UIO-66 as Nucleating Agent on the Crystallization Behavior and Properties of Poly(Ethylene Terephthalate)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments
2.3. Characteristics
3. Results and Discussions
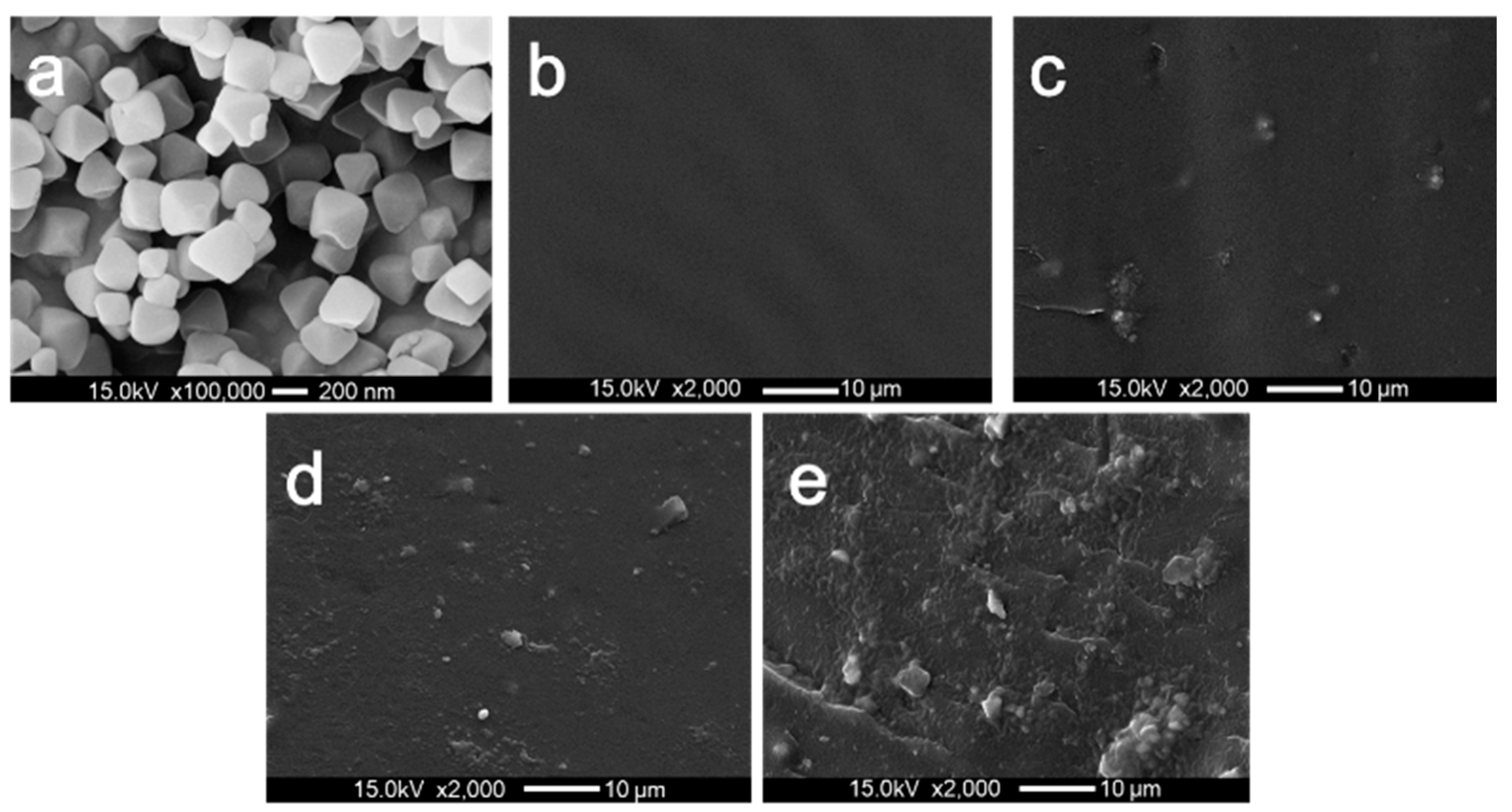
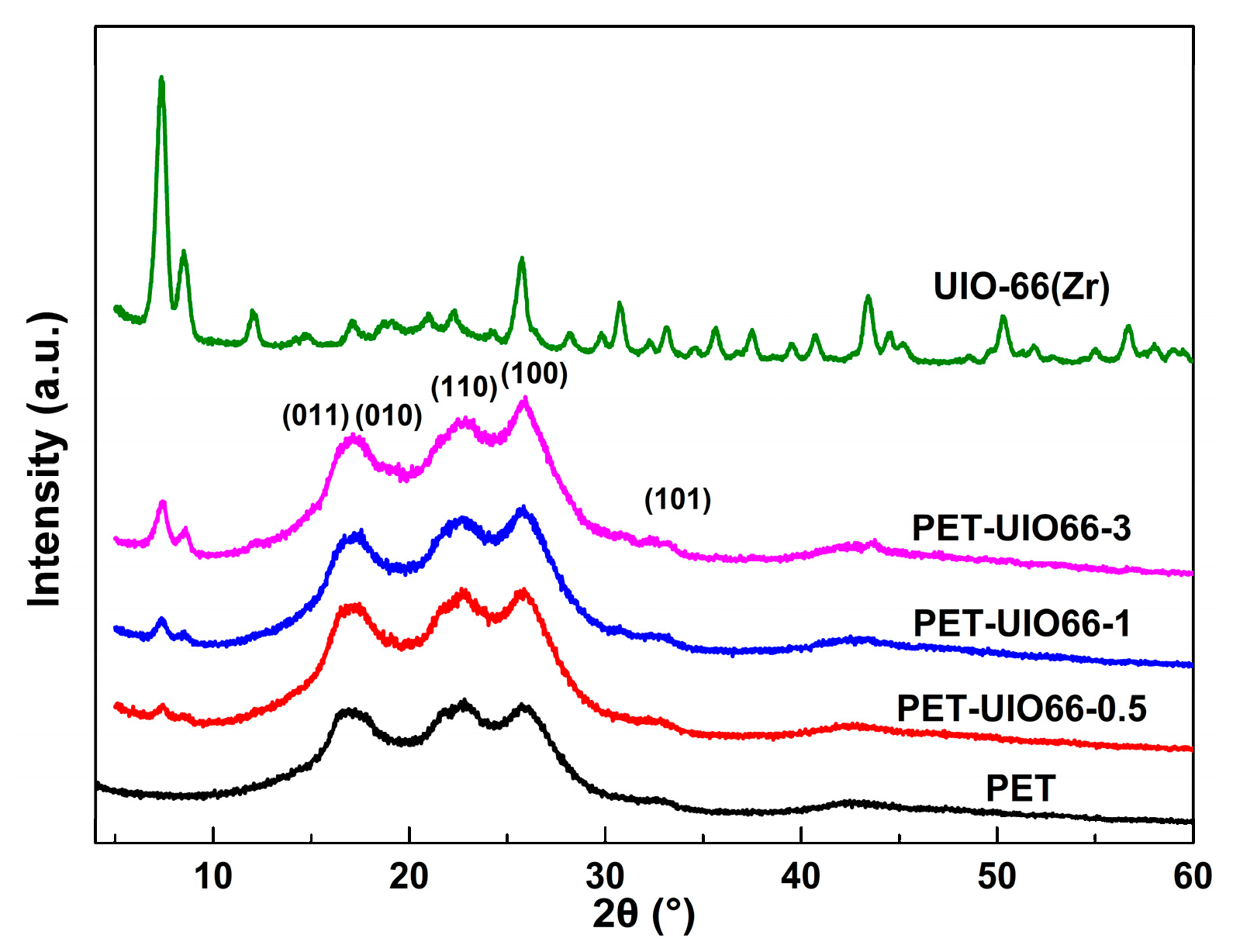
3.1. Dispersion of UIO66 within PET/UIO-66 Nanocomposites
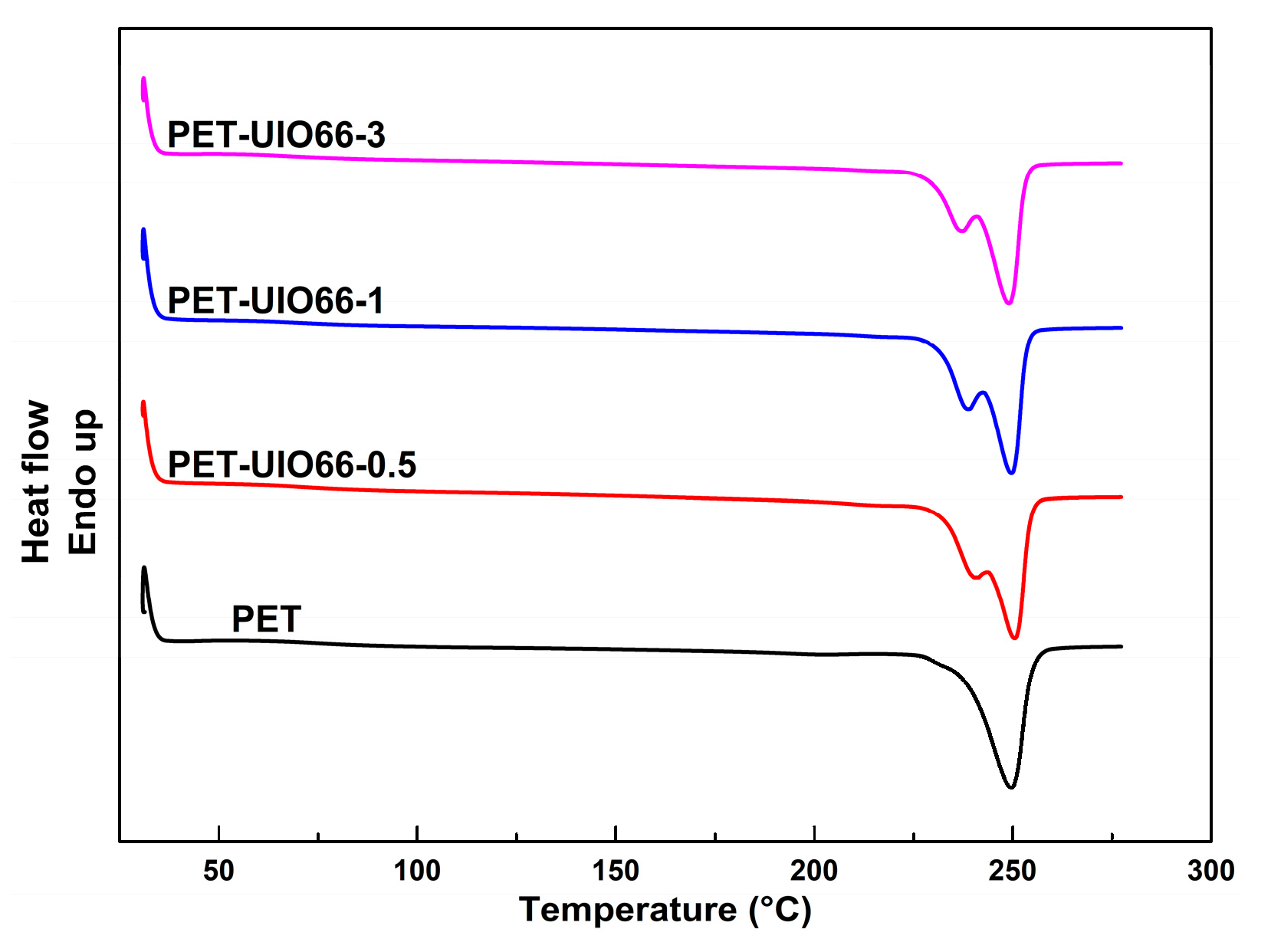
3.2. Melting Behavior and Crystallization of the PET/UIO-66 Nanocomposites
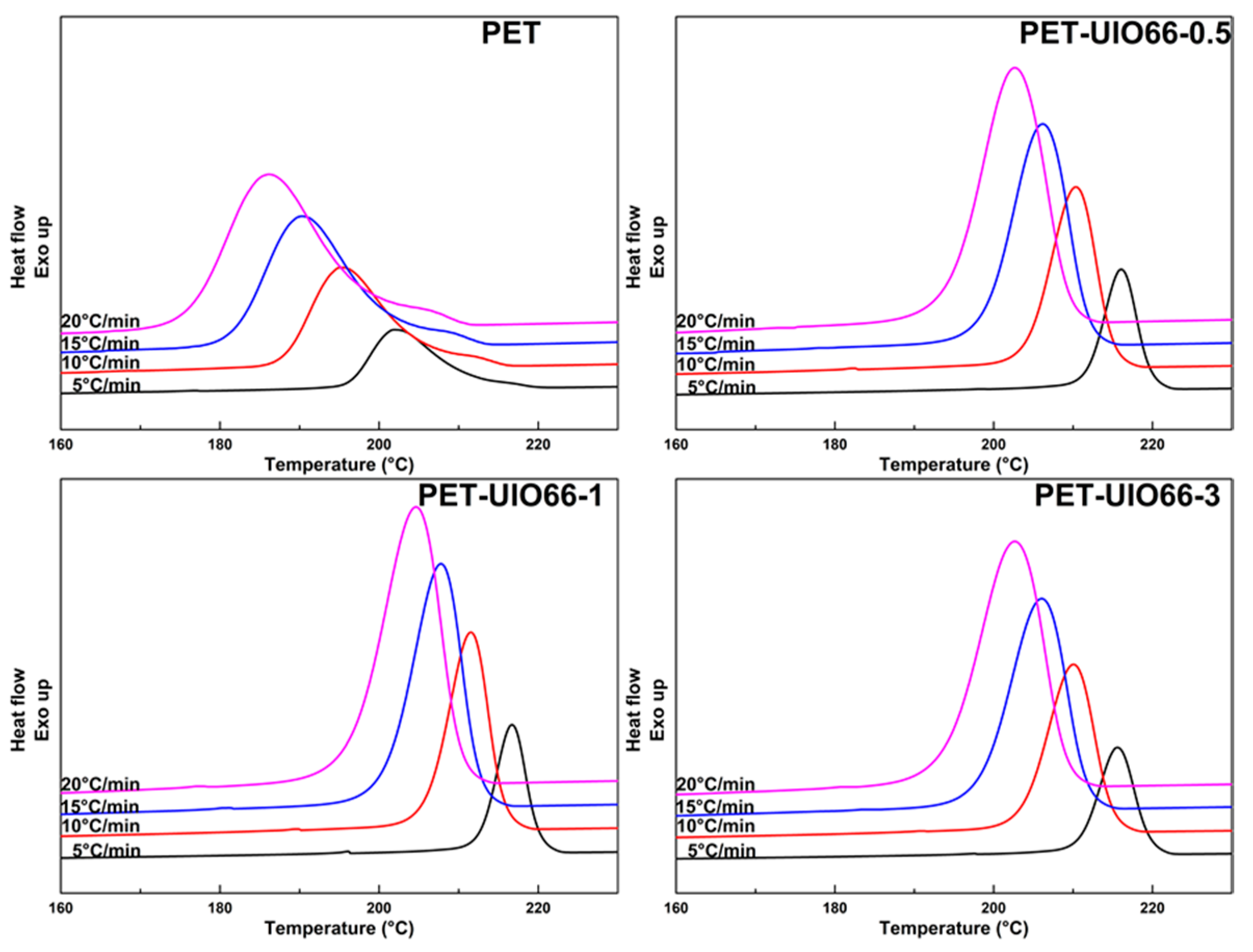
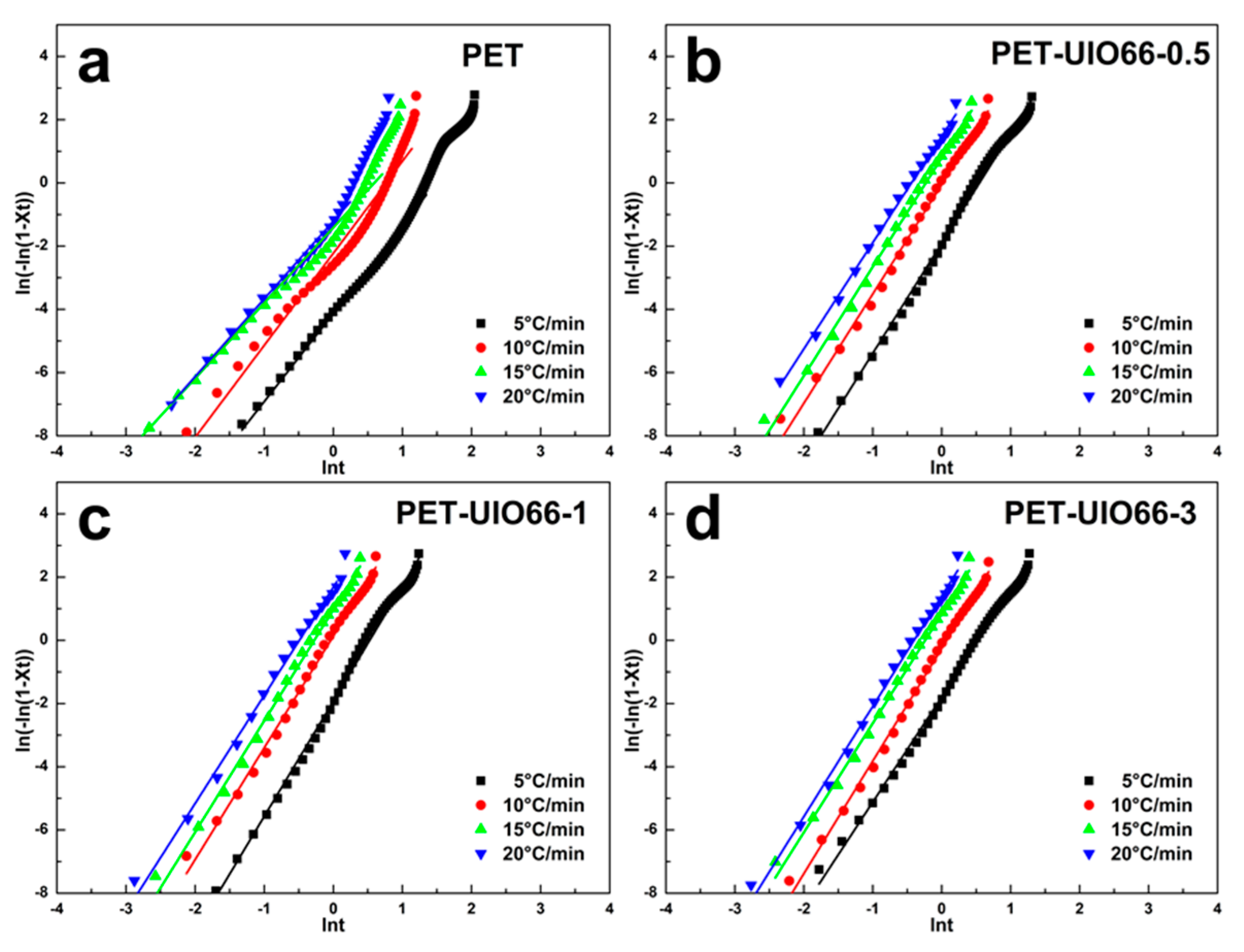
3.3. Non-Isothermal Crystallization Kinetics of the PET/UIO-66 Nanocomposites
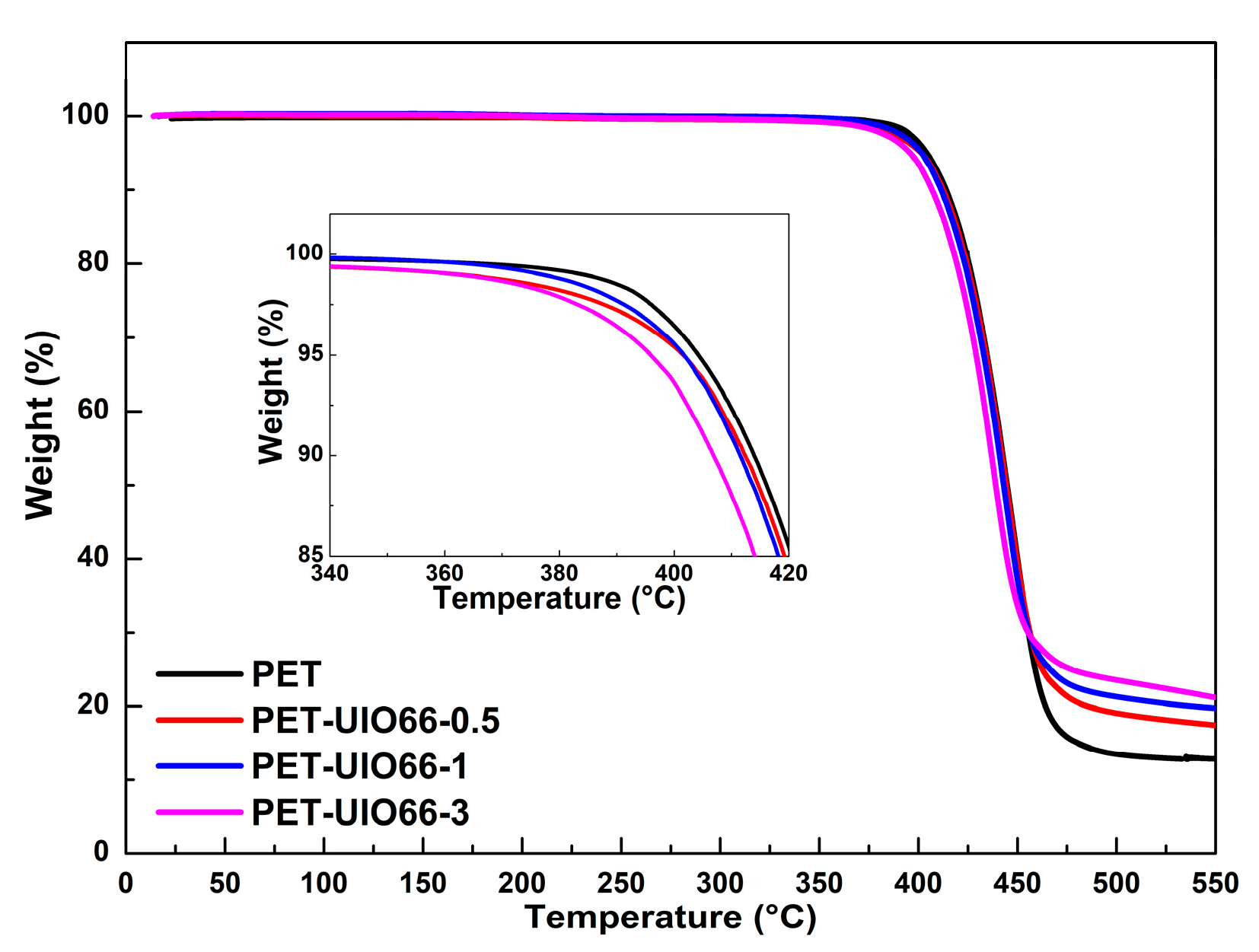
3.4. Thermal Stability of the PET and the PET/UIO-66 Nanocomposites
3.5. Mechanical Property of the PET and the PET/UIO-66 Nanocomposites
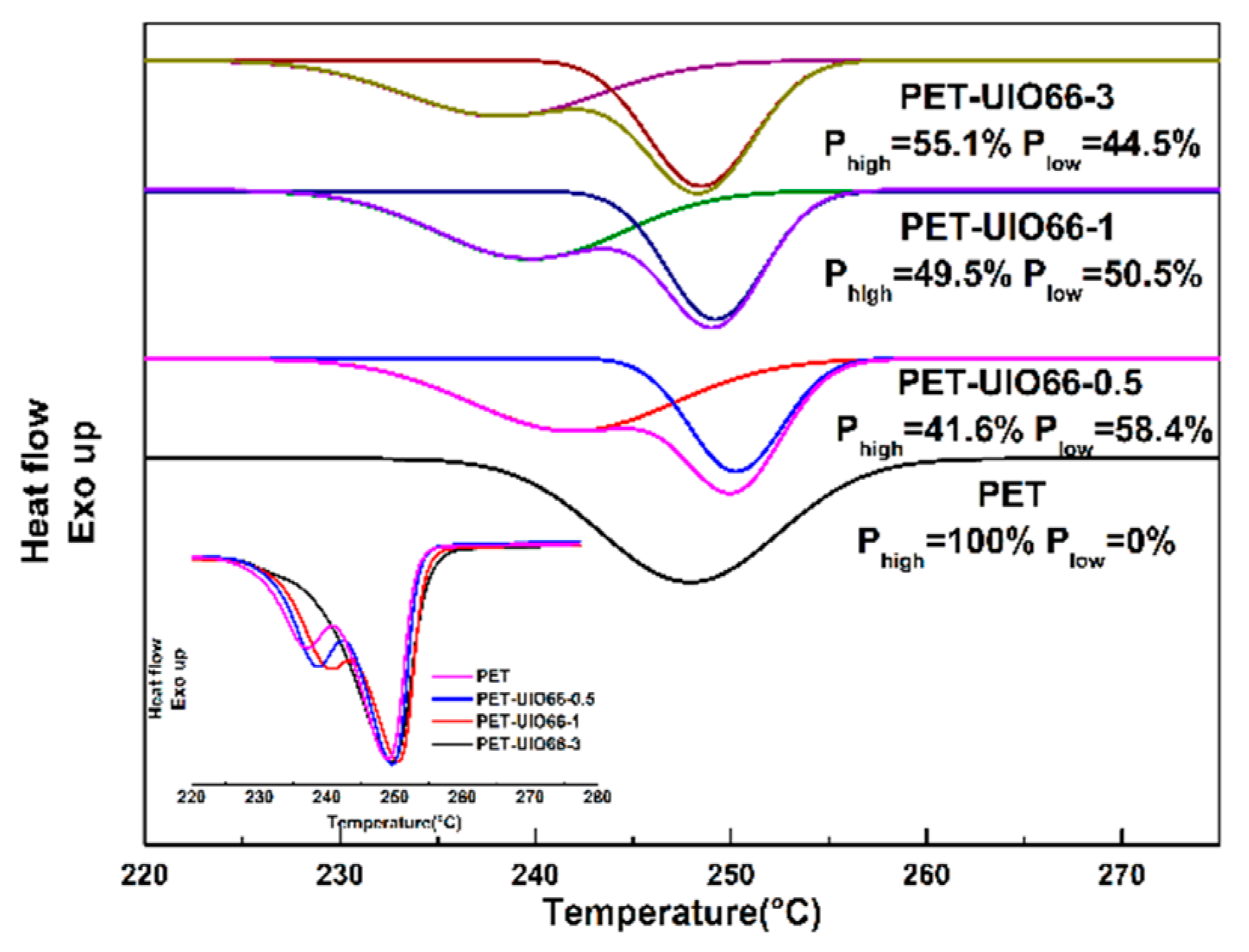
3.6. Three-Phase Model of the PET and the PET/UIO-66 Nanocomposites
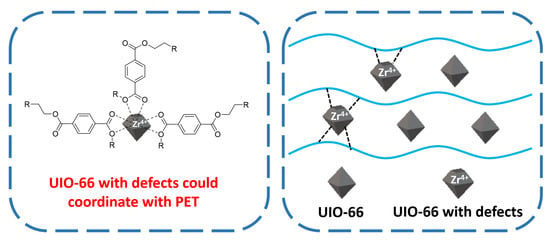
3.7. Crystallization Mechanism of PET and PET/UIO-66 Nanocomposites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, K.; Li, J.; Chen, X.; Yao, J.; Shao, Z. Synthesis of novel multi-hydroxyl N-halamine precursors based on barbituric acid and their applications in antibacterial poly(ethylene terephthalate) (PET) materials. J. Mater. Chem. B 2020, 8, 8695–8701. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-H.; Wang, C.-C.; Chen, C.-Y. Nucleation effect of aliphatic polycarbonate in its blends with poly(ethylene terephthalate). Mater. Chem. Phys. 2020, 248, 122920. [Google Scholar] [CrossRef]
- Pinto, G.M.; Silva, G.d.C.; Santillo, C.; Lavorgna, M.; Maia, J.M.; Fechine, G.J.M. Crystallization kinetics, structure, and rheological behavior of poly(ethylene terephthalate)/multilayer graphene oxide nanocomposites. Polym. Eng. Sci. 2020, 60, 2841–2851. [Google Scholar] [CrossRef]
- Tian, Y.; Tan, J.; Wu, Y.; Song, Z.; Tao, Q.; Zheng, X.; Hu, T.; Gong, X.; Wu, C. Influence of the ion content of CaCl 2-ionised polyamide-66 nucleator on the crystalline nucleation of poly (ethylene terephthalate) through ion–dipole interactions. RSC Adv. 2020, 10, 34177–34186. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Yang, Y.; Tang, P. Poly(maleic acid-co-acrylic acid) ionomer as nucleating agent on the crystallization behavior and properties of poly(ethylene terephthalate). Polym. Bull. 2021. [Google Scholar] [CrossRef]
- Yin, Y.; Jiang, B.; Meng, L. Research on synthesis and thermal properties of poly(ethylene terephthalate) sulfonate group containing ionomer. J. Appl. Polym. Sci. 2021, 138, 49966. [Google Scholar] [CrossRef]
- Han, Z.; Wang, Y.; Wang, J.; Wang, S.; Zhuang, H.; Liu, J.; Huang, L.; Wang, Y.; Wang, W.; Belfiore, L.A.; et al. Preparation of Hybrid Nanoparticle Nucleating Agents and Their Effects on the Crystallization Behavior of Poly(ethylene terephthalate). Materials 2018, 11, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anis, A.; Elnour, A.Y.; Alam, M.A.; Al-Zahrani, S.M.; AlFayez, F.; Bashir, Z. Aluminum-Filled Amorphous-PET, a Composite Showing Simultaneous Increase in Modulus and Impact Resistance. Polymers 2020, 12, 2038. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wirasaputra, A.; Jiang, Z.; Liu, S.; Zhao, J.; Fu, Y. Fabrication of improved overall properties of poly (ethylene terephthalate) by simultaneous chain extension and crystallization promotion. J. Therm. Anal. Calorim. 2018, 133, 1447–1454. [Google Scholar] [CrossRef]
- Tianbin, W.; Yangchuan, K. Preparation of silica–PS composite particles and their application in PET. Eur. Polym. J. 2006, 42, 274–285. [Google Scholar] [CrossRef]
- Antoniadis, G.; Paraskevopoulos, K.M.; Bikiaris, D.; Chrissafis, K. Non-isothermal crystallization kinetic of poly(ethylene terephthalate)/fumed silica (PET/SiO2) prepared by in situ polymerization. Thermochim. Acta 2010, 510, 103–112. [Google Scholar] [CrossRef]
- Ke, Y.-C.; Wu, T.-B.; Xia, Y.-F. The nucleation, crystallization and dispersion behavior of PET–monodisperse SiO2 composites. Polymer 2007, 48, 3324–3336. [Google Scholar] [CrossRef]
- Cayuela, D.; Cot, M.; Algaba, I.; Manich, A.M. Effect of different dispersing agents in the non-isothermal kinetics and thermomechanical behavior of PET/TiO2 composites. J. Macromol. Sci. Part A 2016, 53, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Zhang, H.; Ke, M.; Xiao, L.; Zuo, D.; Qian, Q.; Chen, Q. Non-isothermal crystallization kinetics of poly(ethylene terephthalate)/mica composites. Polym. Bull. 2014, 71, 2287–2301. [Google Scholar] [CrossRef]
- Yang, F.; Mubarak, C.; Keiegel, R.; Kannan, R.M. Supercritical carbon dioxide (scCO2) dispersion of poly(ethylene terephthalate)/clay nanocomposites: Structural, mechanical, thermal, and barrier properties. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Chowreddy, R.R.; Nord-Varhaug, K.; Rapp, F. Recycled poly (ethylene terephthalate)/clay nanocomposites: Rheology, thermal and mechanical properties. J. Polym. Environ. 2019, 27, 37–49. [Google Scholar] [CrossRef]
- Miri, F.S.; Ehsani, M.; Khonakdar, H.A.; Kavyani, B. A Comprehensive Study on Physical, Mechanical, and Thermal Properties of Poly(Ethylene Terephthalate) Filled by Micro- and Nanoglass Flakes. J. Vinyl Addit. Technol. 2020, 26, 380–389. [Google Scholar] [CrossRef]
- Gilmer, J.W.; Neu, R.P.; Liu, Y.J.; Jen, A.K.-Y. The use of sodium salts as nucleation agents for polyethylene terephthalate with minimal molecular weight reduction. Polym. Eng. Sci. 1995, 35, 1407–1412. [Google Scholar] [CrossRef]
- Dekoninck, J.M.; Legras, R.; Mercier, J.P. Nucleation of poly(ethylene terephthalate) by sodium compounds: A unique mechanism. Polymer 1989, 30, 910–913. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, S.; Mohamed, M.G.; Kuo, S.-W.; Xin, Z. Crystallization behaviors of poly(ethylene terephthalate) (PET) with monosilane isobutyl-polyhedral oligomeric silsesquioxanes (POSS). J. Mater. Sci. 2020, 55, 14642–14655. [Google Scholar] [CrossRef]
- Ozdemir, E.; Arenas, D.R.; Kelly, N.L.; Hanna, J.V.; van Rijswijk, B.; Degirmenci, V.; McNally, T. Ethylene methyl acrylate copolymer (EMA) assisted dispersion of few-layer graphene nanoplatelets (GNP) in poly(ethylene terephthalate) (PET). Polymer 2020, 205, 122836. [Google Scholar] [CrossRef]
- Xing, L.; Wang, Y.; Wang, S.; Zhang, Y.; Mao, S.; Wang, G.; Liu, J.; Huang, L.; Li, H.; Belfiore, L.A.; et al. Effects of Modified Graphene Oxide on Thermal and Crystallization Properties of PET. Polymers 2018, 10, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, Y.; Li, H.; Gong, X.; Liu, J.; Huang, L.; Wang, W.; Wang, Y.; Zhao, Z.; Belfiore, L.A.; et al. Fluorescent SiO2@Tb3+(PET-TEG)3Phen Hybrids as Nucleating Additive for Enhancement of Crystallinity of PET. Polymers 2020, 12, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Wang, Y.; Liu, H.; Belfiore, L.A. Effects of organic nucleating agents and zinc oxide nanoparticles on isotactic polypropylene crystallization. Polymer 2004, 45, 2081–2091. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef]
- De Vos, A.; Hendrickx, K.; Van Der Voort, P.; Van Speybroeck, V.; Lejaeghere, K. Missing Linkers: An Alternative Pathway to UiO-66 Electronic Structure Engineering. Chem. Mater. 2017, 29, 3006–3019. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Ubaidullah, M.; Ahmed, J.; Ahamad, T.; Ahmad, T.; Shaikh, S.F.; Naushad, M. Synthesis of NiOx@NPC composite for high-performance supercapacitor via waste PET plastic-derived Ni-MOF. Compos. Part B: Eng. 2020, 183, 107655. [Google Scholar] [CrossRef]
- Deleu, W.P.; Stassen, I.; Jonckheere, D.; Ameloot, R.; De Vos, D.E. Waste PET (bottles) as a resource or substrate for MOF synthesis. J. Mater. Chem. A 2016, 4, 9519–9525. [Google Scholar] [CrossRef]
- Dominici, F.; Sarasini, F.; Luzi, F.; Torre, L.; Puglia, D. Thermomechanical and Morphological Properties of Poly(ethylene terephthalate)/Anhydrous Calcium Terephthalate Nanocomposites. Polymers 2020, 12, 276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, J.; Feng, Y.; Zhang, X.; Jia, M.; Yao, J. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: Adsorption performance and mechanisms. J. Colloid Interface Sci. 2017, 499, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Gaonkar, A.A.; Murudkar, V.V.; Deshpande, V.D. Comparison of crystallization kinetics of polyethylene terephthalate (PET) and reorganized PET. Thermochim. Acta 2020, 683, 178472. [Google Scholar] [CrossRef]
- Monti, M.; Scrivani, M.T.; Kociolek, I.; Larsen, Å.G.; Olafsen, K.; Lambertini, V. Enhanced Impact Strength of Recycled PET/Glass Fiber Composites. Polymers 2021, 13, 1471. [Google Scholar] [CrossRef]
- Aoyama, S.; Ismail, I.; Park, Y.T.; Macosko, C.W.; Ougizawa, T. Higher-Order Structure in Amorphous Poly(ethylene terephthalate)/Graphene Nanocomposites and Its Correlation with Bulk Mechanical Properties. ACS Omega 2019, 4, 1228–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wunderlich, B. Thermal Analysis of Polymeric Materials; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Mayoral, B.; Hornsby, P.R.; McNally, T.; Schiller, T.L.; Jack, K.; Martin, D.J. Quasi-solid state uniaxial and biaxial deformation of PET/MWCNT composites: Structural evolution, electrical and mechanical properties. RSC Adv. 2013, 3, 5162–5183. [Google Scholar] [CrossRef]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 22, 6632–6640. [Google Scholar] [CrossRef]
- Xanthos, M.; Baltzis, B.C.; Hsu, P.P. Effects of carbonate salts on crystallization kinetics and properties of recycled poly(ethylene terephthalate). J. Appl. Polym. Sci. 1997, 64, 1423–1435. [Google Scholar] [CrossRef]
- Wu, H.; Chua, Y.S.; Krungleviciute, V.; Tyagi, M.; Chen, P.; Yildirim, T.; Zhou, W. Unusual and Highly Tunable Missing-Linker Defects in Zirconium Metal–Organic Framework UiO-66 and Their Important Effects on Gas Adsorption. J. Am. Chem. Soc. 2013, 135, 10525–10532. [Google Scholar] [CrossRef]
- Katz, M.J.; Brown, Z.J.; Colón, Y.J.; Siu, P.W.; Scheidt, K.A.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. A facile synthesis of UiO-66, UiO-67 and their derivatives. Chem. Commun. 2013, 49, 9449–9451. [Google Scholar] [CrossRef]





| Samples | 1st Heating | Cooling | |||||
|---|---|---|---|---|---|---|---|
| Tg (°C) | Tcc (°C) | ΔHcc (J/g) | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | |
| PET | 76.6 | 135.6 | 26.9 | 252.5 | 37.2 | 194.3 | 52.1 |
| PET-UIO66-0.5 | 72.3 | 132.5 | 29.5 | 249.6 | 35.5 | 209.8 | 53.4 |
| PET-UIO66-1 | 72.6 | 129.5 | 27.5 | 249.3 | 29.4 | 211.6 | 53.1 |
| PET-UIO66-3 | 71.4 | 128.5 | 27.0 | 249.5 | 37.5 | 210.4 | 51.6 |
| Sample | Tm1 (°C) | Tm2 (°C) | ΔHm (J/g) | χc (%) |
|---|---|---|---|---|
| PET | - | 249.7 | 46.8 | 33.4 |
| PET-UIO66-0.5 | 240.9 | 250.6 | 53.5 | 38.4 |
| PET-UIO66-1 | 238.6 | 249.7 | 49.5 | 35.7 |
| PET-UIO66-3 | 237.3 | 249.1 | 48.1 | 35.4 |
| Cooling Rate | PET | PET-UIO66-0.5 | PET-UIO66-1 | PET-UIO66-3 | ||||
|---|---|---|---|---|---|---|---|---|
| n | Zc | n | Zc | n | Zc | n | Zc | |
| 5 | 2.78 | 0.44 | 3.48 | 0.68 | 3.65 | 0.68 | 3.32 | 0.70 |
| 10 | 2.91 | 0.80 | 3.46 | 0.99 | 3.53 | 1.01 | 3.56 | 0.97 |
| 15 | 2.38 | 0.91 | 3.45 | 1.05 | 3.52 | 1.07 | 3.45 | 1.06 |
| 20 | 2.46 | 0.94 | 3.37 | 1.07 | 3.42 | 1.09 | 3.48 | 1.07 |
| Sample | T5% (°C) | T10% (°C) | Tmax (°C) | Residue (%) |
|---|---|---|---|---|
| PET | 405.2 | 414.6 | 446.2 | 13.2 |
| PET-UIO66-0.5 | 401.6 | 412.4 | 446.0 | 17.4 |
| PET-UIO66-1 | 401.7 | 411.5 | 444.8 | 19.7 |
| PET-UIO66-3 | 395.9 | 406.9 | 438.8 | 21.2 |
| Sample | Yield Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| PET | 41.2 ± 3.5 | 217.0 ± 27.8 |
| PET-UIO66-0.5 | 50.0 ± 1.2 | 229.6 ± 33.5 |
| PET-UIO66-1 | 51.9 ± 0.6 | 207.5 ± 43.5 |
| PET-UIO66-3 | 57.6 ± 4.3 | 14.6 ± 8.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Y.; Wang, Y.; Meng, L. UIO-66 as Nucleating Agent on the Crystallization Behavior and Properties of Poly(Ethylene Terephthalate). Polymers 2021, 13, 2266. https://doi.org/10.3390/polym13142266
Yin Y, Wang Y, Meng L. UIO-66 as Nucleating Agent on the Crystallization Behavior and Properties of Poly(Ethylene Terephthalate). Polymers. 2021; 13(14):2266. https://doi.org/10.3390/polym13142266
Chicago/Turabian StyleYin, Yue, Yuan Wang, and Linghui Meng. 2021. "UIO-66 as Nucleating Agent on the Crystallization Behavior and Properties of Poly(Ethylene Terephthalate)" Polymers 13, no. 14: 2266. https://doi.org/10.3390/polym13142266
APA StyleYin, Y., Wang, Y., & Meng, L. (2021). UIO-66 as Nucleating Agent on the Crystallization Behavior and Properties of Poly(Ethylene Terephthalate). Polymers, 13(14), 2266. https://doi.org/10.3390/polym13142266