH-SY5Y cells. J. Biochem. Mol. Toxicol. 2018, 32, e22024. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Li, M.; Yin, R. Phytochemical Content, Health Benefits, and Toxicology of Common Edible Flowers: A Review (2000–2015). Crit. Rev. Food Sci. Nutr. 2016, 56, S130–S148. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Review of dried fruits: Phytochemicals, antioxidant efficacies, and health benefits. J. Funct. Foods 2016, 21, 113–132. [Google Scholar] [CrossRef]
- Son, Y.-R.; Choi, E.-H.; Kim, G.-T.; Park, T.-S.; Shim, S.-M. Bioefficacy of Graviola leaf extracts in scavenging free radicals and upregulating antioxidant genes. Food Funct. 2016, 7, 861–871. [Google Scholar] [CrossRef]
- Vico, T.A.; Arce, V.B.; Fangio, M.F.; Gende, L.B.; Bertran, C.A.; Mártire, D.O.; Churio, M.S. Two choices for the functionalization of silica nanoparticles with gallic acid: Characterization of the nanomaterials and their antimicrobial activity against Paenibacillus larvae. J. Nanoparticle Res. 2016, 18, 348. [Google Scholar] [CrossRef]
- Pu, S.-B.; Ma, Z.-J.; Wang, Q. Anti-Staphylococcus aureus evaluation of gallic acid by isothermal microcalorimetry and principle component analysis. J. Therm. Anal. Calorim. 2018, 136, 1425–1432. [Google Scholar] [CrossRef]
- Díaz-Gómez, R.; Toledo-Araya, H.; López-Solís, R.; Obreque-Slier, E. Combined effect of gallic acid and catechin against Escherichia coli. LWT 2014, 59, 896–900. [Google Scholar] [CrossRef]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial activity of gallic acid against food-related Pseudomonas strains and its use as biocontrol tool to improve the shelf life of fresh black truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef]
- Daduang, J.; Klaynongsruang, S.; Leelayuwat, C.; Limpaiboon, T.; Lulitanond, A.; Boonsiri, P.; Srichan, S.; Soontaranon, S.; Rugmai, S.; Rattanata, N. Gallic acid conjugated with gold nanoparticles: Antibacterial activity and mechanism of action on foodborne pathogens. Int. J. Nanomed. 2016, ume 11, 3347–3356. [Google Scholar] [CrossRef]
- Kim, K.; Bae, O.-N.; Lim, K.-M.; Noh, J.-Y.; Kang, S.; Chung, K.Y.; Chung, J.-H. Novel Antiplatelet Activity of Protocatechuic Acid through the Inhibition of High Shear Stress-Induced Platelet Aggregation. J. Pharmacol. Exp. Ther. 2012, 343, 704–711. [Google Scholar] [CrossRef]
- Thoppil, R.J. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J. Hepatol. 2011, 3, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Vemula, V.R.; Lagishetty, V.; Lingala, S. Solubility enhancement techniques. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 41–51. [Google Scholar]
- Sivakumar, M.; Tang, S.Y.; Tan, K.W. Cavitation technology–A greener processing technique for the generation of pharmaceutical nanoemulsions. Ultrason. Sonochem. 2014, 21, 2069–2083. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Hashimoto, Y. Improvement in Aqueous Solubility in Small Molecule Drug Discovery Programs by Disruption of Molecular Planarity and Symmetry. J. Med. Chem. 2011, 54, 1539–1554. [Google Scholar] [CrossRef]
- Feriyanto, D.; Idris, M.; Sebayang, D. Effect of Cr to Fe on the Solid Solubility, Lattice Parameter and Strain of Fe80Cr20 Alloy Powder. Appl. Mech. Mater. 2014, 660, 280–284. [Google Scholar] [CrossRef]
- Jadhav, P.B.; Pandey, P.S. Phase solubility analysis: A technique of purity determination. World Res. J. Pharm. Res. 2013, 1, 5–11. [Google Scholar]
- Ravve, A. Free-radical chain-growth polymerization. In Principles of Polymer Chemistry, 2nd ed.; Springer: New York, NY, USA, 2013; p. 41102. ISBN1 9781461368984. ISBN2 9781461542278. [Google Scholar]
- Raza, K. Polymorphism: The Phenomenon Affecting the Performance of Drugs. SOJ Pharm. Pharm. Sci. 2014, 1, 1–10. [Google Scholar] [CrossRef]
- Kale, B.B.; Aloorkar, N.H.; Deshmukh, S.M.; Sulake, S.P.; Humbe, P.V.; Mane, P.P. Recent advancements in particle engi-neering techniques for pharmaceutical applications. Indo. Am. J. Pharm. Res. 2014, 4, 2027–2049. [Google Scholar]
- Koshy, P.; Pacharane, S.; Chaudhry, A.; Jadhav, K.; Kadam, V. Drug particle engineering of poorly water soluble drugs. Der. Pharm. Lett. 2010, 2, 65–76. [Google Scholar]
- Morales, J.O.; Watts, A.B.; McConville, J.T. Mechanical particle-size reduction techniques. In Formulating Poorly Water Soluble Drugs; Williams, R.O., III, Watts, A.B., Miller, D.A., Eds.; Springer: New York, NY, USA, 2016; pp. 133–170. ISBN 9781461411444. [Google Scholar]
- He, X.; Deng, H.; Hwang, H.-M. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef]
- Avachat, A.M.; Patel, V.G. Self nanoemulsifying drug delivery system of stabilized ellagic acid–phospholipid complex with improved dissolution and permeability. Saudi Pharm. J. 2015, 23, 276–289. [Google Scholar] [CrossRef]
- Madrigal-Carballo, S.; Lim, S.; Rodriguez, G.; Vila, A.O.; Krueger, C.G.; Gunasekaran, S.; Reed, J.D. Biopolymer coating of soybean lecithin liposomes via layer-by-layer self-assembly as novel delivery system for ellagic acid. J. Funct. Foods 2010, 2, 99–106. [Google Scholar] [CrossRef]
- Hajipour, H.; Hamishehkar, H.; Rahmati-Yamchi, M.; Shanehbandi, D.; Ahmad, S.N.S.; Hasani, A. Enhanced Anti-Cancer Capability of Ellagic Acid Using Solid Lipid Nanoparticles (SLNs). Int. J. Cancer Manag. 2018, 11. [Google Scholar] [CrossRef]
- Bulani, V.D.; Kothavade, P.S.; Kundaikar, H.; Gawali, N.B.; Chowdhury, A.A.; Degani, M.S.; Juvekar, A.R. Inclusion complex of ellagic acid with β-cyclodextrin: Characterization and in vitro anti-inflammatory evaluation. J. Mol. Struct. 2016, 1105, 308–315. [Google Scholar] [CrossRef]
- Mady, F.; Ibrahim, S.R.-M. Cyclodextrin-based nanosponge for improvement of solubility and oral bioavailability of Ellagic acid. Pak. J. Pharm. Sci. 2018, 31, 2069–2076. [Google Scholar] [PubMed]
- Alfei, S.; Turrini, F.; Catena, S.; Zunin, P.; Parodi, B.; Zuccari, G.; Pittaluga, A.M.; Boggia, R. Preparation of ellagic acid micro and nano formulations with amazingly increased water solubility by its entrapment in pectin or non-PAMAM dendrimers suitable for clinical applications. New J. Chem. 2019, 43, 2438–2448. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K.; Liu, B.; Feng, S.-S. A strategy for precision engineering of nanoparticles of biodegradable copolymers for quantitative control of targeted drug delivery. Biomaterials 2010, 31, 9145–9155. [Google Scholar] [CrossRef]
- Sha, X.; Guo, J.; Chen, Y.; Fang, X. Effect of phospholipid composition on pharmacokinetics and biodistribution of epirubicin liposomes. J. Liposome Res. 2011, 22, 80–88. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B.; Drechsler, M.; Lesieur, S. Neurotrophin delivery using nanotechnology. Drug Discov. Today 2013, 18, 1263–1271. [Google Scholar] [CrossRef]
- De Paz, E.; Martín, Á; Estrella, A.; Rodríguez-Rojo, S.; Matias, A.A.; Duarte, C.M.; Cocero, M.J. Formulation of β-carotene by precipitation from pressurized ethyl acetate-on-water emulsions for application as natural colorant. Food Hydrocoll. 2012, 26, 17–27. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Huang, Q. Quercetin Nanosuspensions Produced by High-Pressure Homogenization. J. Agric. Food Chem. 2014, 62, 1852–1859. [Google Scholar] [CrossRef]
- Campardelli, R.; Reverchon, E. α-Tocopherol nanosuspensions produced using a supercritical assisted process. J. Food Eng. 2015, 149, 131–136. [Google Scholar] [CrossRef]
- Agrawal, Y.K.; Patel, V.R. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar] [CrossRef]
- Ezhilarasi, P.N.; Karthik, P.; Chhanwal, N.; Anandharamakrishnan, C. Nanoencapsulation Techniques for Food Bioactive Components: A Review. Food Bioprocess Technol. 2013, 6, 628–647. [Google Scholar] [CrossRef]
- Salazar, J.; Müller, R.H.; Möschwitzer, J.P. Combinative Particle Size Reduction Technologies for the Production of Drug Nanocrystals. J. Pharm. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, I.; Bose, S.; Vippagunta, R.; Harmon, F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int. J. Pharm. 2011, 409, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Lakshimi, P.; Kumar, G.A. Nanosuspension technology: A review. Int. J. Pharm. 2010, 409, 260–269. [Google Scholar]
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Precipitation followed by high pressure homogenization as a combinative approach to prepare drug nanocrystals. In Tag der Pharmazie; Abstract V2, Booklet Page 6; FU Berlin: Berlin, Germany, 2012. [Google Scholar]
- Liu, T.; Müller, R.H.; Möschwitzer, J.P. Process optimization of a novel particle size reduction technology H 42. In Tag der Pharmazie; Abstract P33, Booklet Page 25; FU Berlin: Berlin, Germany, 2012. [Google Scholar]
- Al Shaal, L.; Müller, R.H.; Shegokar, R. SmartCrystal Combination Technology–Scale up from Lab to Pilot Scale and Long Term Stability. Die Pharm. Int. J. Pharm. Sci. 2010, 65, 877–884. [Google Scholar] [CrossRef]
- McClements, D.; Li, Y. Review of in vitro digestion models for rapid screening of emulsion-based systems. Food Funct. 2010, 1, 32–59. [Google Scholar] [CrossRef]
- Chime, S.A.; Kenechukwu, F.C.; Attama, A.A. Nanoemulsions–Advances in Formulation, Characterization and Applications in Drug Delivery; IntechOpen: London, UK, 2014; ISBN 9789535116288. [Google Scholar]
- Odriozola-Serrano, I.; Oms-Oliu, G.; Martã n-Belloso, O. Nanoemulsion-Based Delivery Systems to Improve Functionality of Lipophilic Components. Front. Nutr. 2014, 1, 24. [Google Scholar] [CrossRef]
- Qian, C.; McClements, D.J. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: Factors affecting particle size. Food Hydrocoll. 2011, 25, 1000–1008. [Google Scholar] [CrossRef]
- Lee, D.R.; Ho, M.J.; Choi, Y.W.; Kang, M.J. A Polyvinylpyrrolidone-Based Supersaturable Self-Emulsifying Drug Delivery System for Enhanced Dissolution of Cyclosporine A. Polymers 2017, 9, 124. [Google Scholar] [CrossRef]
- Magnuson, B.A.; Jonaitis, T.S.; Card, J.W. A Brief Review of the Occurrence, Use, and Safety of Food-Related Nanomaterials. J. Food Sci. 2011, 76, R126–R133. [Google Scholar] [CrossRef]
- Zamora, A.; Guamis, B. Opportunities for Ultra-High-Pressure Homogenisation (UHPH) for the Food Industry. Food Eng. Rev. 2015, 7, 130–142. [Google Scholar] [CrossRef]
- Singh, B.; Beg, S.; Khurana, R.K.; Sandhu, P.S.; Kaur, R.; Katare, O.P. Recent advances in self-emulsifying drug delivery systems (SEDDS). Crit. Rev. Ther. Drug Carr. Syst. 2014, 31, 121–185. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, G.; Xia, Q.; Sun, R. Development and characterization of a self-double-emulsifying drug delivery system containing both epigallocatechin-3-gallate and α-lipoic acid. J. Mater. Sci. 2015, 50, 6567–6577. [Google Scholar] [CrossRef]
- Simion, V.; Stan, D.; Constantinescu, C.A.; Deleanu, M.; Dragan, E.; Tucureanu, M.M.; Gan, A.M.; Butoi, E.; Constantin, A.; Manduteanu, I.; et al. Conjugation of curcumin-loaded lipid nanoemulsions with cell-penetrating peptides increases their cellular uptake and enhances the anti-inflammatory effects in endothelial cells. J. Pharm. Pharmacol. 2016, 68, 195–207. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Koutelidakis, A.E.; Argyri, K.; Sevastou, Z.; Lamprinaki, D.; Panagopoulou, E.; Paximada, E.; Sali, A.; Papalazarou, V.; Mallouchos, A.; Evageliou, V.; et al. Bioactivity of Epigallocatechin Gallate Nanoemulsions Evaluated in Mice Model. J. Med. Food 2017, 20, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and Controlled Delivery for Bioactive Compounds: Outlining Challenges for New “Smart-Foods” for Health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Chow, P.Y.; Gue, S.Z.; Leow, S.K.; Goh, L.B. Solid self-microemulsifying system (S-SMECS) for enhanced bioavailability and pigmentation of highly lipophilic bioactive carotenoid. Powder Technol. 2015, 274, 199–204. [Google Scholar] [CrossRef]
- Yoo, J.H.; Shanmugam, S.; Thapa, P.; Lee, E.-S.; Balakrishnan, P.; Baskaran, R.; Yoon, S.-K.; Choi, H.-G.; Yong, C.S.; Yoo, B.K.; et al. Novel self-nanoemulsifying drug delivery system for enhanced solubility and dissolution of lutein. Arch. Pharm. Res. 2010, 33, 417–426. [Google Scholar] [CrossRef]
- Shanmugam, S.; Baskaran, R.; Balakrishnan, P.; Thapa, P.; Yong, C.S.; Yoo, B.K. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) containing phosphatidylcholine for enhanced bioavailability of highly lipophilic bioactive carotenoid lutein. Eur. J. Pharm. Biopharm. 2011, 79, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, J.; Xiao, H.; Mcclements, D. Nanoemulsion-Based Delivery Systems for Poorly Water-Soluble Bioactive Com-pounds: Influence of Formulation Parameters on Polymethoxyflavone Crystallization. Food Hydrocoll. 2012, 272, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.; Decker, E.; Xiao, H.; McClements, D.J. Nanoemulsion delivery systems: Influence of carrier oil on β-carotene bioaccessibility. Food Chem. 2012, 135, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.V.A.; Kim, S.; Choi, Y.; Kwak, H.-S.; Lee, S.J.; Wen, J.; Oey, I.; Ko, S. Antioxidant activity and bioaccessibility of size-different nanoemulsions for lycopene-enriched tomato extract. Food Chem. 2015, 178, 115–121. [Google Scholar] [CrossRef]
- Tran, T.H.; Guo, Y.; Song, D.; Bruno, R.; Lu, X. Quercetin-Containing Self-Nanoemulsifying Drug Delivery System for Improving Oral Bioavailability. J. Pharm. Sci. 2014, 103, 840–852. [Google Scholar] [CrossRef]
- Baccarin, T.; Lemos-Senna, E. Pomegranate Seed Oil Nanoemulsions Encapsulating Pomegranate Peel Polyphenol-Rich Ethyl Acetate Fraction: Development and Antioxidant Assessment. J. Nanopharm. Drug Deliv. 2014, 2, 333–343. [Google Scholar] [CrossRef]
- Kim, I.-H.; Lee, H.; Kim, J.E.; Bin Song, K.; Lee, Y.S.; Chung, D.S.; Min, S.C. Plum Coatings of Lemongrass Oil-incorporating Carnauba Wax-based Nanoemulsion. J. Food Sci. 2013, 78, E1551–E1559. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Xiao, J.; Nian, S.; Huang, Q. Assembly of kafirin/carboxymethyl chitosan nanoparticles to enhance the cellular uptake of curcumin. Food Hydrocoll. 2015, 51, 166–175. [Google Scholar] [CrossRef]
- Kumar, V.D.; Verma, P.R.P.; Singh, S.K. Development and evaluation of biodegradable polymeric nanoparticles for the effective delivery of quercetin using a quality by design approach. LWT 2015, 61, 330–338. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q.; Baek, S.J. Enhanced Dispersibility and Bioactivity of Curcumin by Encapsulation in Casein Nanocapsules. J. Agric. Food Chem. 2013, 61, 6036–6043. [Google Scholar] [CrossRef]
- Hu, B.; Ting, Y.; Yang, X.; Tang, W.; Zeng, X.; Huang, Q. Nanochemoprevention by encapsulation of (−)-epigallocatechin-3-gallate with bioactive peptides/chitosan nanoparticles for enhancement of its bioavailability. Chem. Commun. 2012, 48, 2421–2423. [Google Scholar] [CrossRef]
- Zou, L.; Zheng, B.; Zhang, R.; Zhang, Z.; Liu, W.; Liu, C.; Xiao, H.; McClements, D.J. Food-grade nanoparticles for encapsulation, protection and delivery of curcumin: Comparison of lipid, protein, and phospholipid nanoparticles under simulated gastrointestinal conditions. RSC Adv. 2015, 6, 3126–3136. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Donsì, F.; Senatore, B.; Huang, Q.; Ferrari, G. Development of Novel Pea Protein-Based Nanoemulsions for Delivery of Nutraceuticals. J. Agric. Food Chem. 2010, 58, 10653–10660. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Ma, P.X. Synthetic biodegradable functional polymers for tissue engineering: A brief review. Sci. China Ser. B Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Castellaro, S.; Taptue, G.B. Synthesis and NMR characterization of dendrimers based on 2, 2-bis-(hydroxymethyl)-propanoic acid (bis-HMPA) containing peripheral amino acid residues for gene transfection. Org. Commun. 2017, 10, 144–177. [Google Scholar] [CrossRef]
- Alfei, S.; Castellaro, S. Synthesis and characterization of polyester-based dendrimers containing peripheral arginine or mixed amino acids as potential vectors for gene and drug delivery. Macromol. Res. 2017, 25, 1172–1186. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S. Synthesis and characterization of versatile amphiphilic dendrimers peripherally decorated with positively charged amino acids. Polym. Int. 2018, 67, 1572–1584. [Google Scholar] [CrossRef]
- Alfei, S.; Catena, S. Synthesis and characterization of fourth generation polyester-based dendrimers with cationic amino acids-modified crown as promising water soluble biomedical devices. Polym. Adv. Technol. 2018, 29, 2735–2749. [Google Scholar] [CrossRef]
- Zuccari, G.; Carosio, R.; Fini, A.; Montaldo, P.; Orienti, I. Modified polyvinylalcohol for encapsulation of all-trans-retinoic acid in polymeric micelles. J. Control. Release 2005, 103, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, G.; Bergamante, V.; Carosio, R.; Gotti, R.; Montaldo, P.; Orienti, I. Micellar complexes of all-trans retinoic acid with polyvinylalcohol-nicotinoyl esters as new parenteral formulations in neuroblastoma. Drug Deliv. 2009, 16, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Zuccari, G.; Baldassari, S.; Alfei, S.; Marengo, B.; Valenti, G.; Domenicotti, C.; Ailuno, G.; Villa, C.; Marchitto, L.; Caviglioli, G. D-α-Tocopherol-Based Micelles for Successful Encapsulation of Retinoic Acid. Pharmaceuticals 2021, 14, 212. [Google Scholar] [CrossRef]
- Alfei, S.; Taptue, G.B.; Catena, S.; Bisio, A. Synthesis of Water-soluble, Polyester-based Dendrimer Prodrugs for Exploiting Therapeutic Properties of Two Triterpenoid Acids. Chin. J. Polym. Sci. 2018, 36, 999–1010. [Google Scholar] [CrossRef]
- Schito, A.; Schito, G.; Alfei, S. Synthesis and Antibacterial Activity of Cationic Amino Acid-Conjugated Dendrimers Loaded with a Mixture of Two Triterpenoid Acids. Polymers 2021, 13, 521. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer Nanodevices and Gallic Acid as Novel Strategies to Fight Chemoresistance in Neuroblastoma Cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef]
- Alfei, S.; Oliveri, P.; Malegori, C. Assessment of the Efficiency of a Nanospherical Gallic Acid Dendrimer for Long-Term Preservation of Essential Oils: An Integrated Chemometric-Assisted FTIR Study. ChemistrySelect 2019, 4, 8891–8901. [Google Scholar] [CrossRef]
- Alfei, S.; Signorello, M.G.; Schito, A.; Catena, S.; Turrini, F. Reshaped as polyester-based nanoparticles, gallic acid inhibits platelet aggregation, reactive oxygen species production and multi-resistant Gram-positive bacteria with an efficiency never obtained. Nanoscale Adv. 2019, 1, 4148–4157. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Neethirajan, S.; Jayas, D.S. Nanotechnology for the Food and Bioprocessing Industries. Food Bioprocess Technol. 2011, 4, 39–47. [Google Scholar] [CrossRef]
- Bengoechea, C.; Jones, O.G.; Guerrero, A.; McClements, D. Formation and characterization of lactoferrin/pectin electrostatic complexes: Impact of composition, pH and thermal treatment. Food Hydrocoll. 2011, 25, 1227–1232. [Google Scholar] [CrossRef]
- Berton-Carabin, C.; Coupland, J.N.; Elias, R. Effect of the lipophilicity of model ingredients on their location and reactivity in emulsions and solid lipid nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 431, 9–17. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Pinheiro, A.C.; Silva, H.; Ramos, P.E.; Azevedo, M.; López, M.L.F.; Rivera, M.C.; Bourbon, A.I.; Ramos, Ó.L.; Vicente, A.A. Design of Bio-nanosystems for Oral Delivery of Functional Compounds. Food Eng. Rev. 2014, 6, 1–19. [Google Scholar] [CrossRef]
- Kaminskas, L.; Boyd, B.J.; Porter, C. Dendrimer pharmacokinetics: The effect of size, structure and surface characteristics on ADME properties. Nanomedicine 2011, 6, 1063–1084. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Pardeshi, C.; Rajput, P.; Belgamwar, V.; Tekade, A.; Patil, G.; Chaudhary, K.; Sonje, A. Solid lipid based nanocarriers: An overview. Acta Pharm. 2012, 62, 433–472. [Google Scholar] [CrossRef] [PubMed]
- Mashaghi, S.; Jadidi, T.; Koenderink, G.; Mashaghi, A. Lipid Nanotechnology. Int. J. Mol. Sci. 2013, 14, 4242–4282. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.; Park, J.W.; Yoon, I.-S. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): Recent advances in drug delivery. J. Pharm. Investig. 2013, 43, 353–362. [Google Scholar] [CrossRef]
- Thatipamula, R.; Palem, C.; Gannu, R.; Mudragada, S.; Yamsani, M.R. Formulation and in vitro characterization of domperidone loaded solid lipid nanoparticles and nanostructured lipid carriers. DARU J. Pharm. Sci. 2011, 19, 23–32. [Google Scholar]
- Gong, J.; Chen, M.; Zheng, Y.; Wang, S.; Wang, Y. Polymeric micelles drug delivery system in oncology. J. Control. Release 2012, 159, 312–323. [Google Scholar] [CrossRef]
- Chhipa, H. Chapter 6—Applications of Nanotechnology in Agriculture. In Methods in Microbiology; Gurtler, V., Ball, A.S., Soni, S., Eds.; Academic Press: New York, NY, USA, 2019; Volume 46, pp. 115–142. ISBN 0580-9517. [Google Scholar]
- Junyaprasert, V.B.; Singhsa, P.; Suksiriworapong, J.; Chantasart, D. Physicochemical properties and skin permeation of Span 60/Tween 60 niosomes of ellagic acid. Int. J. Pharm. 2012, 423, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Patil, J.S.; Kadam, D.V.; Marapur, S.C.; Kamalapur, M.V. Inclusion complex system; a novel technique to improve the solubility and bioavailability of poorly soluble drugs: A review. Int. J. Pharm. Sci. Rev. Res. 2010, 2, 29–34. [Google Scholar]
- Recharla, N.; Riaz, M.; Ko, S.; Park, S. Novel technologies to enhance solubility of food-derived bioactive compounds: A review. J. Funct. Foods 2017, 39, 63–73. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Petito, N.D.L.; Dias, D.D.S.; Costa, V.G.; Falcão, D.Q.; Araujo, K.G.D.L. Increasing solubility of red bell pepper carotenoids by complexation with 2-hydroxypropyl-β-cyclodextrin. Food Chem. 2016, 208, 124–131. [Google Scholar] [CrossRef]
- Nerome, H.; Machmudah, S.; Diono, W.; Fukuzato, R.; Higashiura, T.; Youn, Y.-S.; Lee, Y.-W.; Goto, M. Nanoparticle formation of lycopene/β-cyclodextrin inclusion complex using supercritical antisolvent precipitation. J. Supercrit. Fluids 2013, 83, 97–103. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols–A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Joye, I.J.; Nelis, V.; McClements, D. Gliadin-based nanoparticles: Stabilization by post-production polysaccharide coating. Food Hydrocoll. 2015, 43, 236–242. [Google Scholar] [CrossRef]
- Yu, H.; Park, J.-Y.; Kwon, C.W.; Hong, S.-C.; Park, K.-M.; Chang, P.-S. An Overview of Nanotechnology in Food Science: Preparative Methods, Practical Applications, and Safety. J. Chem. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Nitta, S.K.; Numata, K. Biopolymer-Based Nanoparticles for Drug/Gene Delivery and Tissue Engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef]
- Salatin, S.; Jelvehgari, M. Natural Polysaccharide based Nanoparticles for Drug/Gene Delivery. Pharm. Sci. 2017, 23, 84–94. [Google Scholar] [CrossRef]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef]
- Duceppe, N.; Tabrizian, M. Advances in using chitosan-based nanoparticles for in vitro and in vivo drug and gene delivery. Expert Opin. Drug Deliv. 2010, 7, 1191–1207. [Google Scholar] [CrossRef] [PubMed]
- Ojea-Jiménez, I.; Tort, O.; Lorenzo, J.; Puntes, V.F. Engineered Nonviral Nanocarriers for Intracellular Gene Delivery Appli-cations. Biomed. Mater. 2012, 7, 054106. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-S.; Kim, S.-K.; Ahn, C.-B.; Je, J.-Y. Preparation, Characterization, and Antioxidant Properties of Gallic Ac-id-Grafted-Chitosans. Carbohydr. Polym. 2011, 83, 1617–1622. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Ramana, L.N.; Sethuraman, S.; Krishnan, U.M. Ellagic acid encapsulated chitosan nanoparticles as anti-hemorrhagic agent. Carbohydr. Polym. 2014, 111, 215–221. [Google Scholar] [CrossRef]
- Rocha, D.S.; Casagrande, L.; Model, J.F.A.; Dos Santos, J.T.; Hoefel, A.L.; Kucharski, L.C. Effect of yerba mate (Ilex paraguariensis) extract on the metabolism of diabetic rats. Biomed. Pharmacother. 2018, 105, 370–376. [Google Scholar] [CrossRef]
- Boggia, R.; Turrini, F.; Roggeri, A.; Olivero, G.; Cisani, F.; Bonfiglio, T.; Summa, M.; Grilli, M.; Caviglioli, G.; Alfei, S.; et al. Neuroinflammation in Aged Brain: Impact of the Oral Administration of Ellagic Acid Microdispersion. Int. J. Mol. Sci. 2020, 21, 3631. [Google Scholar] [CrossRef] [PubMed]
- Miladi, K.; Ibraheem, D.; Iqbal, M.; Sfar, S.; Fessi, H.; Elaissari, A. Particles from Preformed Polymers as Carriers for Drug De-livery. EXCLI J. 2014, 13, 28–57. [Google Scholar] [PubMed]
- Tarhini, M.; Greige-Gerges, H.; Elaissari, A. Protein-based nanoparticles: From preparation to encapsulation of active molecules. Int. J. Pharm. 2017, 522, 172–197. [Google Scholar] [CrossRef]
- Zhu, X.-F.; Zheng, J.; Liu, F.; Qiu, C.-Y.; Lin, W.-F.; Tang, C.-H. The influence of ionic strength on the characteristics of heat-induced soy protein aggregate nanoparticles and the freeze–thaw stability of the resultant Pickering emulsions. Food Funct. 2017, 8, 2974–2981. [Google Scholar] [CrossRef]
- Ruan, J.; Yang, Y.; Yang, F.; Wan, K.; Fan, D.; Wang, D. Novel oral administrated ellagic acid nanoparticles for enhancing oral bioavailability and anti-inflammatory efficacy. J. Drug Deliv. Sci. Technol. 2018, 46, 215–222. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef]
- Rajput, S.D.; Mahulikar, P.P.; Gite, V.V. Biobased dimer fatty acid containing two pack polyurethane for wood finished coatings. Prog. Org. Coat. 2014, 77, 38–46. [Google Scholar] [CrossRef]
- Nohra, B.; Candy, L.; Blanco, J.-F.; Guerin, C.; Raoul, Y.; Mouloungui, Z. From Petrochemical Polyurethanes to Biobased Polyhydroxyurethanes. Macromolecules 2013, 46, 3771–3792. [Google Scholar] [CrossRef]
- Guidance for the Use of PURs in Food Contact Applications. Available online: https://polyurethane.americanchemistry.com/Resources-and-Document-Library/10355.pdf (accessed on 20 June 2021).
- Kumari, A.; Singla, R.; Guliani, A.; Yadav, S.K. Nanoencapsulation for drug delivery. EXCLI J. 2014, 13, 265–286. [Google Scholar] [PubMed]
- Boulle, F.; Kenis, G.; Cazorla, M.; Hamon, M.; Steinbusch, H.W.; Lanfumey, L.; Hove, D.L.V.D. TrkB inhibition as a therapeutic target for CNS-related disorders. Prog. Neurobiol. 2012, 98, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Mady, F.M.; A Shaker, M. Enhanced anticancer activity and oral bioavailability of ellagic acid through encapsulation in biodegradable polymeric nanoparticles. Int. J. Nanomed. 2017, 12, 7405–7417. [Google Scholar] [CrossRef]
- Mo, J.; Kaewnopparat, N.; Songkro, S.; Panichayupakaranant, P.; Reanmongkol, W. Physicochemical properties, in vitro release and skin permeation studies of a topical formulation of standardized pomegranate rind extract. Pak. J. Pharm. Sci. 2015, 28, 29–36. [Google Scholar]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Songkro, S.; Reanmongkol, W. Topical Anti-inflammatory Potential of Standardized Pomegranate Rind Extract and Ellagic Acid in Contact Dermatitis. Phytother. Res. 2013, 28, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Abd-Rabou, A.A.; Ahmed, H.H. CS-PEG decorated PLGA nano-prototype for delivery of bioactive compounds: A novel approach for induction of apoptosis in HepG2 cell line. Adv. Med Sci. 2017, 62, 357–367. [Google Scholar] [CrossRef]
- Amin, F.U.; Shah, S.A.; Badshah, H.; Khan, M.; Kim, M.O. Anthocyanins encapsulated by PLGA@PEG nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Aβ1-42-induced oxidative stress. J. Nanobiotechnol. 2017, 15, 12. [Google Scholar] [CrossRef]
- Jiménez-Aguilar, D.; Ortega-Regules, A.; Lozada-Ramírez, J.; Pérez-Pérez, M.; Vernon-Carter, E.J.; Welti-Chanes, J. Color and chemical stability of spray-dried blueberry extract using mesquite gum as wall material. J. Food Compos. Anal. 2011, 24, 889–894. [Google Scholar] [CrossRef]
- Li, L.; Li, W.; Jung, S.W.; Lee, Y.W.; Kim, Y.H. Protective effects of decursin and decursinol angelate against amy-loid-protein-induced oxidative stress in the PC12 cell line: The role of Nrf2 and antioxidant enzymes. Biosci. Biotechnol. Biochem. 2011, 75, 434–442. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, Z.; Kessler, M.R.; Brehm-Stecher, B.; LaRock, R.C. Antibacterial Soybean-Oil-Based Cationic Polyurethane Coatings Prepared from Different Amino Polyols. ChemSusChem 2012, 5, 2221–2227. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Zuccari, G. Nanotechnology application in food packaging: A plethora of opportunities versus pending risks assessment and public concerns. Food Res. Int. 2020, 137, 109664. [Google Scholar] [CrossRef] [PubMed]
- Enescu, D.; Cerqueira, M.A.; Fuciños, P.; Pastrana, L. Recent advances and challenges on applications of nanotechnology in food packaging. A literature review. Food Chem. Toxicol. 2019, 134, 110814. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Ko, S. Nano-Food Packaging: An Overview of Market, Migration Research, and Safety Regulations. J. Food Sci. 2015, 80, R910–R923. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Katiyar, V.; Plackett, D.; Larsen, E.H.; Gerds, N.; Koch, C.B.; Petersen, J.H. Migration of nanosized layered double hydroxide platelets from polylactide nanocomposite films. Food Addit. Contam. Part A 2011, 28, 956–966. [Google Scholar] [CrossRef]
- Bott, J.; Störmer, A.; Franz, R. Migration of nanoparticles from plastic packaging materials containing carbon black into foodstuffs. Food Addit. Contam. Part A 2014, 31, 1769–1782. [Google Scholar] [CrossRef]
- Gallocchio, F.; Cibin, V.; Biancotto, G.; Roccato, A.; Muzzolon, O.; Carmen, L.; Simone, B.; Manodori, L.; Fabrizi, A.; Patuzzi, I.; et al. Testing nano-silver food packaging to evaluate silver migration and food spoilage bacteria on chicken meat. Food Addit. Contam. Part A 2016, 33, 1063–1071. [Google Scholar] [CrossRef]
- Tiimob, B.J.; Mwinyelle, G.; Abdela, W.; Samuel, T.; Jeelani, S.; Rangari, V.K. Nanoengineered Eggshell–Silver Tailored Copolyester Polymer Blend Film with Antimicrobial Properties. J. Agric. Food Chem. 2017, 65, 1967–1976. [Google Scholar] [CrossRef]
- Su, Q.-Z.; Lin, Q.-B.; Chen, C.-F.; Wu, L.-B.; Wang, Z.-W. Effect of organic additives on silver release from nanosilver–polyethylene composite films to acidic food simulant. Food Chem. 2017, 228, 560–566. [Google Scholar] [CrossRef]
- Han, W.; Yu, Y.; Li, N.; Wang, L. Application and safety assessment for nano-composite materials in food packaging. Chin. Sci. Bull. 2011, 56, 1216–1225. [Google Scholar] [CrossRef]
- Maisanaba, S.; Pichardo, S.; Puerto, M.; Gutiérrez-Praena, D.; Cameán, A.M.; Jos, A. Toxicological evaluation of clay minerals and derived nanocomposites: A review. Environ. Res. 2015, 138, 233–254. [Google Scholar] [CrossRef]
- Fukushima, S.; Kasai, T.; Umeda, Y.; Ohnishi, M.; Sasaki, T.; Matsumoto, M. Carcinogenicity of multi-walled carbon nanotubes: Challenging issue on hazard assessment. J. Occup. Health 2018, 60, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Becaro, A.A.; Siqueira, M.C.; Puti, F.C.; De Moura, M.R.; Correa, D.; Marconcini, J.M.; Mattoso, L.H.C.; Ferreira, M.D. Cytotoxic and genotoxic effects of silver nanoparticle/carboxymethyl cellulose on Allium cepa. Environ. Monit. Assess. 2017, 189, 352. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Martucci, N.; Liu, Y.; Yoo, E.; Tako, E.; Mahler, G.J. Silicon dioxide nanoparticle exposure affects small intestine function in an in vitro model. Nanotoxicology 2018, 12, 485–508. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Senapati, V.A.; Gupta, G.S.; Pandey, A.K.; Shanker, R.; Dhawan, A.; Kumar, A. Zinc oxide nanoparticle induced age dependent immunotoxicity in BALB/c mice. Toxicol. Res. 2017, 6, 342–352. [Google Scholar] [CrossRef]
- Ansar, S.; Abudawood, M.; Hamed, S.S.; Aleem, M.M. Exposure to Zinc Oxide Nanoparticles Induces Neurotoxicity and Proinflammatory Response: Amelioration by Hesperidin. Biol. Trace Elem. Res. 2017, 175, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Olivas, F.; Tako, E.; Mahler, G.J. Retracted Article: ZnO nanoparticles affect intestinal function in anin vitromodel. Food Funct. 2018, 9, 1475–1491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, R.; Xiao, H.; Bhattacharya, K.; Bitounis, D.; Demokritou, P.; McClements, D.J. Development of a Stand-ardized Food Model for Studying the Impact of Food Matrix Effects on the Gastrointestinal Fate and Toxicity of Ingested Nanomaterials. NanoImpact 2019, 13, 13–25. [Google Scholar] [CrossRef]
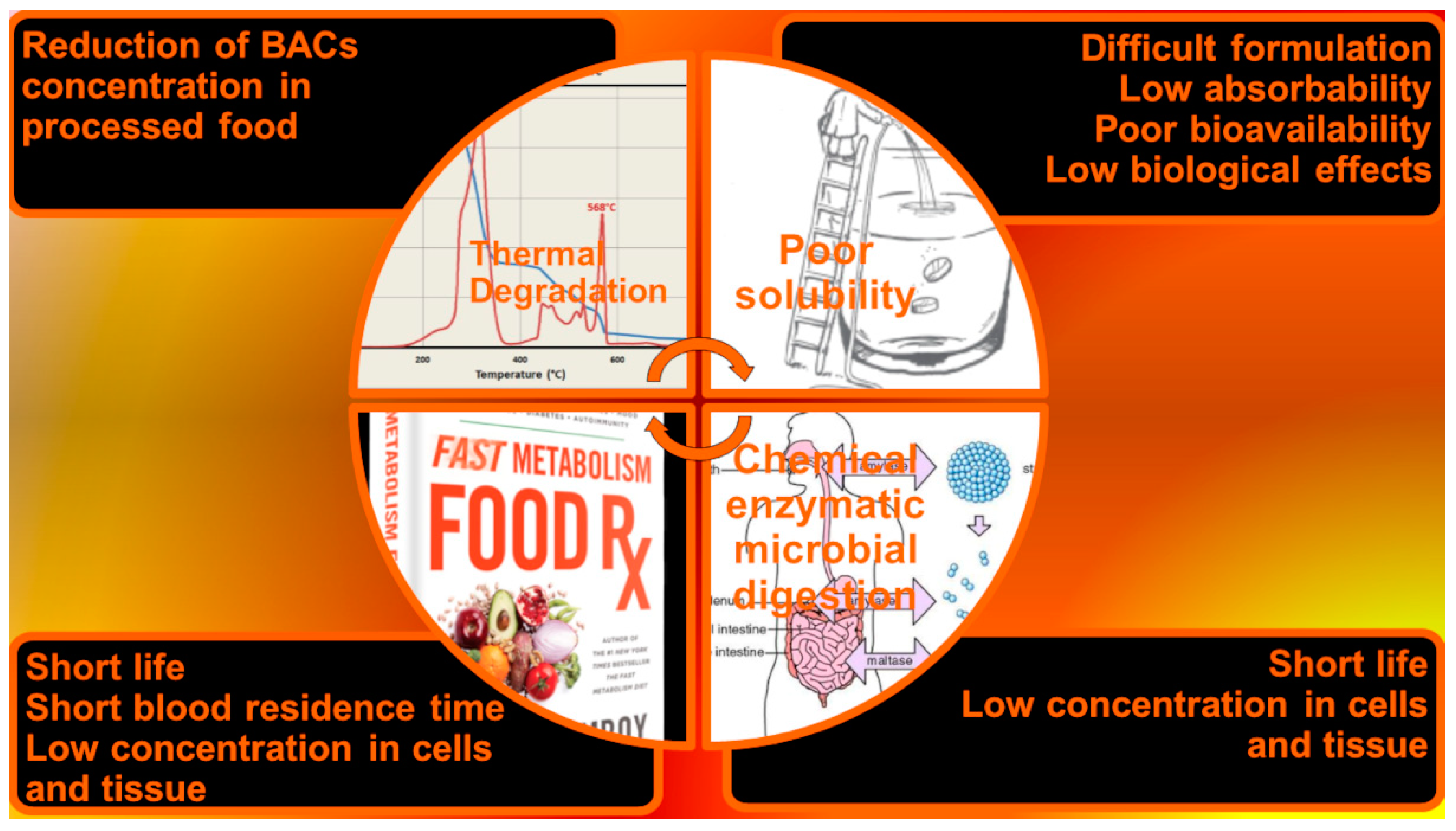
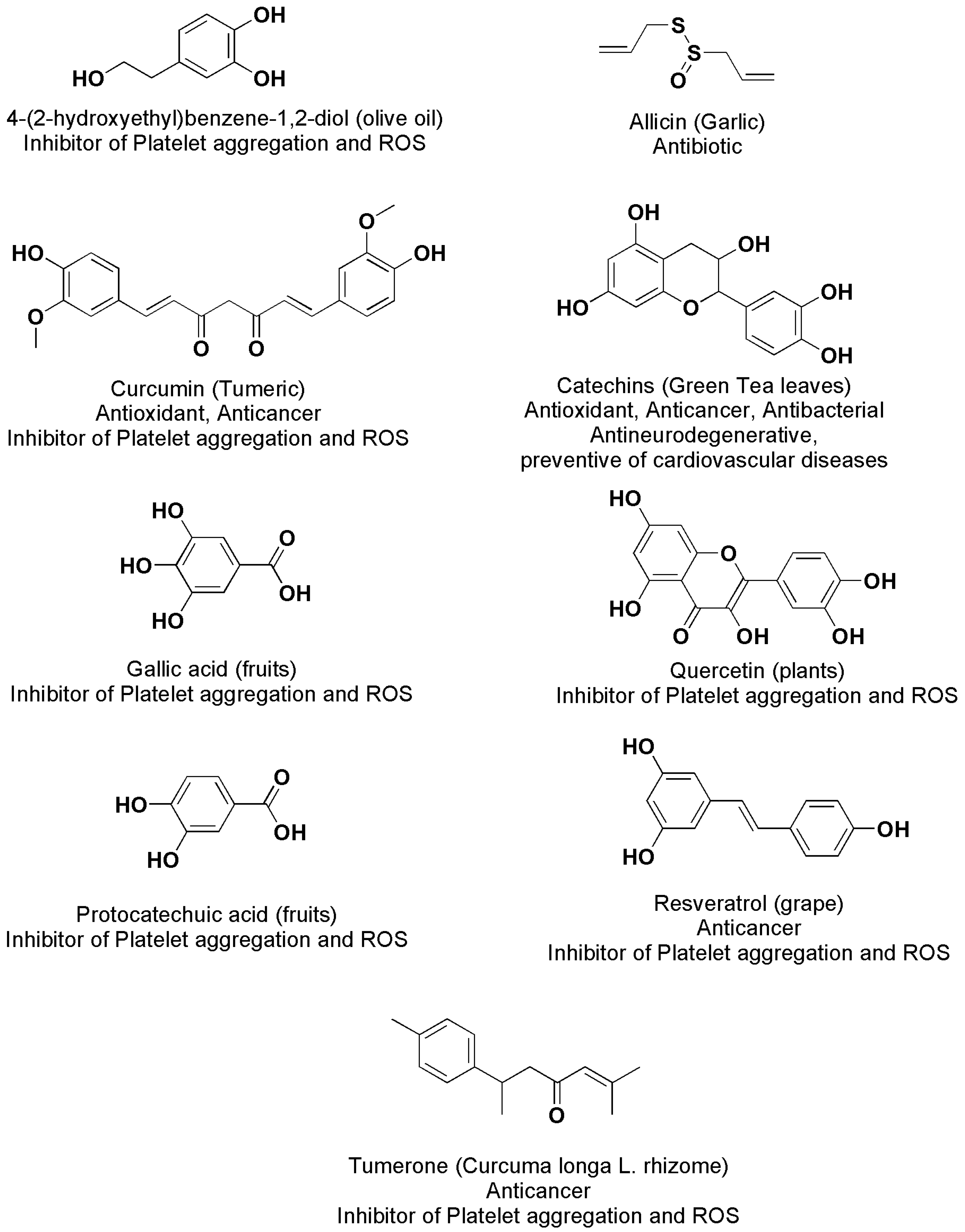

| Chemical Category | Description | Source | Nutritional Function | Healthy Properties |
|---|---|---|---|---|
| Carotenoids | Plant pigments Lipid nature | Carrots, pumpkins, melon Apricots, tomatoes Watermelons, peppers, Spinach, cabbage, parsley | Vitaminic activity (retinol) | Photoprotective agents Antioxidants Immune system reinforcers  precancerous disease progress precancerous disease progress |
| Hydro-soluble vitamins (HVs) Liposoluble vitamins (LV) | Hydro-soluble/Liposoluble Organic Compounds | Fruits, vegetables, fish Meat, eggs, dairy products Milk | Health of cells, organ, tissues Promote the use of the the energy supplied by food | Prevention of many pathologies Treatment of post-operative debilitation, Treatment of severe stress Bone reinforcement |
| Phytosterols | Saturated/not saturated sterols and stanols | Plants, cereal products Vegetables, fruit, berries Vegetable oils | N.P. | ↧ LDL cholesterol |
| Polyunsaturated lipids | Unsaturated long chain fatty acids | Fatty fish, plant-based oils Seeds, nuts | Supply calories ↑ absorption of LVs Provide essential nutrients | Crucial for brain development and function ↧ Age-related mental decline |
| Curcuminoids | Linear diarylheptanoids | Turmeric, curry powder Mango, ginger | N.P. | Analgesic, anti-inflammatory Anti-cancer, antioxidative Anti-depressive Against hay fever, depression ↧ Cholesterol and itching risk |
| Polyphenols | Flavonoids Tannic acid Ellagitannins Phenolic acids | Fruits, vegetables, grains bark, roots, stems, flowers Tea, wine | N.P. | Anti-oxidative, anti-inflammatory, Anti-mutagenic, anti-carcinogenic Modulate cellular enzyme functions |
| Indole compounds (indole-3-carbinol) | Organic compounds containing the indole structure | Cabbage, cauliflower Broccoli, kale Brussels sprouts | N.P. | Strong antioxidant, DNA protector Chemo-preventive, anti-cancer ↑ Heart health |
| Alkaloids | Basic organic compounds containing at least one nitrogen atom | Bacteria, fungi, plants Animals | N.P. | Antimalarial, antiasthma, anticancer Cholinomimetics, vasodilatory Antiarrhythmic, analgesic Antibacterial, antihyperglycemic Psychotropic and stimulant activities |
| Phytoprostanes Phytofurans | Oxylipins synthesized by the oxidation of α-linolenic acid | Almonds, vegetal oils Olives, algae, passion fruit Nut kernels, rice | N.P. | Immunomodulators, anti-inflammatory Anti-tumors |
 = stop at; ↑= increasing; ↧ = decreasing.
= stop at; ↑= increasing; ↧ = decreasing.| Factor | Explanation | Reference |
|---|---|---|
| Particle size Shape | Unsymmetrical, ↧↧↧ small size particles with ↑ surface area dissolve better and more quickly | [42] |
| Temperature | ↑ temperatures promote dissolution | [43] |
| Molecular Weight (MW) | ↑ MW = ↧ its solubility | [44,45] |
| Chemical structure | ↑ amount branching in carbon chains = ↑ solubility Branched polymers are ↑ soluble of linear ones with = MW Branched-chain molecules have ↧ volume/dimension ratio in solution and ↑ dissolution rate | [44,45] |
| Molecular Polarity | Polar solvents dissolve polar solutes Non-polar solvents dissolve non-polar substances | N.M. |
| Physical form | Molecules arranged in amorphous forms possess ↑ aqueous solubility than the crystalline ones Different polymorphs have ≠ solubility | [46] |
| pH of the medium | Weak acids and weak bases ionize in solution Ionized forms have ↑ solubility in water | N.M. |
| Presence of surface-active compounds with a hydrophilic head and a lipophilic tail | ↧ the interfacial tension between the oil and water interface = ↑ both phases solubility | [15] |
| Techniques | Goal | Strategies | Methods | Reference | |
| PETs | BACs with modified physicochemical properties | ↧↧↧↧ particles size No stabilizers No surfactants | Mechanical particle-size reduction Wet-milling, dry-milling High-pressure homogenization Ultra-high-pressure homogenization | [47,48] | |
| Cryogenic particle engineering Nanoprecipitation Nanosuspension Supercritical fluid processing | [47,49] | ||||
| Freeze-drying, spray-freezing | |||||
| FAs | Solid formulations Lipid formulations | Use of mixture of water/oil phases, stabilizers, solvents/co-solvents | Spray-drying Milling | [15] |
| Approach | Technique Type | Instruments | Advantages | Limitations |
|---|---|---|---|---|
| Solvents | ||||
| Additives/Polymers | ||||
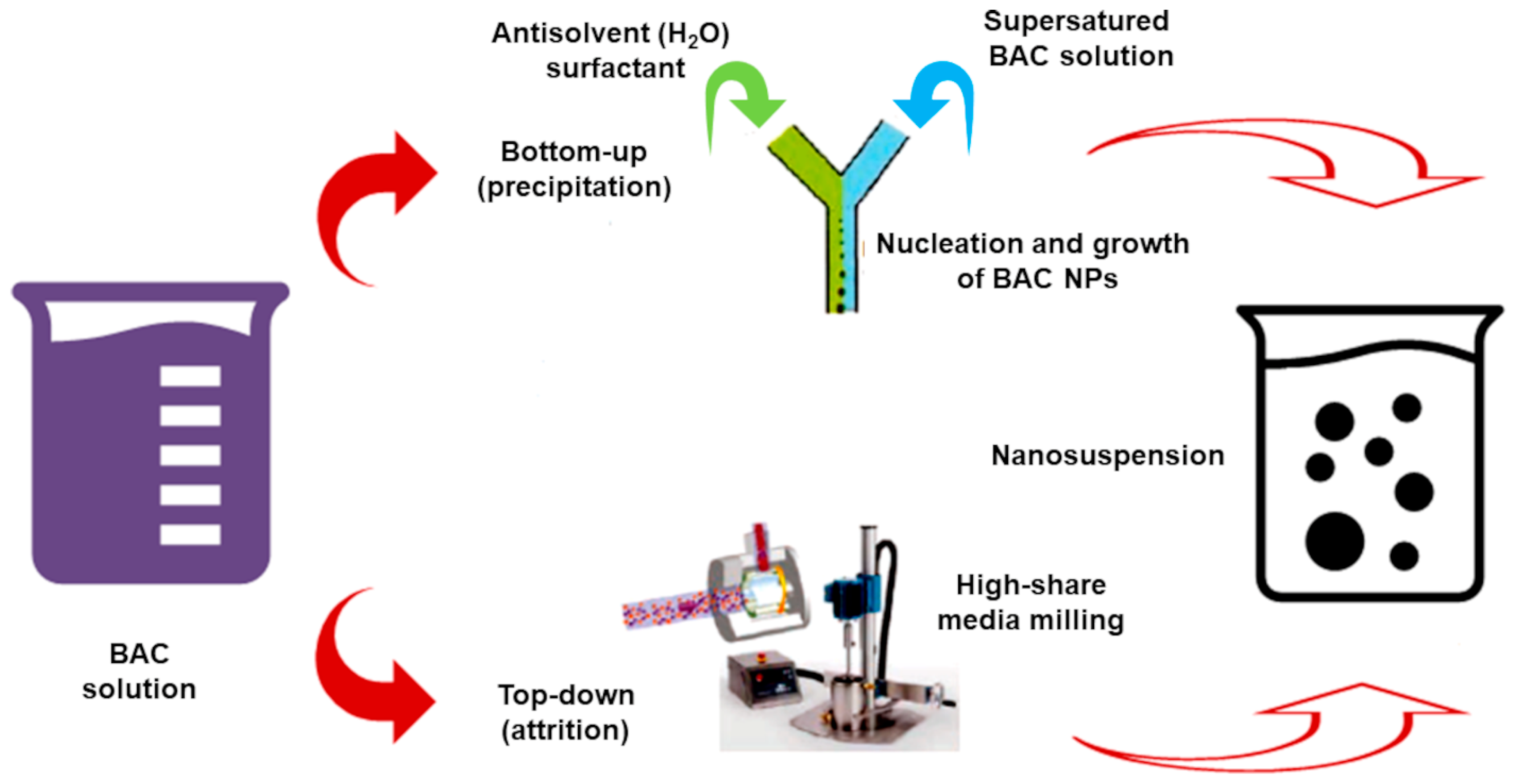
| Bottom-up | Nanoprecipitation (solvent/anti-solvent) [64] | Mixer (pre-grinding) | Nanosized particles (Ps) Simple ↓ Cost equipment | Only for BACs soluble in organic solvents Residual organic solvents |
| Organic solvent Water (anti-solvent) | ||||
| Surfactants | ||||
| Bottom-up | Supercritical fluid extraction (SFE) | Syringe or diaphragm CO2 pumps | Nanosized Ps ↑ Selectivity ↑ Speedy | ↑ Costs |
| Solvents/co-solvents | ||||
| No | ||||
| Bottom-up | Inclusion complexation (IC) [65] 1 | No | Nanosized Ps Masking of odors/flavors Aroma’s preservation ↑ EE% ↑ Stability | For few materials |
| Organic solvent, water | ||||
| β-cyclodextrin β-lactogloglobulin | ||||
| Bottom-up | Coacervation [65] | No | Nanosized Ps ↑↑↑ payloads (99%) Controlled release Sustained release | ↑↑↑ Influencing variables |
| Organic/aqueous solvents | ||||
| Polymers Chemicals/enzymatic cross-linkers (glutaraldehyde, transglutaminase) [62] | ||||
| Top-down (dissocubes) | HPH at r.t. [15] | Piston-gap homogenizers | Nanosized Ps No material erosion For solving both organic and aqueous solubility drawbacks | Pre-processing micronization Thermic degradation ↑ Cost instruments |
| Aqueous media | ||||
| Surfactants | ||||
| Top-down (Nanopure®) | Deep-freeze Homogenization | Piston-gap homogenizers | Nanosized Ps No crystals grow ↓ Operation times ↑ Stability | Pre-micronization ↑ Cost instruments |
| Non aqueous media Water with water-miscible liquids | ||||
| PEG-400, PEG-1000 | ||||
| Top-down (IDD-P) 2 [15] | Jet stream Homogenization [66] | Z-type or Y-type Collision chamber | Nanosized Ps No crystals grow ↓ Operation times ↑ Stability | Pre-micronization Thermic degradation ↑ Cost instruments |
| Aqueous media | ||||
| Phospholipids, surfactants, stabilizers | ||||
| Top-down | Media milling technique collision [67] | High-shear media mills/Pearl mills | Nanosized Ps ↓ Batch-to-batch variation | ↑ Operation times Thermic degradation |
| Aqueous media | ||||
| Excipients |
| BACs | Method | Surfactant(s) | Results | Reference |
|---|---|---|---|---|
| Carotenoids (Paprika Oleoresin) | Solid self-microemulsifying carotenoid system (S-SMECS) | Tween 80 | ↑ Solubility | [85] |
| Lutein | SMEDDS | Tween 80 Labrasol TranscutolHP/Lutro-E400 1 | ↑ Solubility ↑ Bioavailability | [86,87] |
| Polymethoxyflavones (PMFs) | HPH (NEs-based) | Tween 20 Tween 85 | ↑ Dissolution rate | [88] |
| β-Carotene | HPH (o/w NEs-based) | Tween 20 | ↑ Emulsion stability ↑ Solubility ↑ Bioaccessibility | [89] |
| Lycopene | MEs-based method | Ethoxylated sorbitan esters 3GIO SML | ↑ Solubility | [90] |
| Quercetin | SNEDDS | Tween 80 PEG 400 | ↑ Solubility | [91] |
| SNDSs | ||
|---|---|---|
| Requisite | Description | Refs. |
| Food grade ingredients | Manufactured with food-grade/natural ingredients No use of solvents | [15] |
| Food incorporation | Able to physically incorporate or covalently bind BACs Able to compact BACs in more soluble and stable NPs suitable for being incorporated into the food matrix with ↑ EE% and ↓ impact on the sensory properties of the derivative product | [100] |
| Protection by degradation | Able to protect BACs from the interaction with the food matrix constituents, temperature, light, pH, during food manufacturing, storage, processing, and from inactivation by digestion | [15] |
| ↑ Uptake ↑ Bioavailability | Able to promote the cell up-take Able to release BACs in a controlled mode responding to specific environmental stimuli. | [15] |
| Industrial scalability | Suitable to be produced on a large scale | [101] |
| Polymers | BAC | Formulation | Properties | Reference |
|---|---|---|---|---|
| Modified PVA | ATRA | Micelles | ↧ ATRA release ↥ Cytotoxic activity on NB cells | [107,108] |
| TPGS | ATRA | Micelles | ↥ Cytotoxic activity on NB cells | [109] |
| Hydrophilic and amphiphilic biodegradable dendrimers | Ursolic acid Oleanolic acid | Dendrimer NPs | ↥ Water solubility ↥ Biocompatibility ↥ Biodegradability ↧ Toxicity ↥ Antibacterial activity | [110,111] |
| EA | ↥ Water solubility ↥ Biocompatibility ↥ Biodegradability ↧ Toxicity ↥ Antioxidant properties ↥ Scavenging activity | [16] | ||
| GA (linked) | ↥ Solubility in lipids ↥ Biocompatibility ↥ Biodegradability ↥ Antioxidant properties ↥ Scavenging activity ↧ Platelet aggregation ↧ ROS production | [112,113,114] | ||
| GA (Linked and encapsulated) | ↥ Solubility in lipids ↥ Biocompatibility ↥ Biodegradability ↧ Toxicity ↥ Antioxidant | [112] |
| Nanomaterial | Requisites | Function | Reference |
|---|---|---|---|
| LNPs | ↥ Functional groups | Host/guest interaction with HBACs and LBACs | [103,116] |
| Inner cavities | Accommodation of LBACs | [103] | |
| Nanocontainers Protective envelopes | Opposite GIT digestion Opposite degradation | [117,118,119] | |
| Permeability enhancers | Promote GIT absorption | [117,118,119] | |
| Polymeric SNPs | ↥ MW | ↥ Systemic retention time | [120] |
| Method | Mixing | Solvent | Drying | Instruments | Steps |
|---|---|---|---|---|---|
| Physical State | |||||
| Physical blending | Mechanical | No | No | Mixer | Mixing |
| Powder | |||||
| Kneading | Mechanical | Water Water/alcohol | Yes | Kneading machine | CDs pasting BAC addition Mixing Drying |
| Paste | |||||
| Co-precipitation | Mechanical | Solvent (BAC) Water (CD) | Yes | Magnetic stirrer Mechanical stirrer | Dissolutions Precipitation Drying |
| Solutions | |||||
| Ball milling | Mechanical | No | No | Mechanical Oscillatory mill | Mixing |
| Solid state | |||||
| SD 1 | N.R. | Solvent (BAC) Water (CD) | No | Spray dryer | Dissolution SD |
| Solutions | |||||
| FD 2 | N.R. | Solvent (BAC) Water (CD) | No | Freeze dryer | Dissolution FD |
| Solution | |||||
| Supercritical anti-solvent | Mechanical | Solvent (BAC) Water (CD) CO2 (anti-solvent) | No | Magnetic stirrer Mechanical stirrer | Dissolution Precipitation Solvent extraction |
| Solutions/gas CO2 |
| Cyclodextrin | BACs | Improvements |
|---|---|---|
| β-CDs | Linoleic acid (LA) | ↥ Thermal stability, ↧ Degradation |
| β-CDs | RES | ↥ Stability, ↥ Solubility |
| Maltosyl-β-CDs | ||
| Hydroxypropyl-β-CD | Carotenoids | ↥ Water solubility |
| β-CDs | Lycopene (Lyc) | ↥ Water dispersibility, ↥ Stability |
| HP-β-CDs | Hesperidin | ↥ Stability, ↥ Solubility |
| β-CDs | Olive leaf extracts 1 | ↥ Water solubility, ↥ Stability, ↥ Antioxidant activity |
| HP- β-CDs Maltosyl-β-CDs β-CDs | Quercetin Myricetin | ↥ Water solubility, ↥ Stability, ↥ Antioxidant activity |
| HP-β-CDs | Kaempferol | ↥ Water solubility, ↥ Stability, ↥ Antioxidant activity |
| α-CDs β-CDs | 3-Hydroxyflavone Morin Quercetin | ↥ Water solubility, ↥ Stability, ↥ Antioxidant activity |
| β-CD | Rutin | ↥ Water solubility, ↥ Stability, ↥ Antioxidant activity |
| HP-β-CDs | Curcumin | ↥ Water solubility, ↥ Stability, ↥ Antioxidant activity |
| α-CDs | Ferulic acid | ↥ Water solubility, ↥ Stability, ↥ Antioxidant activity |
| β-CDs | EA | ↥ Water solubility, ↥ Anti-inflammatory activity |
| β-CDs/DMC | EA | ↥ Water solubility, ↥ DL%, Controlled release, ↥ Oral bioavailability |
| α-CDs | Amino acids Hydrolysed soy pro 2 | ↧ Bitter taste perception |
| Formulation | BAC | Targeted Microorganism | Activity |
|---|---|---|---|
| PLC-based NPs | Tea tree oil | Tricophyton rubrum | ↑ EO effectiveness against fungi infecting nails |
| Zein-Sodium Caseinate NPs | Thymol | E. coli, Salmonella | Good antimicrobial activity Two-phase release |
| PLGA-based NPs | Cinnamaldehyde Eugenol | Salmonella spp. Listeria spp. | Good antimicrobial activity Controlled release |
| Chitosan-based NPs | Carvacrol | E. coli, S aureus, B. cereus | ↑ Antimicrobial activity |
| Chitosan-based NPs | Oregano EO | N.R. | Controlled release |
| Zein-based NPs | Thymol Carvacrol | E. coli | Good antimicrobial activity ↑ Solubility |
| Chitosan-based NPs | Eugenol Carvacrol | S. aureus, E. coli | ↓ Cytotoxicity |
| PLGA-based NPs | Carvacrol | S. epidermidis biofilms | ↓ Elasticity and stability of performed biofilm |
| Methyl/ethylcellulose NPs | Thymol | S. aureus, E. coli P. aeruginosa | Good antimicrobial activity Preventive activity in cosmetic lotions, creams, gels |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S.; Schito, A.M.; Zuccari, G. Nanotechnological Manipulation of Nutraceuticals and Phytochemicals for Healthy Purposes: Established Advantages vs. Still Undefined Risks. Polymers 2021, 13, 2262. https://doi.org/10.3390/polym13142262
Alfei S, Schito AM, Zuccari G. Nanotechnological Manipulation of Nutraceuticals and Phytochemicals for Healthy Purposes: Established Advantages vs. Still Undefined Risks. Polymers. 2021; 13(14):2262. https://doi.org/10.3390/polym13142262
Chicago/Turabian StyleAlfei, Silvana, Anna Maria Schito, and Guendalina Zuccari. 2021. "Nanotechnological Manipulation of Nutraceuticals and Phytochemicals for Healthy Purposes: Established Advantages vs. Still Undefined Risks" Polymers 13, no. 14: 2262. https://doi.org/10.3390/polym13142262
APA StyleAlfei, S., Schito, A. M., & Zuccari, G. (2021). Nanotechnological Manipulation of Nutraceuticals and Phytochemicals for Healthy Purposes: Established Advantages vs. Still Undefined Risks. Polymers, 13(14), 2262. https://doi.org/10.3390/polym13142262









