Contribution to a Circular Economy Model: From Lignocellulosic Wastes from the Extraction of Vegetable Oils to the Development of a New Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Silane Treatment
2.3. Sample Preparation
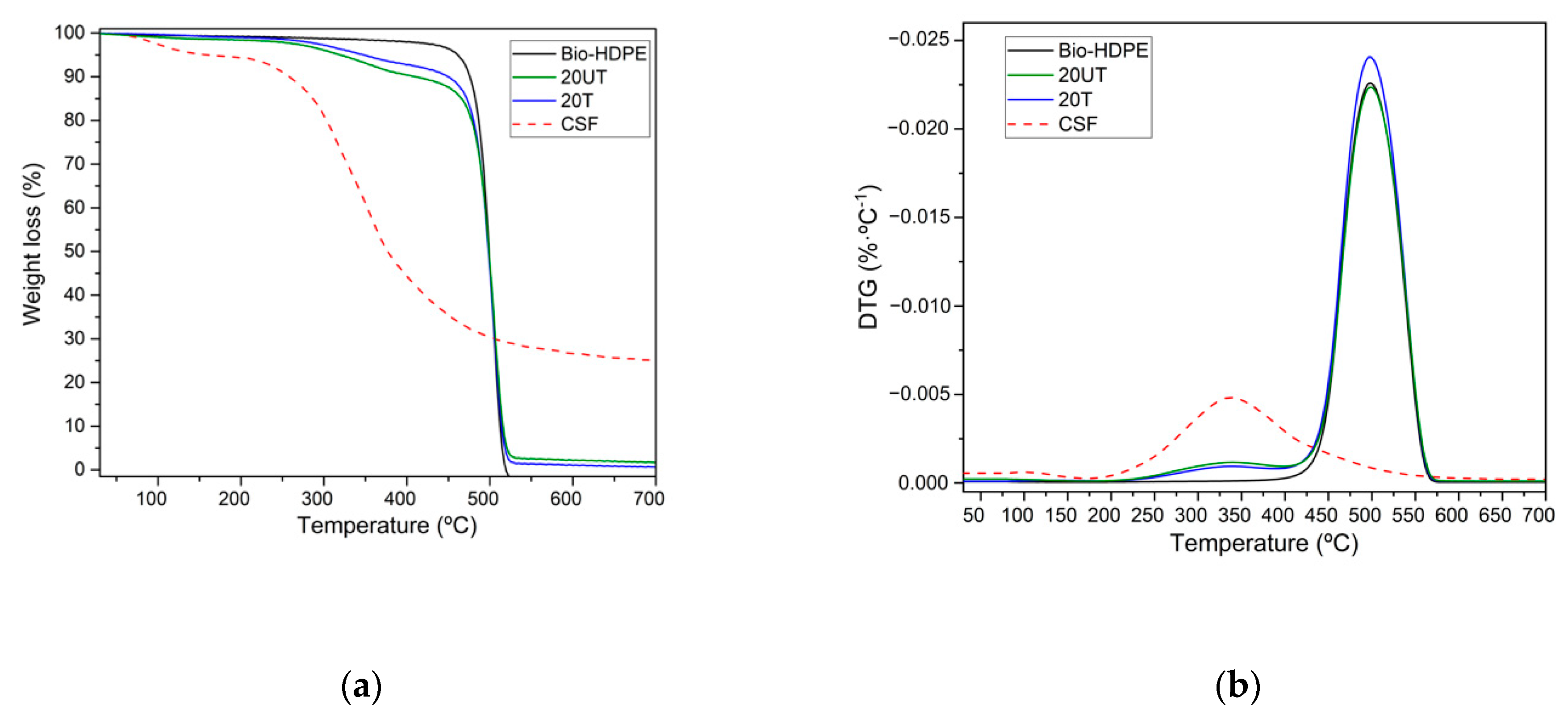
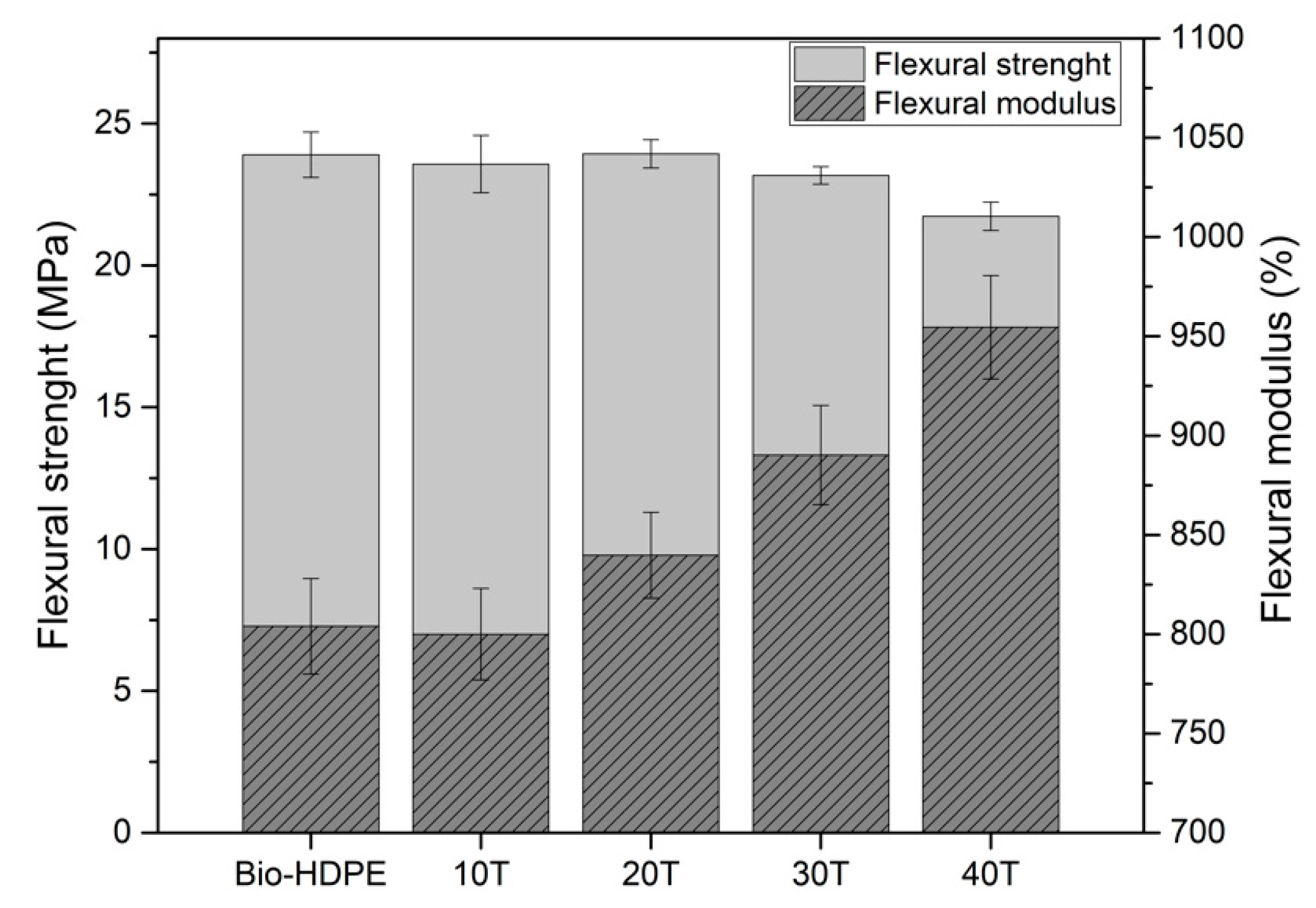
2.4. Mechanical and Thermal Characterization
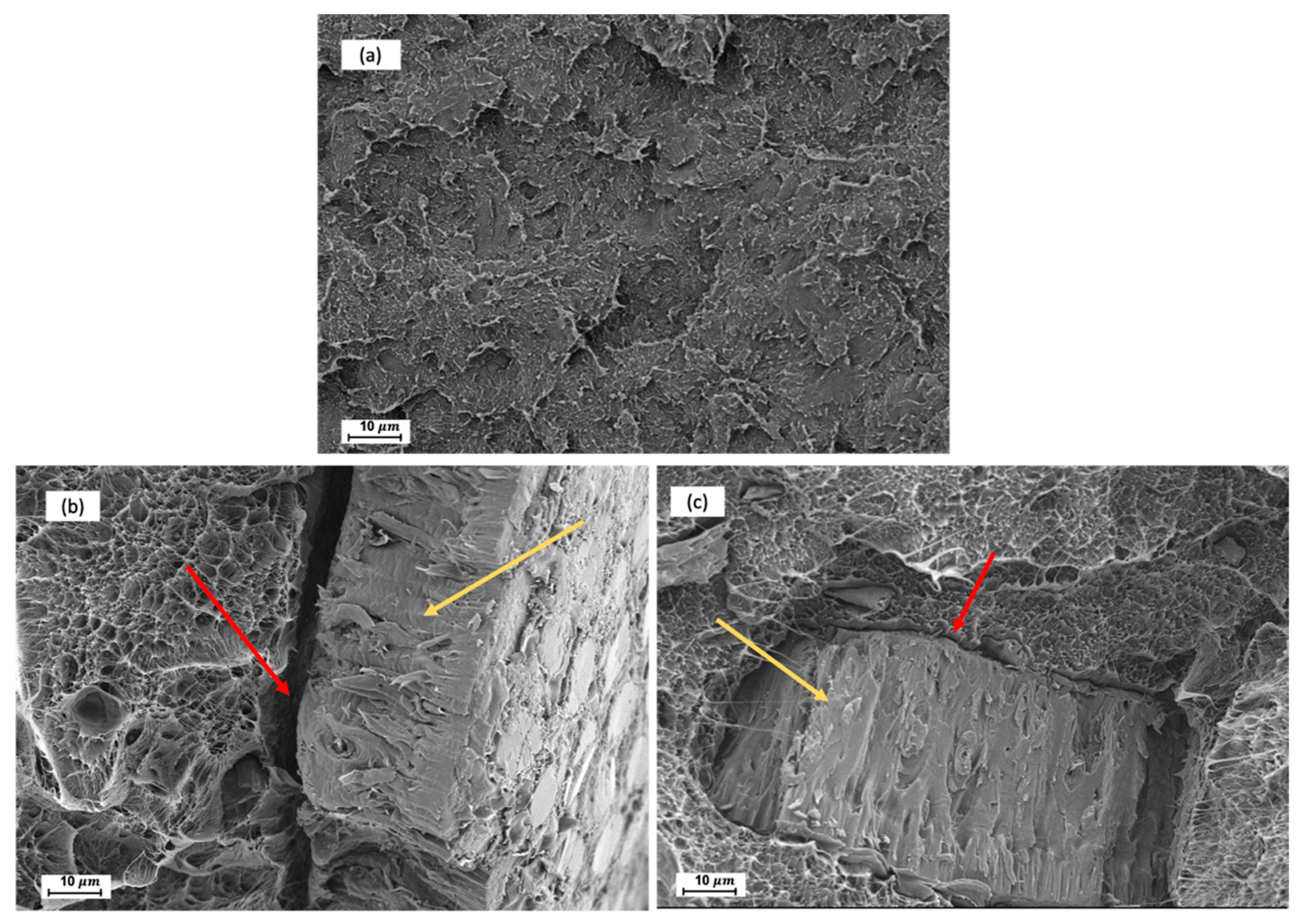
2.5. Morphology Chatacterization
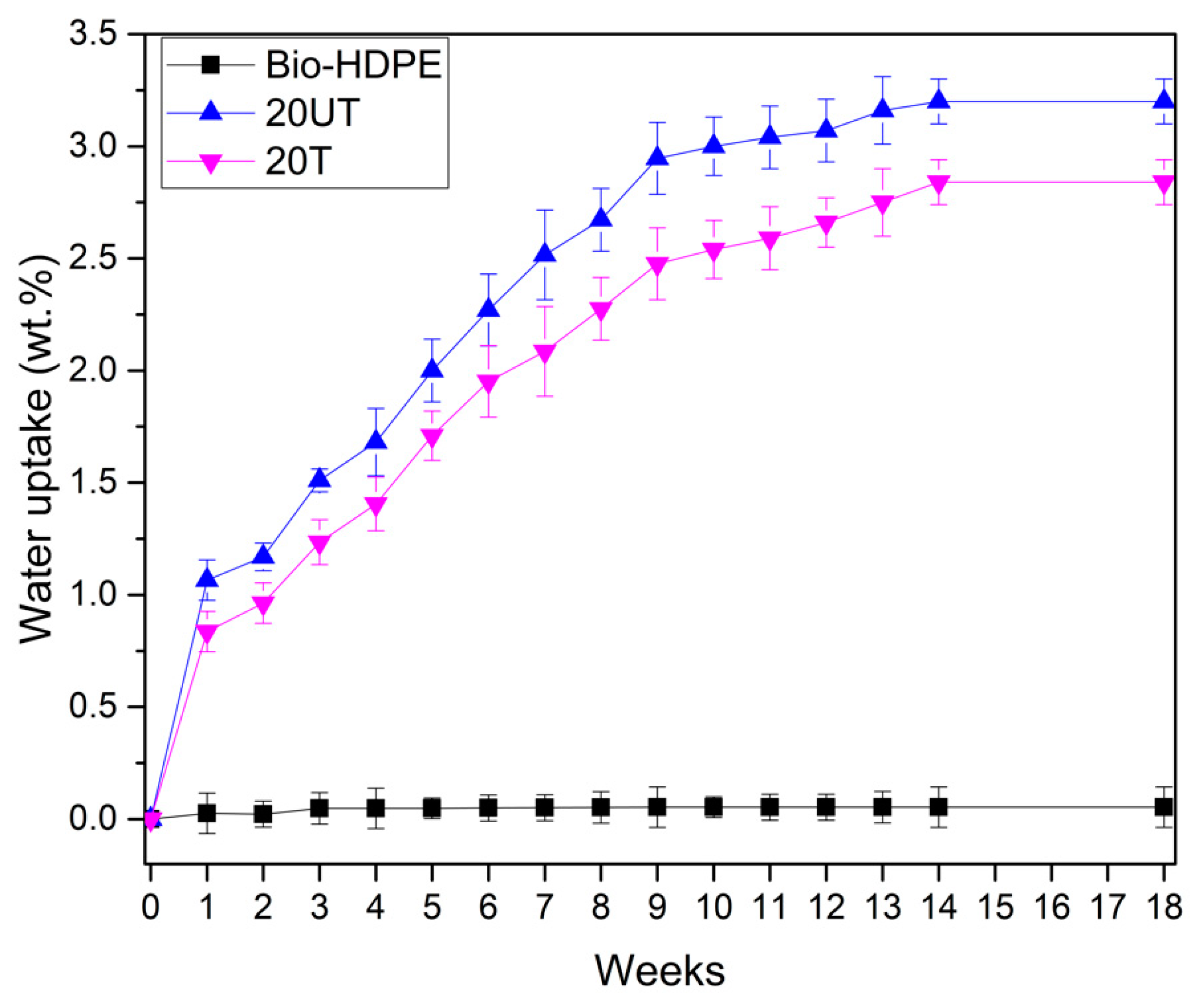
2.6. Water Uptake
2.7. Colour Characterization
2.8. Degradation under Composting Conditions
3. Results
3.1. CSF Extraction Yield
3.2. First Stage. Effect of Silane Treatment
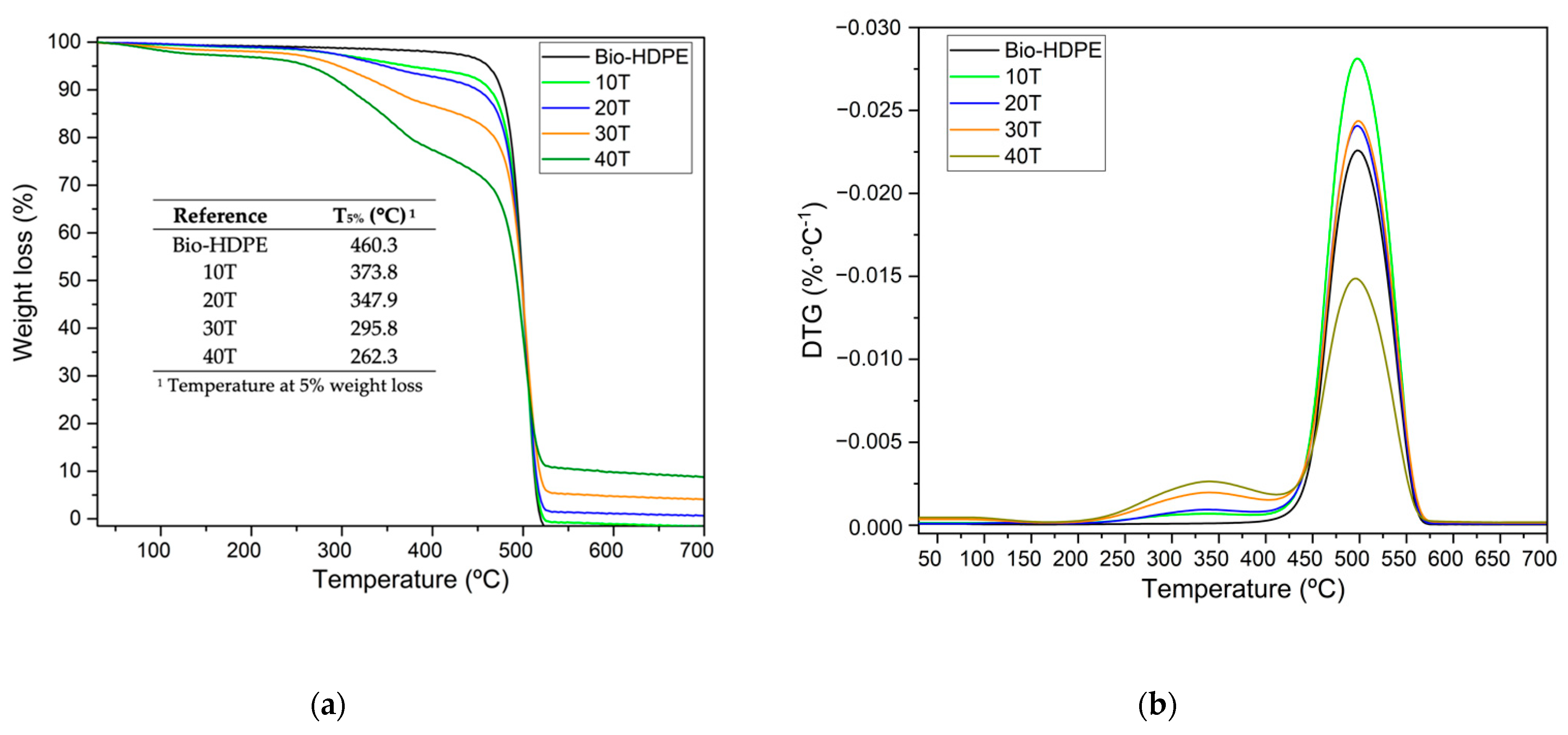
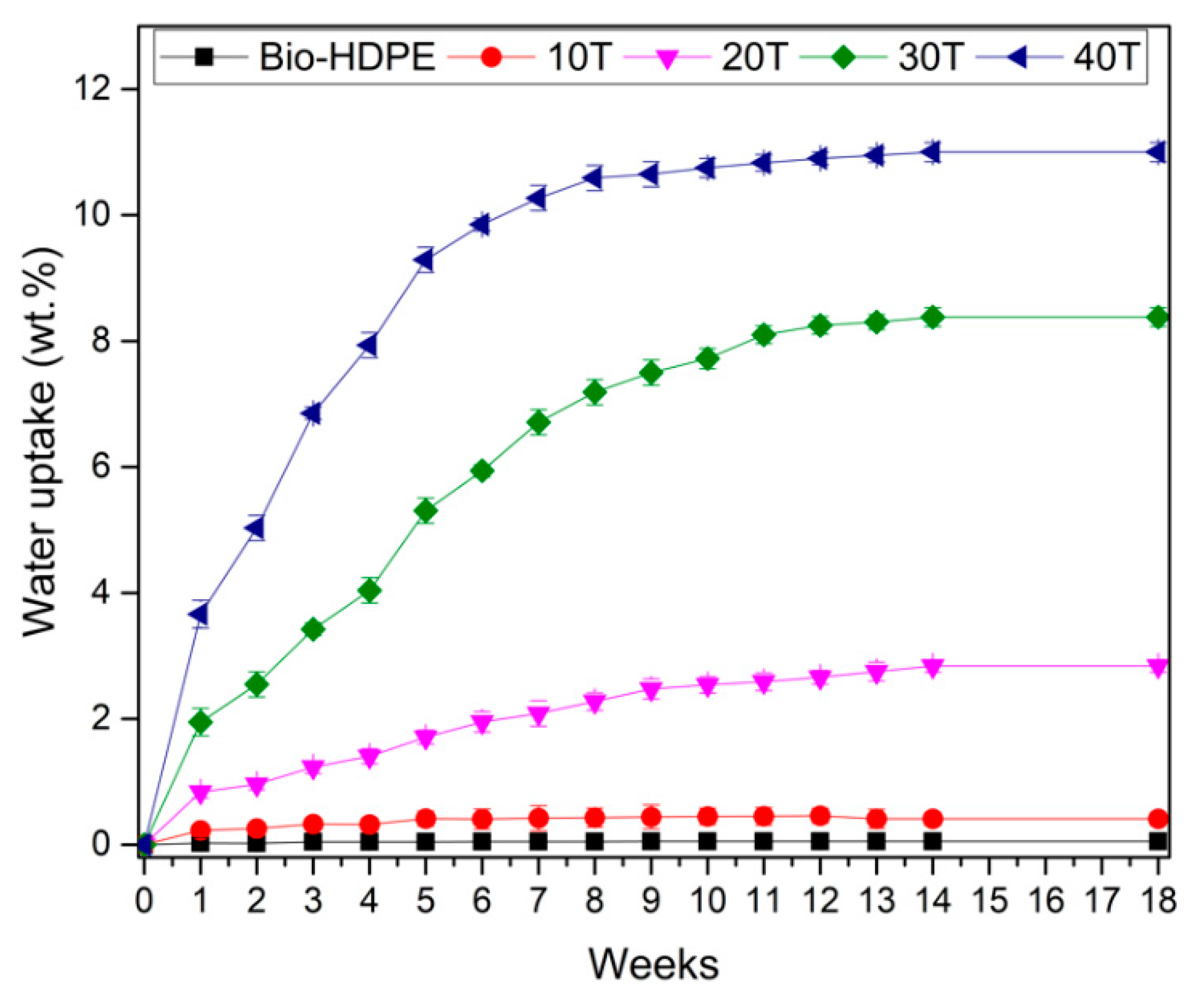
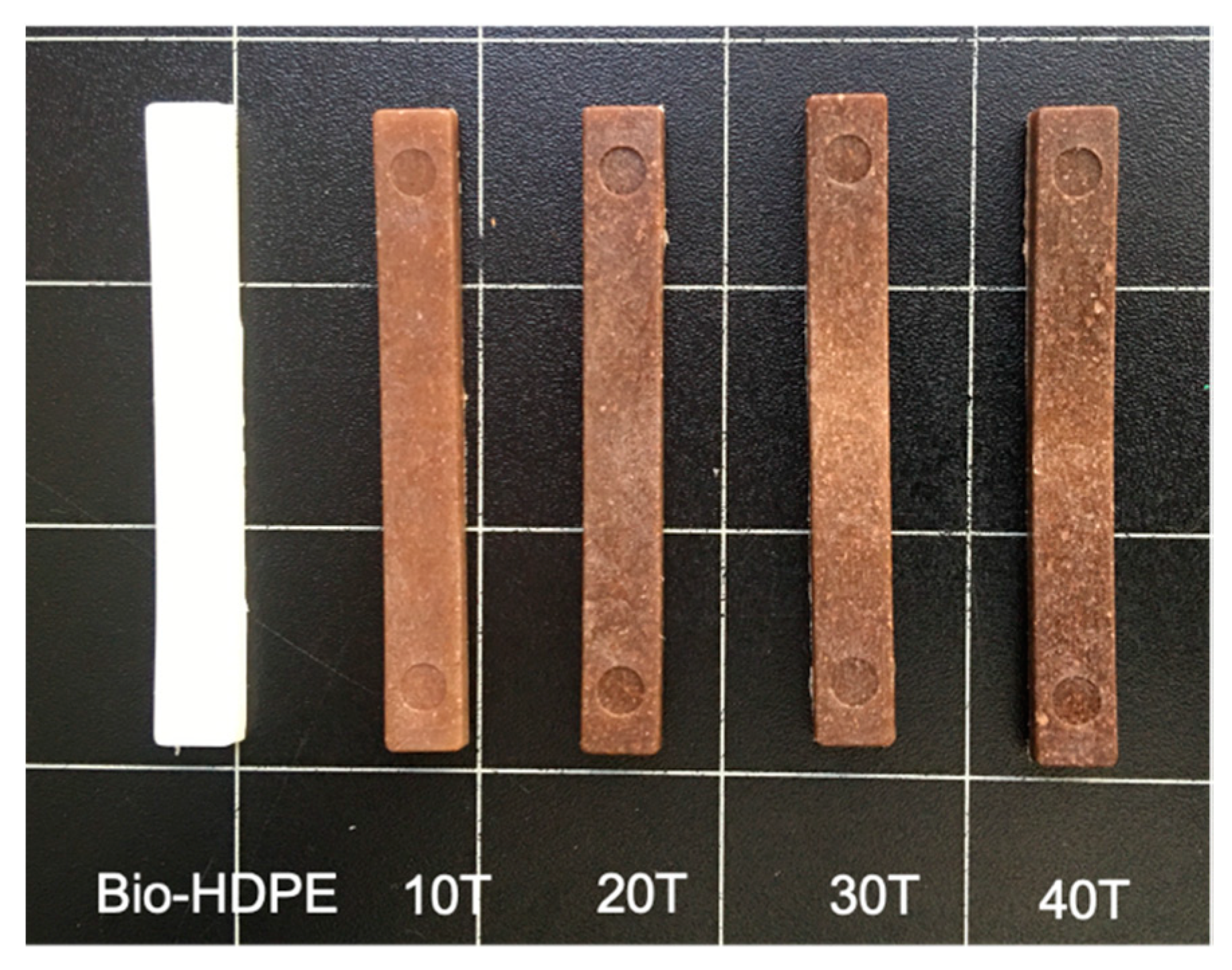
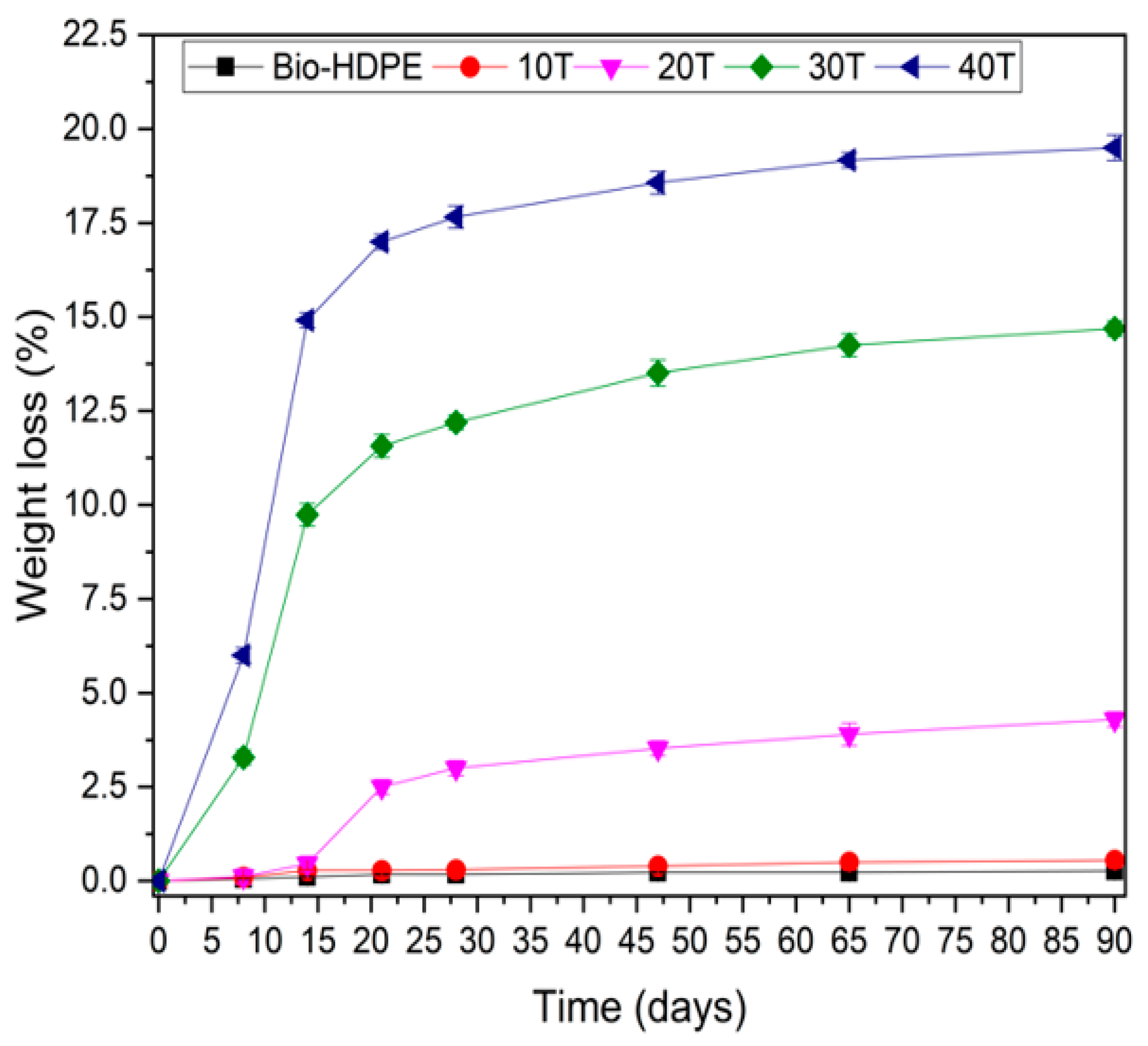
3.3. Second Stage. Evaluation of CSF Filler Percentage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Europe, P. Plastics—The Facts 2020: An Analysis of European Plastics Production, Demand and Waste. Available online: https://www.plasticseurope.org/download_file/force/4829/181 (accessed on 29 April 2021).
- Luzi, F.; Torre, L.; Kenny, J.M.; Puglia, D. Bio- and Fossil-Based Polymeric Blends and Nanocomposites for Packaging: Structure-Property Relationship. Materials 2019, 12, 471. [Google Scholar] [CrossRef] [Green Version]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Ahvenainen, R. Novel Food Packaging Techniques; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.; Mahanti, N.K. Application of biodegradable polymers in food packaging industry: A comprehensive review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Niaounakis, M. Biopolymers: Processing and Products; William Andrew: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Varganici, C.-D.; Rosu, L.; Rosu, D.; Mustata, F.; Rusu, T. Sustainable wood coatings made of epoxidized vegetable oils for ultraviolet protection. Environ. Chem. Lett. 2021, 19, 307–328. [Google Scholar] [CrossRef]
- Carlos Ronda, J.; Lligadas, G.; Galia, M.; Cadiz, V. Vegetable oils as platform chemicals for polymer synthesis. Eur. J. Lipid Sci. Technol. 2011, 113, 46–58. [Google Scholar] [CrossRef]
- De Espinosa, L.M.; Meier, M.A.R. Plant oils: The perfect renewable resource for polymer science?! Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef] [Green Version]
- Production of Vegetable Oils Worldwirde Since 2000. Available online: https://www.statista.com/statistics/263933/production-of-vegetable-oils-worldwide-since-2000/ (accessed on 1 April 2021).
- Lee, S.H.; Ashaari, Z.; Lum, W.C.; Halip, J.A.; Ang, A.F.; Tan, L.P.; Chin, K.L.; Tahir, P.M. Thermal treatment of wood using vegetable oils: A review. Constr. Build. Mater. 2018, 181, 408–419. [Google Scholar] [CrossRef]
- Jin, F.L.; Park, S.J. Thermomechanical behavior of epoxy resins modified with epoxidized vegetable oils. Polym. Int. 2008, 57, 577–583. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Qu, J.P. Mechanical and Rheological Properties of Epoxidized Soybean Oil Plasticized Poly(lactic acid). J. Appl. Polym. Sci. 2009, 112, 3185–3191. [Google Scholar] [CrossRef]
- Fenollar, O.; Garcia-Sanoguera, D.; Sanchez-Nacher, L.; Lopez, J.; Balart, R. Effect of the epoxidized linseed oil concentration as natural plasticizer in vinyl plastisols. J. Mater. Sci. 2010, 45, 4406–4413. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.; Jiménez-López, M.; Aldas, M.; López, J. Combined effect of linseed oil and gum rosin as natural additives for PVC. Ind. Crops Prod. 2017, 99, 196–204. [Google Scholar] [CrossRef]
- Baryeh, E.A. Effects of palm oil processing parameters on yield. J. Food Eng. 2001, 48, 1–6. [Google Scholar] [CrossRef]
- Jokic, S.; Nagy, B.; Zekovic, Z.; Vidovic, S.; Bilic, M.; Velic, D.; Simandi, B. Effects of supercritical CO2 extraction parameters on soybean oil yield. Food Bioprod. Process. 2012, 90, 693–699. [Google Scholar] [CrossRef]
- Kasote, D.M.; Badhe, Y.S.; Hegde, M.V. Effect of mechanical press oil extraction processing on quality of linseed oil. Ind. Crop. Prod. 2013, 42, 10–13. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valero, J.R. Bio-processing of agro-byproducts to animal feed. Crit. Rev. Biotechnol. 2012, 32, 382–400. [Google Scholar] [CrossRef] [PubMed]
- Barczewski, M.; Mysiukiewicz, O.; Klozinski, A. Complex modification effect of linseed cake as an agricultural waste filler used in high density polyethylene composites. Iran. Polym. J. 2018, 27, 677–688. [Google Scholar] [CrossRef] [Green Version]
- Zuhri, M.Y.M.; Sapuan, S.M.; Ismail, N. Oil Palm Fibre Reinforced Polymer Composites: A Review. Prog. Rubber Plast. Recycl. Technol. 2009, 25, 233–246. [Google Scholar] [CrossRef]
- Fuqua, M.A.; Chevali, V.S.; Ulven, C.A. Lignocellulosic byproducts as filler in polypropylene: Comprehensive study on the effects of compatibilization and loading. J. Appl. Polym. Sci. 2013, 127, 862–868. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Isbrandt, M.; Czuprynski, B.; Apiecionek, L. The Use of Waste from the Production of Rapeseed Oil for Obtaining of New Polyurethane Composites. Polymers 2019, 11, 1431. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Mu, B.S.; Yi, X.; Li, S.J.; Wang, Q.W. Sustainable Use of Coffee Husks for Reinforcing Polyethylene Composites. J. Polym. Environ. 2018, 26, 48–58. [Google Scholar] [CrossRef]
- Sanjay, M.R.; Madhu, P.; Jawaid, M.; Senthamaraikannan, P.; Senthil, S.; Pradeep, S. Characterization and properties of natural fiber polymer composites: A comprehensive review. J. Clean. Prod. 2018, 172, 566–581. [Google Scholar] [CrossRef]
- Li, X.; Lei, B.; Lin, Z.; Huang, L.; Tan, S.; Cai, X. The utilization of bamboo charcoal enhances wood plastic composites with excellent mechanical and thermal properties. Mater. Des. 2014, 53, 419–424. [Google Scholar] [CrossRef]
- Ivorra-Martinez, J.; Manuel-Manogil, J.; Boronat, T.; Sanchez-Nacher, L.; Balart, R.; Quiles-Carrillo, L. Development and Characterization of Sustainable Composites from Bacterial Polyester Poly(3-Hydroxybutyrate-co-3-hydroxyhexanoate) and Almond Shell Flour by Reactive Extrusion with Oligomers of Lactic Acid. Polymers 2020, 12, 1097. [Google Scholar] [CrossRef]
- Ali, N.M.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Tan, S.W.; Tan, S.G. The Promising Future of Chia, Salvia hispanica L. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef] [Green Version]
- Sales Volume of Chia Seeds Worldwide in 2017, by Type. Available online: https://www.statista.com/statistics/860314/global-chia-seed-sales-volume-by-type/ (accessed on 15 April 2021).
- Chia Seeds Market Size Worth $4.7 Billion By 2025. Available online: https://www.grandviewresearch.com/press-release/global-chia-seeds-market (accessed on 17 April 2021).
- Shen, Y.B.; Zheng, L.Y.; Jin, J.; Li, X.J.; Fu, J.N.; Wang, M.Z.; Guan, Y.F.; Song, X. Phytochemical and Biological Characteristics of Mexican Chia Seed Oil. Molecules 2018, 23, 3219. [Google Scholar] [CrossRef] [Green Version]
- Cofrades, S.; Santos-López, J.; Freire, M.; Benedí, J.; Sánchez-Muniz, F.; Jiménez-Colmenero, F. Oxidative stability of meat systems made with W1/O/W2 emulsions prepared with hydroxytyrosol and chia oil as lipid phase. LWT Food Sci. Technol. 2014, 59, 941–947. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Vongsvivut, J.; Adhikari, R.; Adhikari, B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017, 228, 394–402. [Google Scholar] [CrossRef]
- Dominguez-Candela, I.; Ferri, J.M.; Cardona, S.C.; Lora, J.; Fombuena, V. Dual Plasticizer/Thermal Stabilizer Effect of Epoxidized Chia Seed Oil (Salvia hispanica L.) to Improve Ductility and Thermal Properties of Poly (Lactic Acid). Polymers 2021, 13, 1283. [Google Scholar] [CrossRef]
- Darie, R.N.; Bercea, M.; Kozlowski, M.; Spiridon, I. Evaluation of properties of LDPE/oak wood composites exposed to artificial ageing. Cellul. Chem. Technol. 2011, 45, 127–135. [Google Scholar]
- Shah, H.L.; Matuana, L.M. Novel coupling agents for PVC/wood-flour composites. J. Vinyl Addit. Technol. 2005, 11, 160–165. [Google Scholar] [CrossRef]
- Kaseem, M.; Hamad, K.; Deri, F.; Ko, Y.G. Effect of Wood Fibers on the Rheological and Mechanical Properties of Polystyrene/Wood Composites. J. Wood Chem. Technol. 2017, 37, 251–260. [Google Scholar] [CrossRef]
- Goldemberg, J.; Coelho, S.T.; Guardabassi, P. The sustainability of ethanol production from sugarcane. Energy Policy 2008, 36, 2086–2097. [Google Scholar] [CrossRef]
- Babu, R.P.; O’connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Progress Biomater. 2013, 2, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(ethylene terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef]
- Taşdemır, M.; Biltekin, H.; Caneba, G.T. Preparation and characterization of LDPE and PP—Wood fiber composites. J. Appl. Polym. Sci. 2009, 112, 3095–3102. [Google Scholar] [CrossRef]
- Zahedi, M.; Tabarsa, T.; Ashori, A.; Madhoushi, M.; Shakeri, A. A comparative study on some properties of wood plastic composites using canola stalk, Paulownia, and nanoclay. J. Appl. Polym. Sci. 2013, 129, 1491–1498. [Google Scholar] [CrossRef]
- Petchwattana, N.; Covavisaruch, S.; Chanakul, S. Mechanical properties, thermal degradation and natural weathering of high density polyethylene/rice hull composites compatibilized with maleic anhydride grafted polyethylene. J. Polym. Res. 2012, 19, 1–9. [Google Scholar] [CrossRef]
- Boronat, T.; Fombuena, V.; Garcia-Sanoguera, D.; Sanchez-Nacher, L.; Balart, R. Development of a biocomposite based on green polyethylene biopolymer and eggshell. Mater. Des. 2015, 68, 177–185. [Google Scholar] [CrossRef]
- Fombuena, V.; Petrucci, R.; Dominici, F.; Jorda-Vilaplana, A.; Montanes, N.; Torre, L. Maleinized Linseed Oil as Epoxy Resin Hardener for Composites with High Bio Content Obtained from Linen Byproducts. Polymers 2019, 11, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordá-Vilaplana, A.; Carbonell-Verdú, A.; Samper, M.-D.; Pop, A.; Garcia-Sanoguera, D. Development and characterization of a new natural fiber reinforced thermoplastic (NFRP) with Cortaderia selloana (Pampa grass) short fibers. Compos. Sci. Technol. 2017, 145, 1–9. [Google Scholar] [CrossRef]
- Carbonell-Verdú, A.; García-García, D.; Jordá, A.; Samper, M.; Balart, R. Development of slate fiber reinforced high density polyethylene composites for injection molding. Compos. Part. B Eng. 2015, 69, 460–466. [Google Scholar] [CrossRef]
- Fombuena, V.; Bernardi, L.; Fenollar, O.; Boronat, T.; Balart, R. Characterization of green composites from biobased epoxy matrices and bio-fillers derived from seashell wastes. Mater. Design 2014, 57, 168–174. [Google Scholar] [CrossRef]
- Pickering, K.L.; Abdalla, A.; Ji, C.; McDonald, A.; Franich, R.A. The effect of silane coupling agents on radiata pine fibre for use in thermoplastic matrix composites. Compos. Part. A Appl. Sci. Manuf. 2003, 34, 915–926. [Google Scholar] [CrossRef] [Green Version]
- Salon, M.C.B.; Gerbaud, G.; Abdelmouleh, M.; Bruzzese, C.; Boufi, S.; Belgacem, M.N. Studies of interactions between silane coupling agents and cellulose fibers with liquid and solid-state NMR. Magn. Reson. Chem. 2007, 45, 473–483. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part. A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Zhu, J.; Zhu, H.; Njuguna, J.; Abhyankar, H. Recent development of flax fibres and their reinforced composites based on different polymeric matrices. Materials 2013, 6, 5171–5198. [Google Scholar] [CrossRef]
- Wunderlich, B. Thermal Analysis; Elsevier: New York, NY, USA, 1990. [Google Scholar]
- Quiles-Carrillo, L.; Blanes-Martínez, M.; Montanes, N.; Fenollar, O.; Torres-Giner, S.; Balart, R. Reactive toughening of injection-molded polylactide pieces using maleinized hemp seed oil. Eur. Polym. J. 2018, 98, 402–410. [Google Scholar] [CrossRef]
- Aguero, A.; del Carmen Morcillo, M.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Lascano, D.; Torres-Giner, S.; Fenollar, O. Study of the Influence of the Reprocessing Cycles on the Final Properties of Polylactide Pieces Obtained by Injection Molding. Polymers 2019, 11, 1908. [Google Scholar] [CrossRef] [Green Version]
- Ixtaina, V.Y.; Martinez, M.L.; Spotorno, V.; Mateo, C.M.; Maestri, D.M.; Diehl, B.W.K.; Nolasco, S.M.; Tomas, M.C. Characterization of chia seed oils obtained by pressing and solvent extraction. J. Food Compos. Anal. 2011, 24, 166–174. [Google Scholar] [CrossRef]
- Ge, X.; Li, X.; Meng, Y. Tensile properties, morphology, and thermal behavior of PVC composites containing pine flour and bamboo flour. J. Appl. Polym. Sci. 2004, 93, 1804–1811. [Google Scholar] [CrossRef]
- Abdelmouleh, M.; Boufi, S.; Belgacem, M.N.; Dufresne, A. Short natural-fibre reinforced polyethylene and natural rubber composites: Effect of silane coupling agents and fibres loading. Compos. Sci. Technol. 2007, 67, 1627–1639. [Google Scholar] [CrossRef]
- Xiong, C.; Qi, R.; Wang, Y. Wood-Thermoplastic Composites from Wood Flour and High-Density Polyethylene. J. Appl. Polym. Sci. 2009, 114, 1160–1168. [Google Scholar] [CrossRef]
- Ihamouchen, C.; Djidjelli, H.; Boukerrou, A.; Krim, S.; Kaci, M.; Martinez, J.J. Effect of Surface Treatment on the Physicomechanical and Thermal Properties of High-Density Polyethylene/Olive Husk Flour Composites. J. Appl. Polym. Sci. 2012, 123, 1310–1319. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Carbonell-Verdu, A.; Jorda-Vilaplana, A.; Balart, R.; Garcia-Sanoguera, D. Development and characterization of green composites from bio-based polyethylene and peanut shell. J. Appl. Polymer. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Fonseca, L.P.; Waldman, W.R.; De Paoli, M.A. ABS composites with cellulose fibers: Towards fiber-matrix adhesion without surface modification. Compos. Part. C Open Access 2021, 5, 100142. [Google Scholar] [CrossRef]
- Balart, J.F.; Garcia-Sanoguera, D.; Balart, R.; Boronat, T.; Sanchez-Nacher, L. Manufacturing and properties of biobased thermoplastic composites from poly(lactid acid) and hazelnut shell wastes. Polymer. Compos. 2018, 39, 848–857. [Google Scholar] [CrossRef]
- Maziad, N.A.; El-Nashar, D.E.; Sadek, E.M. The effects of a silane coupling agent on properties of rice husk-filled maleic acid anhydride compatibilized natural rubber/low-density polyethylene blend. J. Mater. Sci. 2009, 44, 2665–2673. [Google Scholar] [CrossRef]
- Gharbi, A.; Hassen, R.B.; Boufi, S. Composite materials from unsaturated polyester resin and olive nuts residue: The effect of silane treatment. Ind. Crops Prod. 2014, 62, 491–498. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, X.; Bao, J.; Xie, J.; Tang, X.; Che, J.; Ma, Y.; Tong, J. Characterization of silane treated and untreated natural cellulosic fibre from corn stalk waste as potential reinforcement in polymer composites. Carbohydr. Polym. 2019, 218, 179–187. [Google Scholar] [CrossRef]
- Rojas-Lema, S.; Torres-Giner, S.; Quiles-Carrillo, L.; Gomez-Caturla, J.; Garcia-Garcia, D.; Balart, R. On the Use of Phenolic Compounds Present in Citrus Fruits and Grapes as Natural Antioxidants for Thermo-Compressed Bio-Based High-Density Polyethylene Films. Antioxidants 2021, 10, 14. [Google Scholar] [CrossRef]
- Toro, P.; Quijada, R.; Arias, J.L.; Yazdani-Pedram, M. Mechanical and morphological studies of poly(propylene)-filled eggshell composites. Macromol. Mater. Eng. 2007, 292, 1027–1034. [Google Scholar] [CrossRef]
- Bijaisoradat, O.; Yue, L.; Manas-Zloczower, I.; Manuspiya, H. Wood flour-high density polyethylene composites: Influence of silanization and esterification on mechanical properties. J. Appl. Polym. Sci. 2021, 138. [Google Scholar] [CrossRef]
- Petinakis, E.; Yu, L.; Edward, G.; Dean, K.; Liu, H.; Scully, A.D. Effect of matrix–particle interfacial adhesion on the mechanical properties of poly (lactic acid)/wood-flour micro-composites. J. Polym. Environ. 2009, 17, 83–94. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Montava-Jorda, S.; Boronat, T.; Sammon, C.; Balart, R.; Torres-Giner, S. On the Use of Gallic Acid as a Potential Natural Antioxidant and Ultraviolet Light Stabilizer in Cast-Extruded Bio-Based High-Density Polyethylene Films. Polymers 2020, 12, 31. [Google Scholar] [CrossRef] [Green Version]
- Chun, K.S.; Husseinsyah, S.; Osman, H. Mechanical and thermal properties of coconut shell powder filled polylactic acid biocomposites: Effects of the filler content and silane coupling agent. J. Polym. Res. 2012, 19, 1–18. [Google Scholar] [CrossRef]
- Srubar, W.V., III; Pilla, S.; Wright, Z.C.; Ryan, C.A.; Greene, J.P.; Frank, C.W.; Billington, S.L. Mechanisms and impact of fiber-matrix compatibilization techniques on the material characterization of PHBV/oak wood flour engineered biobased composites. Compos. Sci. Technol. 2012, 72, 708–715. [Google Scholar] [CrossRef]
- Calabia, B.P.; Ninomiya, F.; Yagi, H.; Oishi, A.; Taguchi, K.; Kunioka, M.; Funabashi, M. Biodegradable Poly(butylene succinate) Composites Reinforced by Cotton Fiber with Silane Coupling Agent. Polymers 2013, 5, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Ueno, T.; Nakashima, E.; Takeda, K. Quantitative analysis of random scission and chain-end scission in the thermal degradation of polyethylene. Polym. Degrad. Stab. 2010, 95, 1862–1869. [Google Scholar] [CrossRef]
- Montanes, N.; Garcia-Sanoguera, D.; Segui, V.J.; Fenollar, O.; Boronat, T. Processing and Characterization of Environmentally Friendly Composites from Biobased Polyethylene and Natural Fillers from Thyme Herbs. J. Polym. Environ. 2018, 26, 1218–1230. [Google Scholar] [CrossRef]
- Peng, M.C.; Sethu, V.; Selvarajoo, A. Performance study of chia seeds, chia flour and Mimosa pudica hydrogel as polysaccharide-based superabsorbent polymers for sanitary napkins. Mater. Today Commun. 2021, 26. [Google Scholar] [CrossRef]
- Lee, S.H.; Wang, S.Q. Biodegradable polymers/bamboo fiber biocomposite with bio-based coupling agent. Compos. Part. A Appl. Sci. Manuf. 2006, 37, 80–91. [Google Scholar] [CrossRef]
- Perthue, A.; Bussiere, P.-O.; Baba, M.; Larche, J.-F.; Therias, S.; Karasu, F.; Croutxe-Barghorn, C. Impact of particle size in PE/ATH composites: The relationship between the interphase and water uptake. Prog. Org. Coat. 2018, 114, 145–153. [Google Scholar] [CrossRef]
- Verdu, S.; Vasquez, F.; Ivorra, E.; Sanchez, A.J.; Barat, J.M.; Grau, R. Physicochemical effects of chia (Salvia hispanica) seed flour on each wheat bread-making process phase and product storage. J. Cereal Sci. 2015, 65, 67–73. [Google Scholar] [CrossRef]
- Girones, J.; Mendez, J.A.; Boufi, S.; Vilaseca, F.; Mutje, P. Effect of silane coupling agents on the properties of pine fibers/polypropylene composites. J. Appl. Polym. Sci. 2007, 103, 3706–3717. [Google Scholar] [CrossRef]
- Harshvardhan, K.; Jha, B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea, India. Mar. Pollut. Bull. 2013, 77, 100–106. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.M.B.; Martins, A.B.; Santana, R.M.C. Biodegradability studies of lignocellulosic fiber reinforced composites. In Fiber Reinforced Composites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 241–271. [Google Scholar]
- García-García, D.; Carbonell, A.; Samper, M.; García-Sanoguera, D.; Balart, R. Green composites based on polypropylene matrix and hydrophobized spend coffee ground (SCG) powder. Compos. Part B Eng. 2015, 78, 256–265. [Google Scholar] [CrossRef]
- Zhang, J.; Koubaa, A.; Xing, D.; Liu, W.; Wang, Q.; Wang, X.; Wang, H. Improving lignocellulose thermal stability by chemical modification with boric acid for incorporating into polyamide. Mater. Des. 2020, 191, 108589. [Google Scholar] [CrossRef]
- Georgopoulos, S.T.; Tarantili, P.; Avgerinos, E.; Andreopoulos, A.; Koukios, E. Thermoplastic polymers reinforced with fibrous agricultural residues. Polym. Degrad. Stab. 2005, 90, 303–312. [Google Scholar] [CrossRef]
- Ndiaye, D.; Matuana, L.M.; Morlat-Therias, S.; Vidal, L.; Tidjani, A.; Gardette, J.L. Thermal and mechanical properties of polypropylene/wood-flour composites. J. Appl. Polym. Sci. 2011, 119, 3321–3328. [Google Scholar] [CrossRef]
- Ramos, M.; Dominici, F.; Luzi, F.; Jiménez, A.; Garrigós, M.C.; Torre, L.; Puglia, D. Effect of almond shell waste on physicochemical properties of polyester-based biocomposites. Polymers 2020, 12, 835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hejna, A.; Barczewski, M.; Kosmela, P.; Mysiukiewicz, O.; Kuzmin, A. Coffee silverskin as a multifunctional waste filler for high-density polyethylene green composites. J. Compos. Sci. 2021, 5, 44. [Google Scholar] [CrossRef]
- Liang, Z.; Pan, P.; Zhu, B.; Dong, T.; Inoue, Y. Mechanical and Thermal Properties of Poly(butylene succinate)/Plant Fiber Biodegradable Composite. J. Appl. Polym. Sci. 2010, 115, 3559–3567. [Google Scholar] [CrossRef]
- Hejna, A.; Sulyman, M.; Przybysz, M.; Saeb, M.R.; Klein, M.; Formela, K. On the correlation of lignocellulosic filler composition with the performance properties of poly (ε-caprolactone) based biocomposites. Waste Biomass Valorization 2020, 11, 1467–1479. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Zhu, S.; Chen, Y.; Li, D. Thermal Properties of Wood-Plastic Composites with Different Compositions. Materials 2019, 12, 881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseinihashemi, S.K.; Arwinfar, F.; Najafi, A.; Nemli, G.; Ayrilmis, N. Long-term water absorption behavior of thermoplastic composites produced with thermally treated wood. Measurement 2016, 86, 202–208. [Google Scholar] [CrossRef]
- Chen, R.S.; Ab Ghani, M.H.; Ahmad, S.; Salleh, M.N.; Tarawneh, M.a.A. Rice husk flour biocomposites based on recycled high-density polyethylene/polyethylene terephthalate blend: Effect of high filler loading on physical, mechanical and thermal properties. J. Compos. Mater. 2015, 49, 1241–1253. [Google Scholar] [CrossRef]
- Liu, R.; Peng, Y.; Cao, J.; Chen, Y. Comparison on properties of lignocellulosic flour/polymer composites by using wood, cellulose, and lignin flours as fillers. Compos. Sci. Technol. 2014, 103, 1–7. [Google Scholar] [CrossRef]
- Tajvidi, M.; Najafi, S.K.; Moteei, N. Long-term water uptake behavior of natural fiber/polypropylene composites. J. Appl. Polym. Sci. 2006, 99, 2199–2203. [Google Scholar] [CrossRef]
- Tamrakar, S.; Lopez-Anido, R.A. Water absorption of wood polypropylene composite sheet piles and its influence on mechanical properties. Constr. Build. Mater. 2011, 25, 3977–3988. [Google Scholar] [CrossRef]
- Hatamian, M.; Noshad, M.; Abdanan-Mehdizadeh, S.; Barzegar, H. Effect of roasting treatment on functional and antioxidant properties of chia seed flours. NFS J. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Jorda-Reolid, M.; Gomez-Caturla, J.; Ivorra-Martinez, J.; Stefani, P.M.; Rojas-Lema, S.; Quiles-Carrillo, L. Upgrading Argan Shell Wastes in Wood Plastic Composites with Biobased Polyethylene Matrix and Different Compatibilizers. Polymers 2021, 13, 922. [Google Scholar] [CrossRef] [PubMed]
- Restrepo-Flórez, J.-M.; Bassi, A.; Thompson, M.R. Microbial degradation and deterioration of polyethylene—A review. Int. Biodeterior. Biodegrad. 2014, 88, 83–90. [Google Scholar] [CrossRef]






| Reference | Parts by Weight (wt.%) | ||
|---|---|---|---|
| Bio-HDPE1 | UTCSF2 | TCSF3 | |
| Bio-HDPE | 100 | 0 | 0 |
| 10T | 90 | 0 | 10 |
| 20UT | 80 | 20 | 0 |
| 20T | 80 | 0 | 20 |
| 30T | 70 | 0 | 30 |
| 40T | 60 | 0 | 40 |
| Reference | Tensile Strength (MPa) | Tensile Modulus (MPa) | Elongation at Break (%) | Flexural Strength (MPa) | Flexural Modulus (MPa) | Shore D Hardness | Impact Absorbed Energy (kJ·m−2) |
|---|---|---|---|---|---|---|---|
| Bio-HDPE 1 | 19.01.2 | 373.016.0 | 520.018.5 | 23.91.21 | 804.038.0 | 56.60.5 | 2.750.2 |
| 20UT 2 | 10.70.5 | 374.014.7 | 35.95.7 | 23.130.76 | 784.934.5 | 61.00.7 | 1.670.08 |
| 20T 3 | 12.00.4 | 396.514.5 | 50.85.4 | 24.10.5 | 839.821.6 | 62.21.0 | 1.880.1 |
| Reference | Tm (°C) 1 | Hm (J g−1) 2 | Xc (%) 3 |
|---|---|---|---|
| Bio-HDPE 4 | 131.0 | 154.2 | 55.8 |
| 20UT 5 | 131.8 | 105.8 | 49.5 |
| 20T 6 | 131.5 | 126.1 | 57.8 |
| Reference | Shore D Hardness | Impact Absorbed Energy (kJ·m−2) |
|---|---|---|
| Bio-HDPE 1 | 56.60.7 | 2.750.20 |
| 10T 2 | 59.40.9 | 1.920.10 |
| 20T 2 | 61.00.7 | 1.880.08 |
| 30T 2 | 62.21.1 | 1.650.12 |
| 40T 2 | 63.00.8 | 1.620.10 |
| Reference | Tm (°C) 1 | Hm (J g−1) 2 | Xc (%) 3 |
|---|---|---|---|
| Bio-HDPE 4 | 131.0 | 154.2 | 55.8 |
| 10T 5 | 131.8 | 151.4 | 59.2 |
| 20T 5 | 131.5 | 126.1 | 57.8 |
| 30T 5 | 130.8 | 105.7 | 51.5 |
| 40T 5 | 131.0 | 86.3 | 49.1 |
| Reference | L* 1 | a* 2 | b* 3 | ΔEab* 4 |
|---|---|---|---|---|
| Bio-HDPE | 71.3 ± 0.3 | −2.6 ± 0.1 | −2.68 ± 0.02 | |
| 10T 5 | 42.5 ± 0.1 | 4.15 ± 0.08 | 6.92 ± 0.1 | 31.1 |
| 20T 5 | 42.1 ± 0.2 | 4.89 ± 0.2 | 7.96 ± 0.1 | 32.0 |
| 30T 5 | 36.6 ± 0.1 | 4.24 ± 0.07 | 5.72 ± 0.09 | 36.3 |
| 40T 5 | 37.2 ± 0.1 | 4.65 ± 0.1 | 6.73 ± 0.08 | 36.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez-Candela, I.; Garcia-Garcia, D.; Perez-Nakai, A.; Lerma-Canto, A.; Lora, J.; Fombuena, V. Contribution to a Circular Economy Model: From Lignocellulosic Wastes from the Extraction of Vegetable Oils to the Development of a New Composite. Polymers 2021, 13, 2269. https://doi.org/10.3390/polym13142269
Dominguez-Candela I, Garcia-Garcia D, Perez-Nakai A, Lerma-Canto A, Lora J, Fombuena V. Contribution to a Circular Economy Model: From Lignocellulosic Wastes from the Extraction of Vegetable Oils to the Development of a New Composite. Polymers. 2021; 13(14):2269. https://doi.org/10.3390/polym13142269
Chicago/Turabian StyleDominguez-Candela, Ivan, Daniel Garcia-Garcia, Aina Perez-Nakai, Alejandro Lerma-Canto, Jaime Lora, and Vicent Fombuena. 2021. "Contribution to a Circular Economy Model: From Lignocellulosic Wastes from the Extraction of Vegetable Oils to the Development of a New Composite" Polymers 13, no. 14: 2269. https://doi.org/10.3390/polym13142269
APA StyleDominguez-Candela, I., Garcia-Garcia, D., Perez-Nakai, A., Lerma-Canto, A., Lora, J., & Fombuena, V. (2021). Contribution to a Circular Economy Model: From Lignocellulosic Wastes from the Extraction of Vegetable Oils to the Development of a New Composite. Polymers, 13(14), 2269. https://doi.org/10.3390/polym13142269










