The Compatibility of Three Silicone Oils with Polydimethylsiloxane and the Microstructure and Properties of Their Composite Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Coating Sample
2.3. Characterization
2.3.1. Phase Separation of Silicone Oil/PDMS Blend Solution
2.3.2. Microscopic Observation of Silicone Oil/PDMS Blend Solution
2.3.3. Fracture Observation of the Coatings
2.3.4. Microscopic Surface Characterization
2.3.5. Mechanical Properties of the Coatings
2.3.6. Surface Properties of Coatings
3. Results
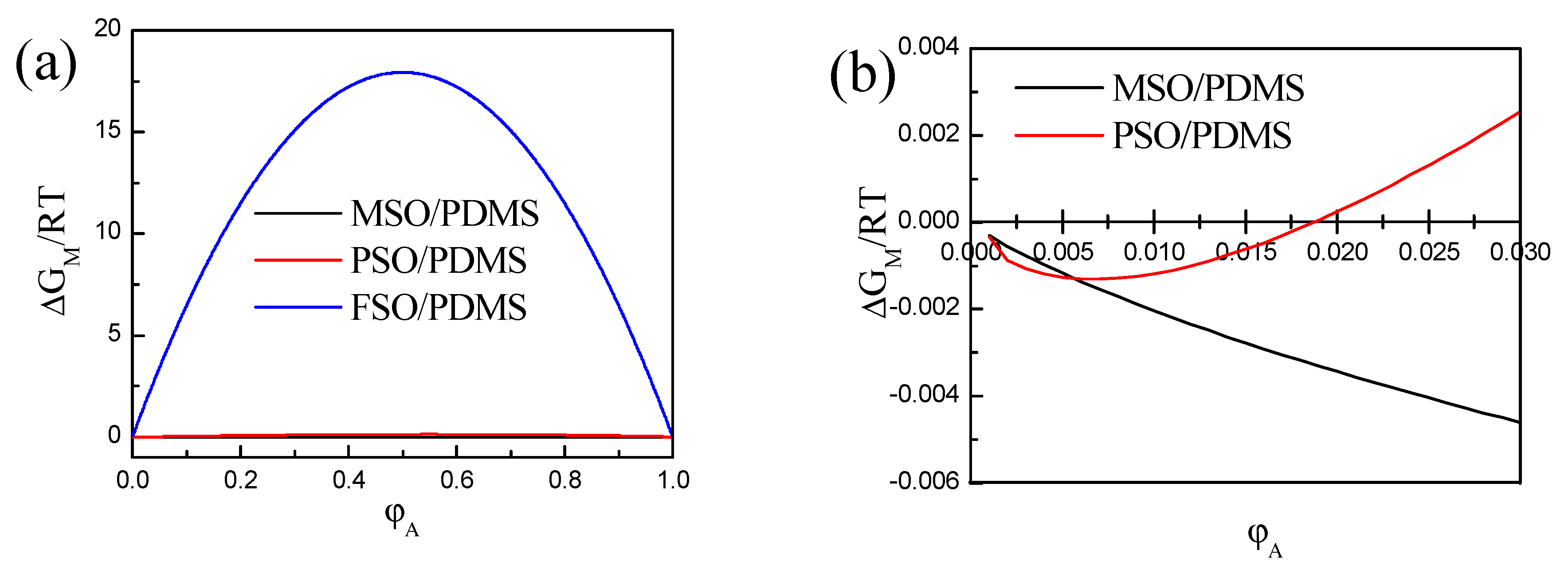
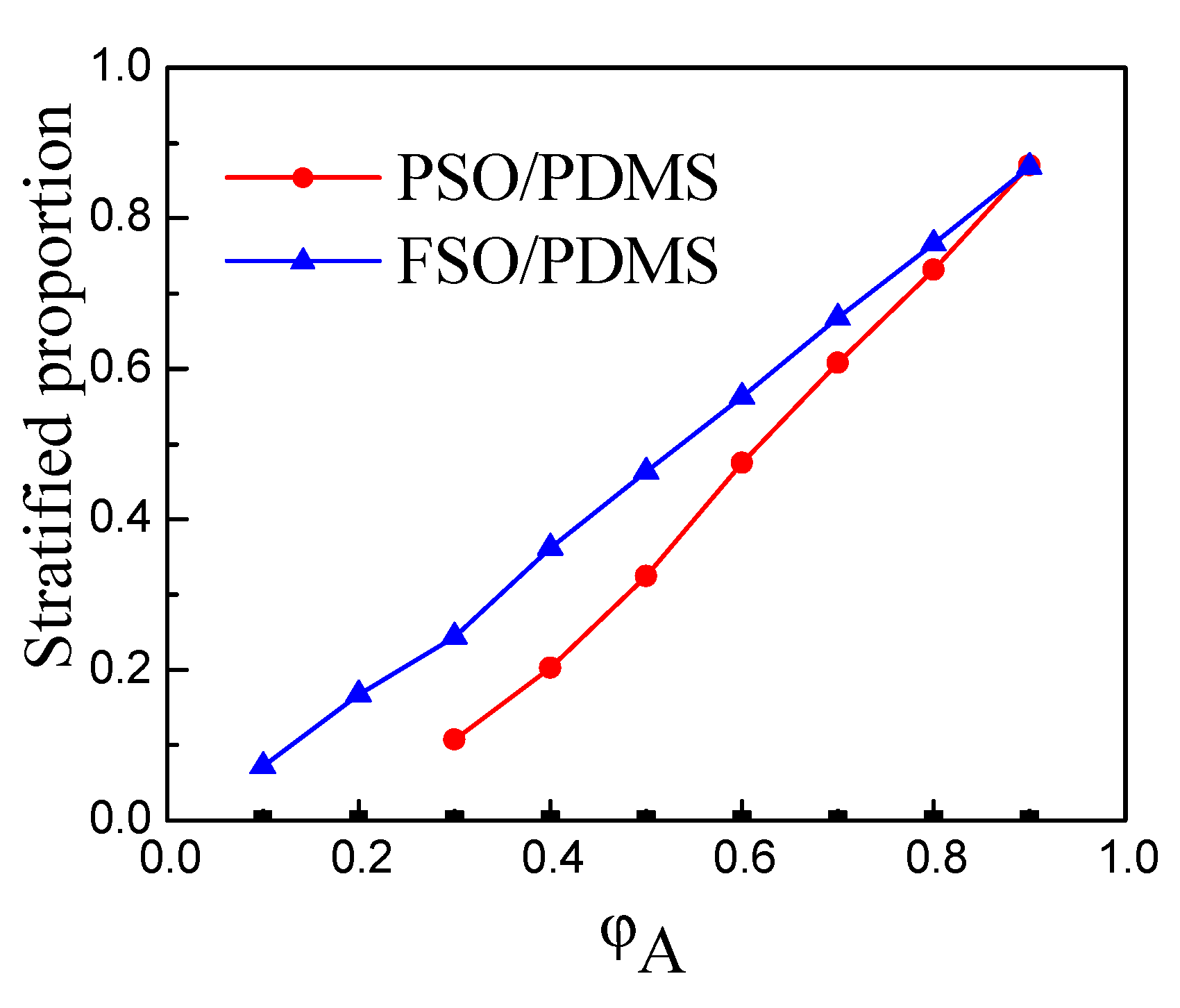
3.1. Phase Separation Thermodynamic of Silicone Oil/PDMS Mixture
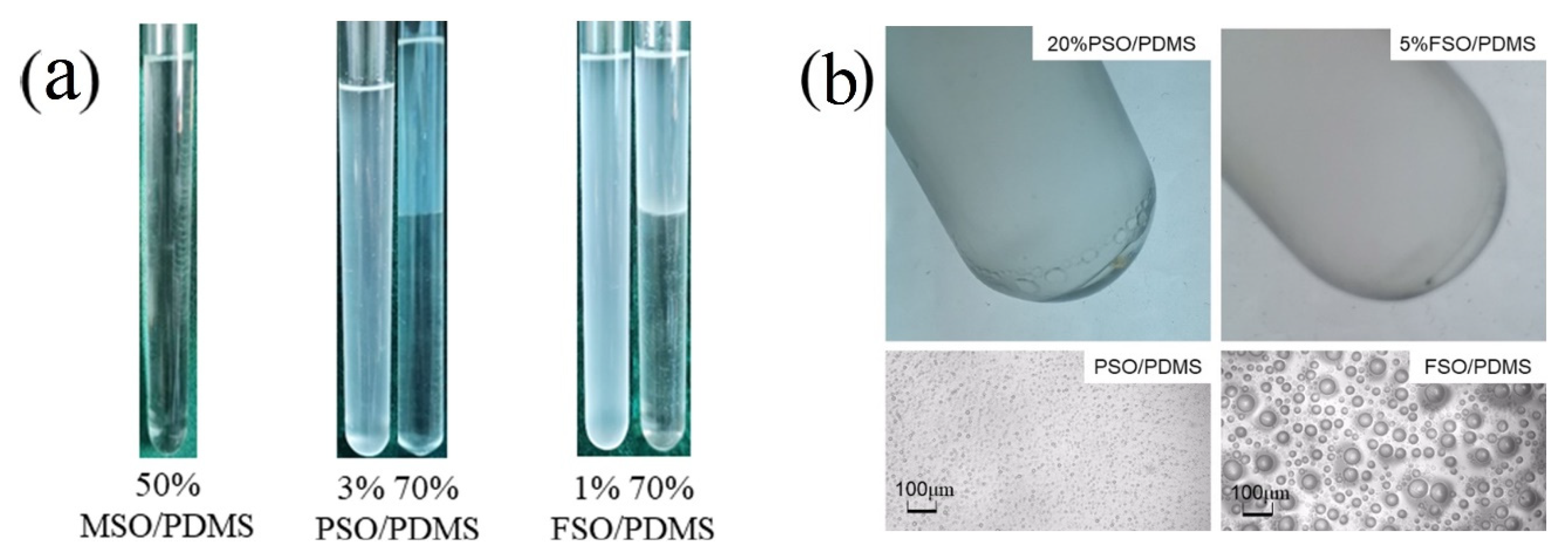
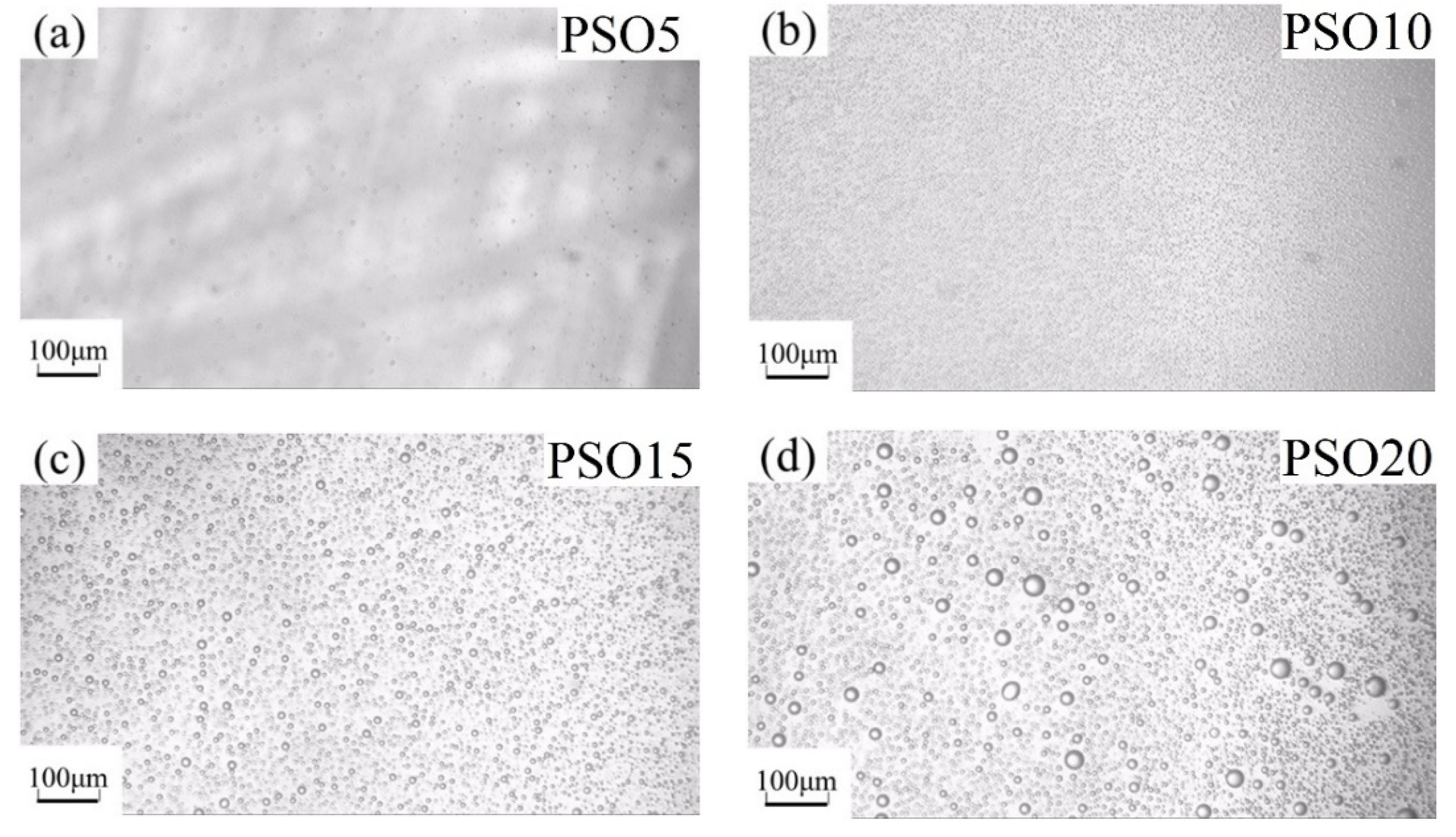
3.2. Experimental Verification of Silicone Oil/PDMS Phase Separation
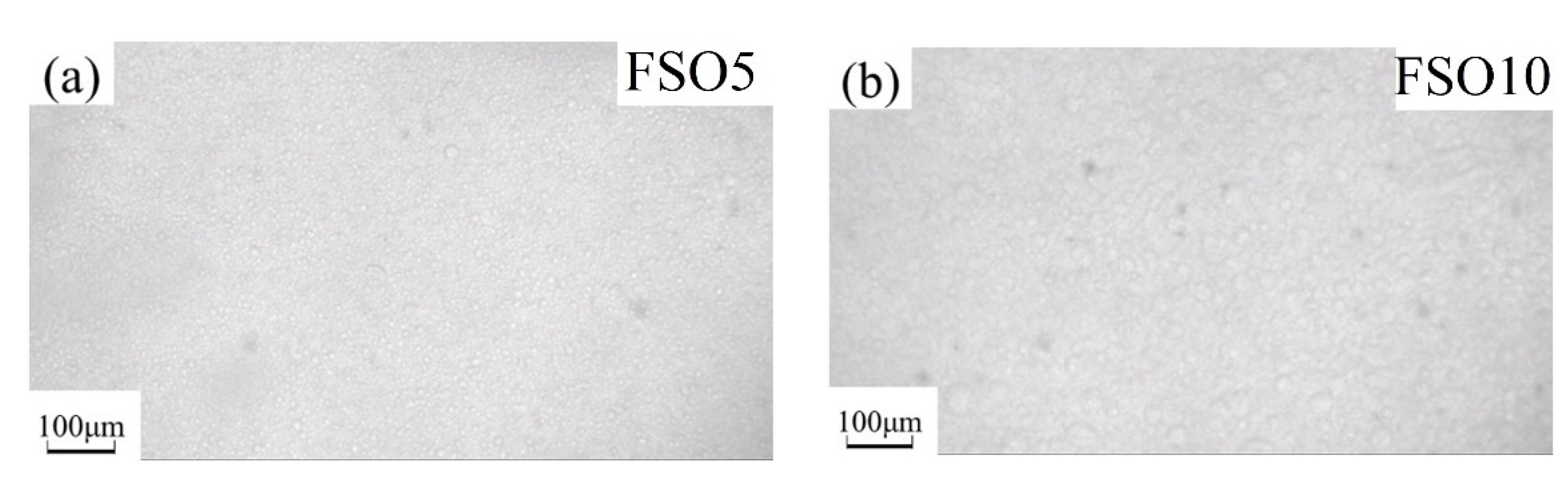
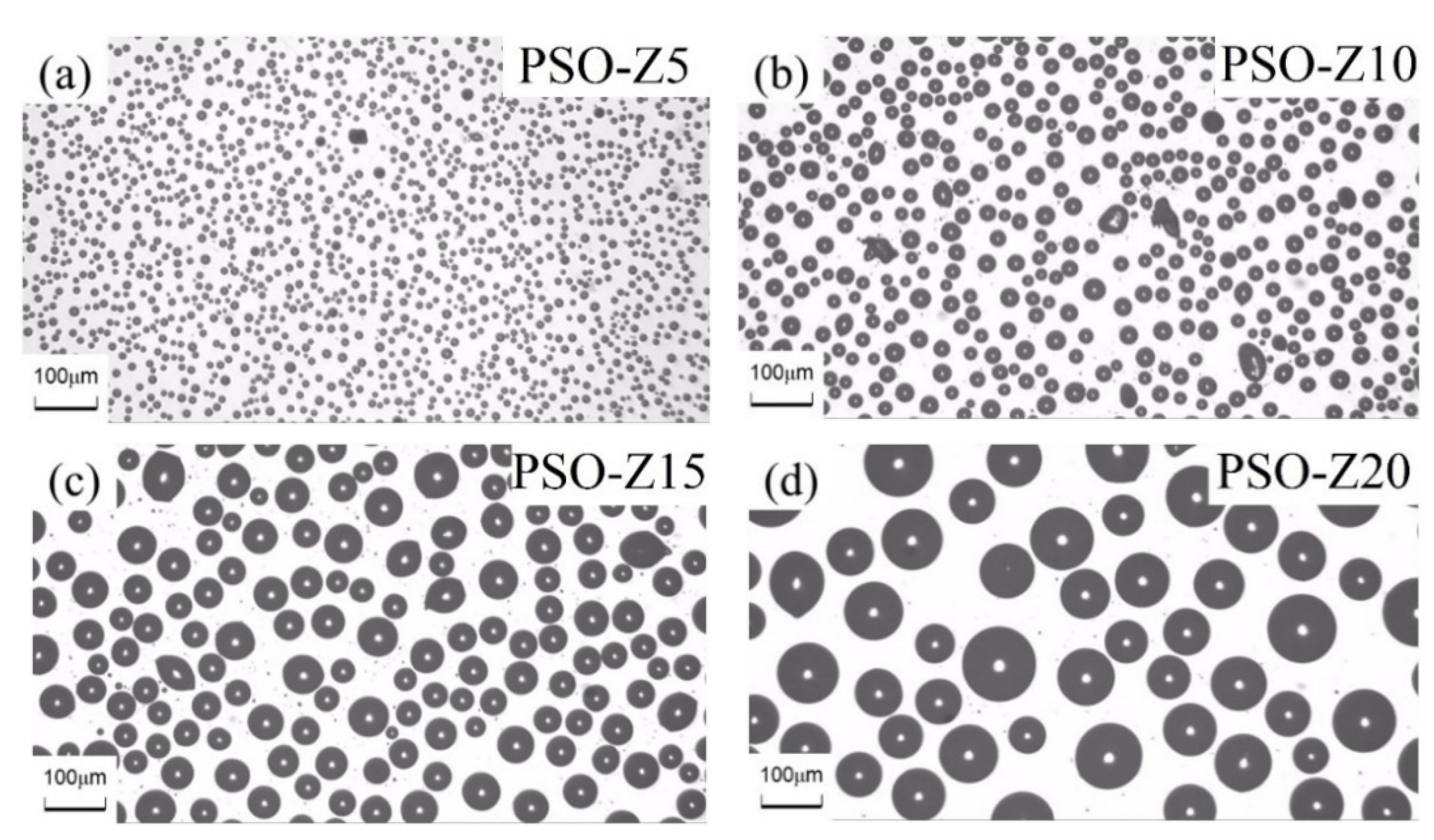
3.3. Fracture Microstructure of the Cured Coatings
3.4. Leaching Behavior of Silicone Oil on the Coating
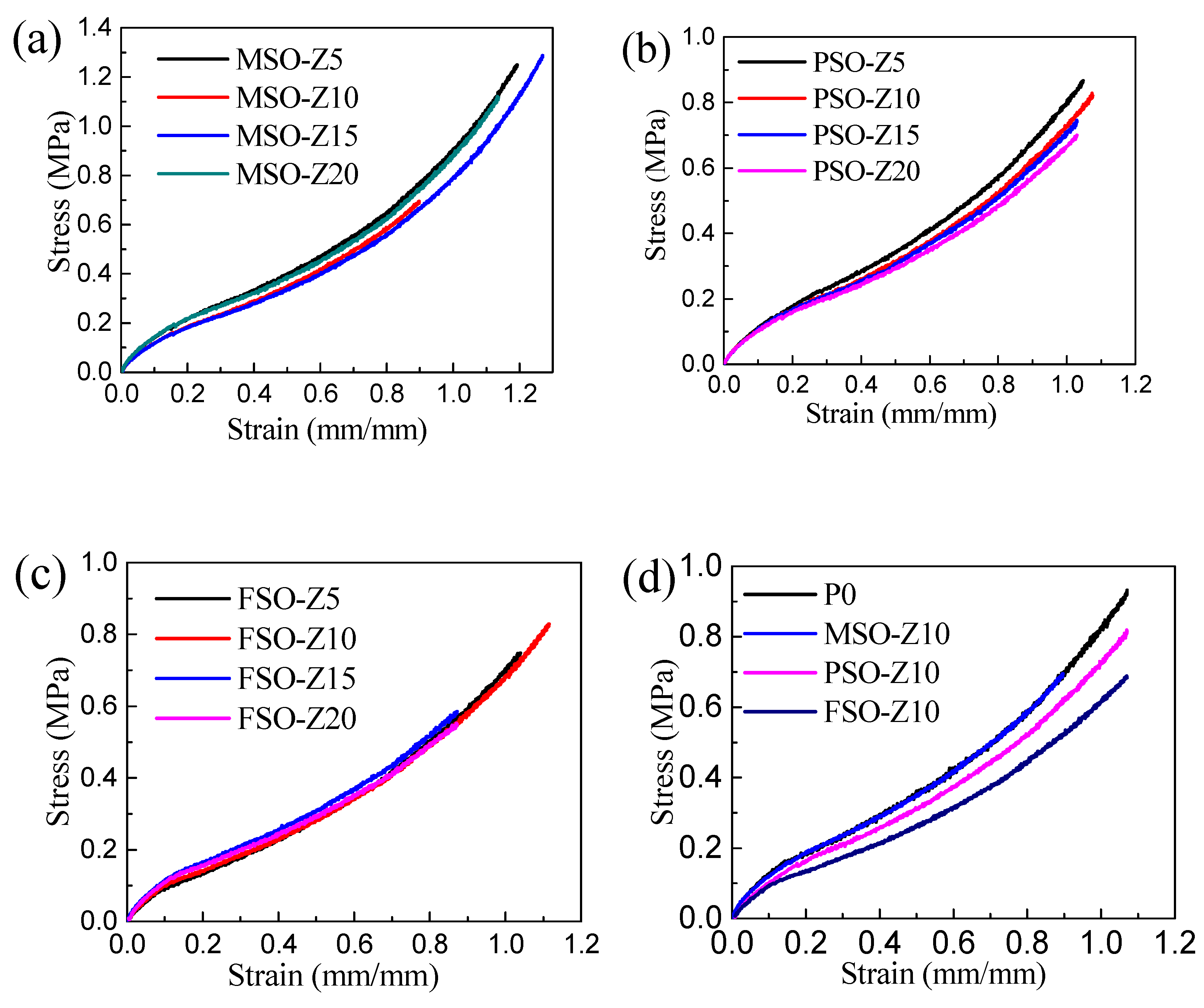
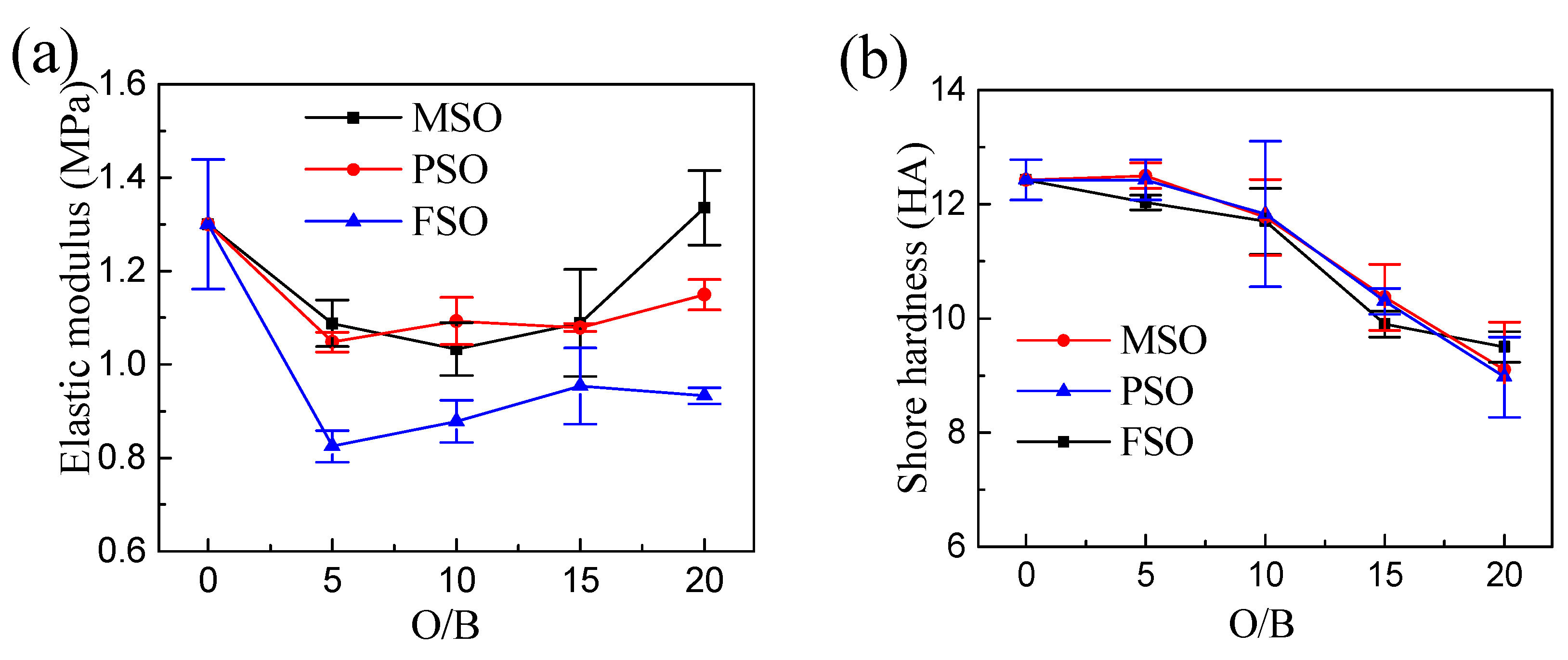
3.5. Mechanical Properties of the Composite Coatings
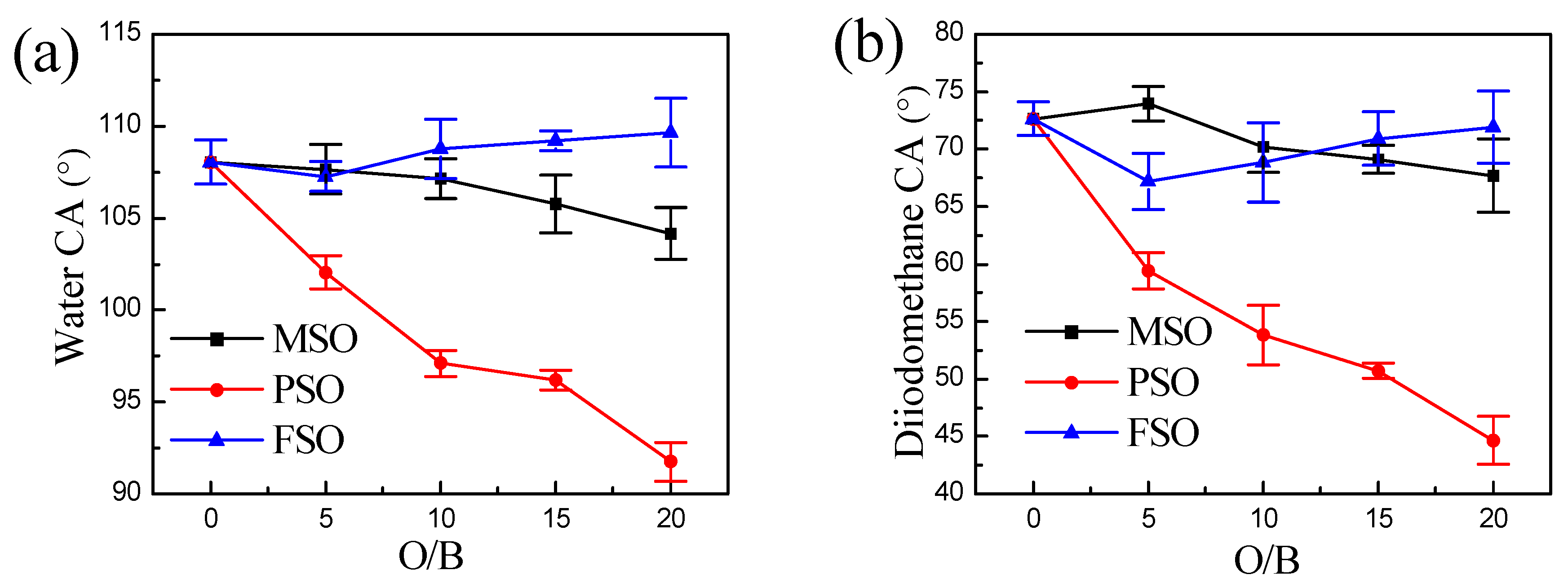
3.6. Surface Energy of the Composite Coatings
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 244. [Google Scholar] [CrossRef]
- Dundua, A.; Franzka, S.; Ulbricht, M. Improved Antifouling Properties of Polydimethylsiloxane Films via Formation of Polysiloxane/Polyzwitterion Interpenetrating Networks. Macromol. Rapid Commun. 2016, 37, 2030–2036. [Google Scholar] [CrossRef]
- Lejars, M.; Margaillan, A.; Bressy, C. Fouling Release Coatings: A Nontoxic Alternative to Biocidal Antifouling Coatings. Chem. Rev. 2012, 112, 4347–4390. [Google Scholar] [CrossRef]
- Yang, W.J.; Neoh, K.G.; Kong, E.T. Polymer brush coatings for combating marine biofouling. Prog. Polym. Sci. 2014, 39, 1017–1042. [Google Scholar] [CrossRef]
- Silva, E.R.; Ferreira, O.; Ramalho, P.A. Eco-friendly non-biocide-release coatings for marine biofouling prevention. Sci. Total Environ. 2019, 650, 2499–2511. [Google Scholar] [CrossRef]
- Ba, M; Zhang, Z.; Qi, Y. The influence of MWCNTs-OH on the properties of the fouling release coatings based on polydimethylsiloxane with the incorporation of phenylmethylsilicone oil. Prog. Org. Coat. 2019, 130, 132–143. [Google Scholar] [CrossRef]
- Nurioglu, A.G.; Esteves, A.C.C.; de With, G. Non-toxic, non-biocide-release antifouling coatings based on molecular structure design for marine applications. J. Mater. Chem. B 2015, 3, 6547–6570. [Google Scholar] [CrossRef] [Green Version]
- Stafslien, S.J.; Bahr, J.A.; Feser, J.M. Combinatorial materials research applied to the development of new surface coatings I: A multiwell plate screening method for the high-throughput assessment of bacterial biofilm retention on surfaces. J. Comb. Chem. 2006, 8, 156–162. [Google Scholar] [CrossRef]
- Chisholm, B.J.; Webster, D.C.; Bennett, J.C. Combinatorial materials research applied to the development of new surface coatings VII: An automated system for adhesion testing. Rev. Sci. Instrum. 2007, 78, 072213. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, S.P.; Zhou, J.L. Controlling Factors and Progress of Low Surface Energy Silicone Antifouling Coatings. Paint Coat. Ind. 2009, 39, 58–61. [Google Scholar]
- Baier, R.E. Surface behaviour of biomaterials: The theta surface for biocompatibility. J. Mater. Sci. Mater. Med. 2006, 17, 1057–1062. [Google Scholar] [CrossRef]
- Brady, R.F.; Singer, I.L. Mechanical factors favoring release from fouling release coatings. Biofouling 2000, 15, 73–81. [Google Scholar] [CrossRef]
- Brady, R.F. Properties which influence marine fouling resistance in polymers containing silicon and fluorine. Prog. Org. Coat. 1999, 35, 31–35. [Google Scholar] [CrossRef]
- Stafslien, S.J.; Christianson, D.; Daniels, J. Combinatorial materials research applied to the development of new surface coatings XVI: Fouling-release properties of amphiphilic polysiloxane coatings. Biofouling 2015, 31, 135–149. [Google Scholar] [CrossRef]
- Ware, C.S.; Smith-Palmer, T.; Peppou-Chapman, S. Marine Antifouling Behavior of Lubricant-Infused Nanowrinkled Polymeric Surfaces. ACS Appl. Mater. Interfaces 2018, 10, 4173–4182. [Google Scholar] [CrossRef]
- Mueller, W.J.; Nowacki, L.J. Ship’s Hull Coated with Antifouling Silicone Rubber. U.S. Patent 3,702,778, 14 November 1972. [Google Scholar]
- Yonehara, Y.; Nanishi, K. Siloxane Polymer Antifouling Paint Composition Containing Polysiloxanes. U.S. Patent 4,910,252, 20 March 1990. [Google Scholar]
- Masato, K.; Makoto, H.; Toshimitsu, M. Antifoul- ing Coating Composition. EP Patent 1,275,705, 28 March 2000. [Google Scholar]
- Takafumi, S. Antifouling Coating Composition and Un-derwater Structure Using the Same. EP Patent 2,143,766, 13 January 2010. [Google Scholar]
- Ba, M.; Zhang, Z.P.; Qi, Y.H. The Dispersion Tolerance of Micro/Nano Particle in Polydimethylsiloxane and Its Influence on the Properties of Fouling Release Coatings Based on Polydimethylsiloxane. Coatings 2017, 7, 7070107. [Google Scholar]
- Chang, J.F.; He, X.Y.; Bai, X.Q. The impact of hydrodynamic shear force on adhesion morphology and biofilm conformation of Bacillus sp. Ocean Eng. 2020, 197, 106860. [Google Scholar] [CrossRef]
- Stein, J.; Truby, K.; Wood, C.D. Silicone foul release coatings: Effect of the interaction of oil and coating functionalities on the magnitude of macrofouling attachment strengths. Biofouling 2003, 19, 71–82. [Google Scholar] [CrossRef]
- Chen, R.R.; Li, Y.K.; Tang, L. Synthesis of zinc-based acrylate copolymers and their marine antifouling application. RSC Adv. 2017, 7, 40020–40027. [Google Scholar] [CrossRef] [Green Version]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Milne, A. Anti-Fouling Marine Compositions. U.S. Patent 4,025,693, 24 May 1977. [Google Scholar]
- Hoipkemeier-Wilson, L.; Schumacher, J.; Carman, M. Antifouling potential of lubricious, micro-engineered, PDMS elastomers against zoospores of the green fouling alga Ulva (Enteromorpha). Biofouling 2004, 20, 53–63. [Google Scholar] [CrossRef]
- Truby, K.; Wood, C.; Stein, J. Evaluation of the performance enhancement of silicone biofouling-release coatings by oil incorporation. Biofouling 2000, 15, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivapooja, P.; Cao, C.Y.; Orihuela, B. Incorporation of silicone oil into elastomers enhances barnacle detachment by active surface strain. Biofouling 2016, 32, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Loriot, M.; Linossier, I.; Vallee-Rehel, K. Influence of Biodegradable Polymer Properties on Antifouling Paints Activity. Polymers 2017, 9, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galhenage, T.P.; Hoffman, D.; Silbert, S.D. Fouling-Release Performance of Silicone Oil-Modified Siloxane-Polyurethane Coatings. ACS Appl. Mater. Interfaces 2016, 8, 29025–29036. [Google Scholar] [CrossRef]
- Oliva, M.; Martinelli, E.; Galli, G. PDMS-based films containing surface-active amphiphilic block copolymers to combat fouling from barnacles B. amphitrite and B. improvises. Polymer 2017, 108, 476–482. [Google Scholar] [CrossRef]
- Wang, X.T.; Olsen, S.M.; Martinez, E.A. Drag resistance of ship hulls: Effects of surface roughness of newly applied fouling control coatings, coating water absorption, and welding seams. J. Coat. Technol. Res. 2018, 15, 657–669. [Google Scholar] [CrossRef] [Green Version]
- Ba, M.; Zhang, Z.P.; Qi, Y.H. Fouling Release Coatings Based on Polydimethylsiloxane with the Incorporation of Phenylmethylsilicone Oil. Coatings 2018, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- ISO 37:2005-Rubber, Vulcanized or Thermoplastic-Determination of Tensile Stress-Strain Properties; International Organization for Standardization: Geneva, Switzerland, 2005.
- Hua, Y.Q.; Jin, R.G. Polymer Solution. Polymer Physics, 4nd ed.; Yang, Q., Li, Y., Eds.; Chemical Industry Publishing Car: Beijing, China, 2013; Volume 3, pp. 101–103. [Google Scholar]
- Liu, D.Z.; Wang, Z.Q. Thermodynamics of Polymer Solution. Solubility Parameter and Its Application in Coating Industry, 1st ed.; Yang, C.X., Liu, Z.H., Eds.; Ocean Press: Beijing, China, 2008; Volume 6, pp. 75–76. [Google Scholar]
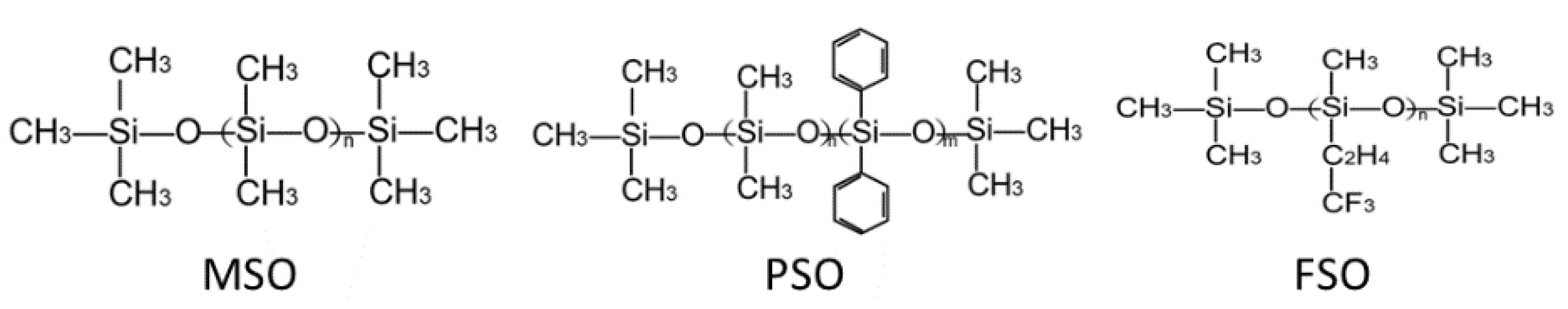



| Types of Silicone Oil | MSO | PSO | FSO |
|---|---|---|---|
| χ1 | 0.0074 | 0.8118 | 71.7798 |
| δ1/(J/cm3)1/2 | 15.10 | 17.22 | 15.83 |
| xA | 22 | 6 | 351 |
| VM1/(mL/mol) | 1770.83 | 432.69 | 17,894.00 |
| Sample | Contact Angle (°) | Surface Free Energy (mJ/m2) | |
|---|---|---|---|
| Water | Diiodomethane | ||
| P0 | 108.05 ± 1.21 | 72.65 ± 1.49 | 21.49 ± 0.86 |
| MSO-Z10 | 107.15 ± 1.09 | 70.15 ± 1.52 | 22.89 ± 0.86 |
| PSO-Z10 | 97.10 ± 0.51 | 53.85 ± 1.83 | 32.39 ± 1.02 |
| FSO-Z10 | 108.75 ± 1.15 | 68.85 ± 2.43 | 23.79 ± 1.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Zhang, Z.; Qi, Y. The Compatibility of Three Silicone Oils with Polydimethylsiloxane and the Microstructure and Properties of Their Composite Coatings. Polymers 2021, 13, 2355. https://doi.org/10.3390/polym13142355
Jiang Y, Zhang Z, Qi Y. The Compatibility of Three Silicone Oils with Polydimethylsiloxane and the Microstructure and Properties of Their Composite Coatings. Polymers. 2021; 13(14):2355. https://doi.org/10.3390/polym13142355
Chicago/Turabian StyleJiang, Yuguo, Zhanping Zhang, and Yuhong Qi. 2021. "The Compatibility of Three Silicone Oils with Polydimethylsiloxane and the Microstructure and Properties of Their Composite Coatings" Polymers 13, no. 14: 2355. https://doi.org/10.3390/polym13142355
APA StyleJiang, Y., Zhang, Z., & Qi, Y. (2021). The Compatibility of Three Silicone Oils with Polydimethylsiloxane and the Microstructure and Properties of Their Composite Coatings. Polymers, 13(14), 2355. https://doi.org/10.3390/polym13142355






