Superparamagnetic, High Magnetic ?-Fe & ??-Fe16N2 Mixture Prepared from Inverse Suspension-Polymerized Fe3O4@polyaniline Composite
Abstract
:1. Introduction
2. Experimental
2.1. Preparation
2.1.1. Synthesis of Fe3O4(OA)
2.1.2. Synthesis of PANI/Fe3O4(OA) Nanocomposite
2.1.3. Calcination of PANI Nanocomposites
2.2. Characterization
2.2.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.2. Ultraviolet and Visible, Near-IR Spectroscopy (UV–Vis–NIR)
2.2.3. TGA (Thermogravimetric Analysis)
2.2.4. Scanning Electron Microscopy (SEM)
2.2.5. Transmission Electron Microscopy (TEM)
2.2.6. Raman Spectroscopy
2.2.7. Powder X-ray Diffraction (Powder XRD)
2.2.8. X-ray Photoelectron Spectroscopy (XPS)
2.2.9. Superconductor Quantum Interference Device (SQUID)
3. Results and Discussion
3.1. FTIR Spectra
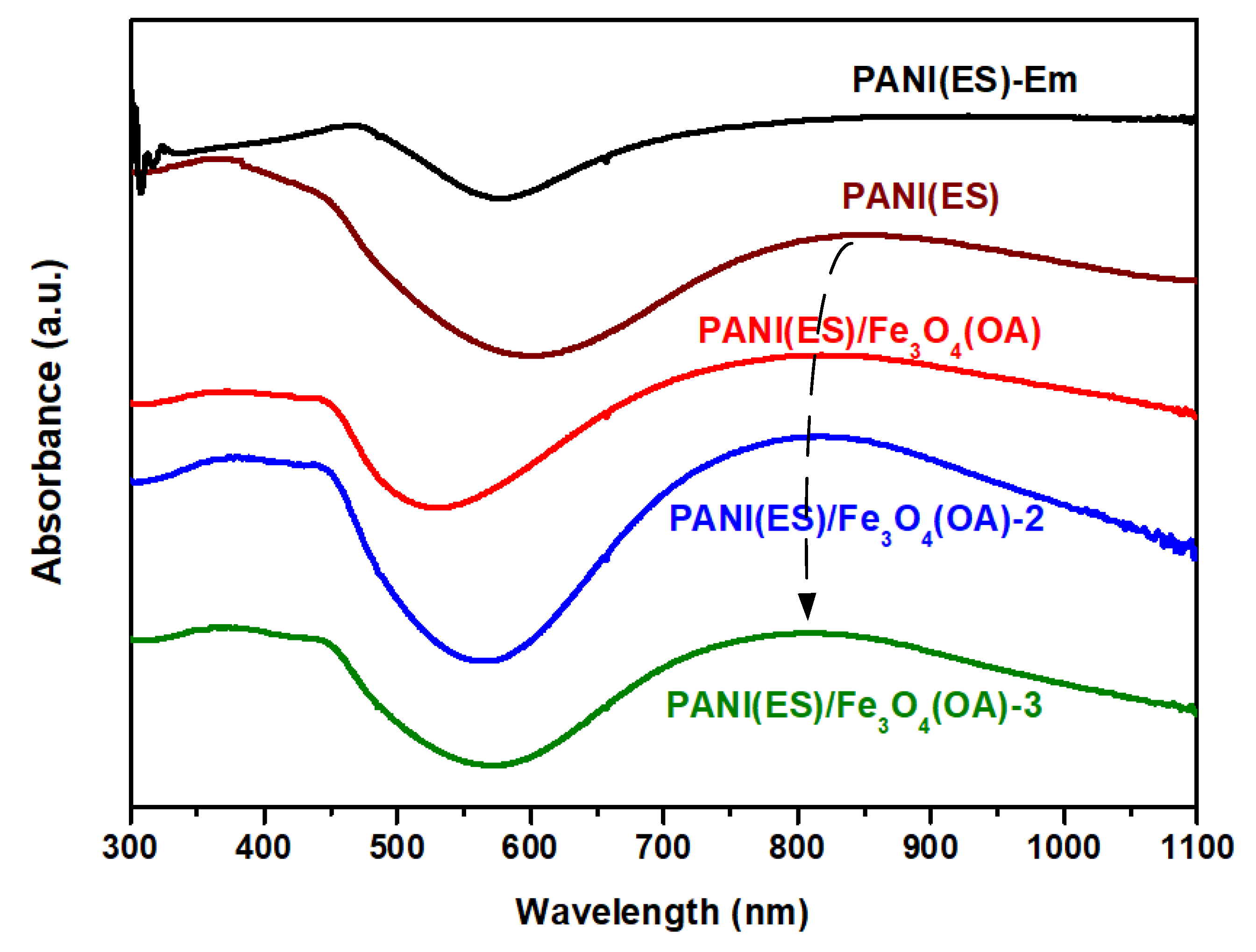
3.2. λmax of PANI in the Nanocomposite Obtained from UV–Vis–NIR Spectra
3.3. TGA Thermogram
3.4. SEM Micropicture
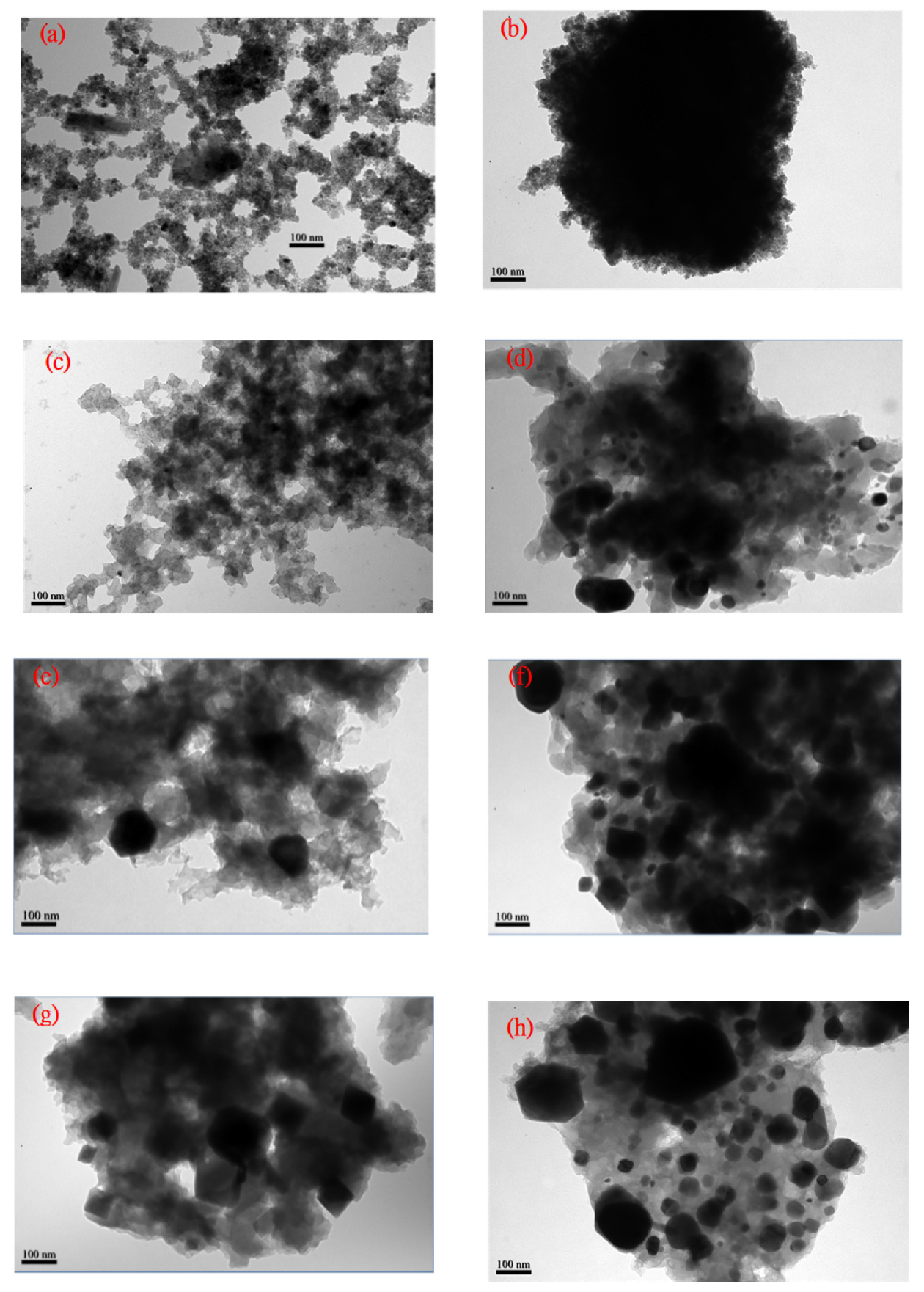
3.5. TEM Micropicture
3.6. Raman Spectra
3.7. Powder XRD Patterns
3.8. XPS Spectra
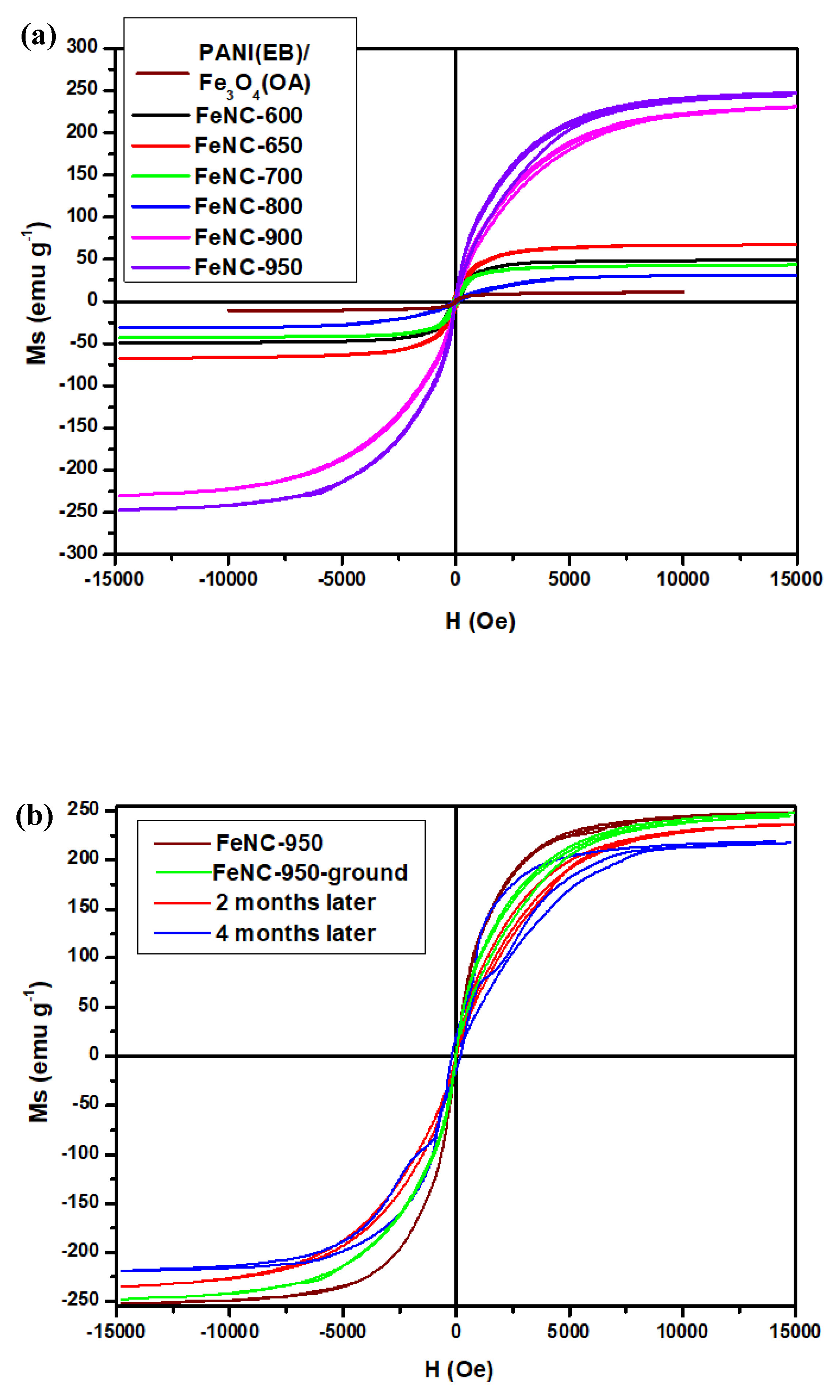
3.9. SQUID Spectra
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fuller, M. Wireless charging in California: Range, recharge, and vehicle electrification. Transp. Res. Part C Emerg. Technol. 2016, 67, 343–356. [Google Scholar] [CrossRef]
- Lin, C.; Wu, G.; Obaidat, M.S.; Yu, C.W. Clustering and splitting charging algorithms for large scaled wireless rechargeable sensor networks. J. Syst. Softw. 2016, 113, 381–394. [Google Scholar] [CrossRef]
- Niu, P.; Liang, X.; Lu, X.; Wang, S.; Li, Y.; Wang, L.; Guo, Y. Preparation of magnetic carbonized polyaniline nanotube and its adsorption behaviors of xanthene colorants in beverage and fish samples. J. Chromatogr. A 2019, 1605, 460369. [Google Scholar] [CrossRef]
- Ghadimi, L.S.; Arsalani, N.; Tabrizi, A.G.; Mohammadi, A.; Ahadzadeh, I. Novel nanocomposite of MnFe2O4 and nitrogen-doped carbon from polyaniline carbonization as electrode material for symmetric ultra-stable supercapacitor. Electrochim. Acta 2018, 282, 116–127. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Tao, L.; Nie, Q.; Meng, F.; Zhu, S.; Cui, L.; Huang, R. Ferromagnetic carbonized polyaniline/nanodiamond hybrids for ultrabroad-band electromagnetic absorption. Carbon 2020, 164, 224–234. [Google Scholar] [CrossRef]
- Sim, B.; Chae, H.S.; Choi, H.J. Fabrication of polyaniline coated iron oxide hybrid particles and their dual stimuli-response under electric and magnetic fields. Express Polym. Lett. 2015, 9, 736–743. [Google Scholar] [CrossRef]
- Siddiqui, M.T.H.; Nizamuddin, S.; Baloch, H.A.; Mubarak, N.M.; Dumbre, D.K.; Inamuddin; Asiri, A.M.; Bhutto, A.W.; Srinivasan, M.; Griffin, G.J. Synthesis of magnetic carbon nanocomposites by hydrothermal carbonization and pyrolysis. Environ. Chem. Lett. 2018, 16, 821–844. [Google Scholar] [CrossRef]
- Prat-Camps, J.; Navau, C.; Sanchez, A. Quasistatic Metamaterials: Magnetic Coupling Enhancement by Effective Space Cancellation. Adv. Mater. 2016, 28, 4898–4903. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, R.; Wang, L.; Tian, X. Preparation of multi-core/single-shell Fe3O4/PANI bifunctional nanoparticles via miniemulsion polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 624–629. [Google Scholar] [CrossRef]
- Zhang, B.; Du, Y.; Zhang, P.; Zhao, H.; Kang, L.; Han, X.; Xu, P. Microwave absorption enhancement of Fe3O4/polyaniline core/shell hybrid microspheres with controlled shell thickness. J. Appl. Polym. Sci. 2013, 130, 1909–1916. [Google Scholar] [CrossRef]
- Yamanaka, K.; Onuma, Y.; Yamashita, S.; Masubuchi, Y.; Takeda, T.; Kikkawa, S. Humidity effects in Fe16N2 fine powder preparation by low-temperature nitridation. J. Solid State Chem. 2010, 183, 2236–2241. [Google Scholar] [CrossRef]
- Li, X.; Xi, S.; Sun, L.; Dou, S.; Huang, Z.; Su, T.; Wang, X. Isolated FeN4 Sites for Efficient Electrocatalytic CO2 Reduction. Adv. Sci. 2020, 7, 2001545. [Google Scholar] [CrossRef]
- Masubuchi, Y.; Yamashita, S.; Motohashi, T.; Kikkawa, S.; Niederberger, M. Magnetite/maghemite mixture prepared in benzyl alcohol for the preparation of α″-Fe16N2 with α-Fe. J. Eur. Ceram. Soc. 2011, 31, 2471–2474. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Z.; Tsai, M.-J.; Hsieh, T.-H.; Tseng, P.-H.; Lu, C.-Y.; Ho, K.-S. Studies on one-dimensional polyanilines prepared with n-dodecylbenzenesulfonic and camphorsulfonic acids. Polym. Int. 2015, 64, 1568–1577. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Chao, L.; Wang, Y.-M.; Ho, K.-S.; Shen, S.-Y.; Hsieh, T.-H.; Wang, Y.-Z. Branched and phenazinized polyaniline nanorod prepared in the presence of meta-phenylenediamine. Synth. Met. 2013, 168, 48–57. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Ho, K.-S.; Cheng, Y.-W.; Chao, L.; Wang, Y.-Z.; Hsieh, T.-H.; Ho, T.-H.; Han, Y.-K. Studies on the synthesis of low molecular weight, one-dimensional polyanilines prepared by fast emulsion polymerization using (n-dodecylbenzenesulfonic acid)/HCl emulsifiers. Polym. Int. 2013, 62, 581–590. [Google Scholar] [CrossRef]
- Chao, L.; Ho, K.-S.; Shen, S.-Y.; Pu, H.-Y.; Hsieh, T.-H.; Kuo, C.-W.; Tseng, B.-H. Short polyaniline nanorod prepared in the presence of para-phenylenediamine. J. Appl. Polym. Sci. 2013, 127, 1853–1862. [Google Scholar] [CrossRef]
- Ho, K.-S.; Han, Y.-K.; Tuan, Y.-T.; Huang, Y.-J.; Wang, Y.-Z.; Ho, T.-H.; Hsieh, T.-H.; Lin, J.-J.; Lin, S.-C. Formation and degradation mechanism of a novel nanofibrous polyaniline. Synth. Met. 2009, 159, 1202–1209. [Google Scholar] [CrossRef]
- Basavaiah, K.; Kumar, Y.P.; Rao, A.V.P. A facile one-pot synthesis of polyaniline/magnetite nanocomposites by micelles-assisted method. Appl. Nanosci. 2013, 3, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Gabunada, J.C.; Vinothkannan, M.; Kim, D.H.; Kim, A.R.; Yoo, D.J. Magnetite Nanorods Stabilized by Polyaniline/Reduced Graphene Oxide as a Sensing Platform for Selective and Sensitive Non-enzymatic Hydrogen Peroxide Detection. Electroanalysis 2019, 31, 1507–1516. [Google Scholar] [CrossRef]
- Subadra, S.T.U.I.; Sutiami, R.; Taufiq, A.; Diantoro, M.; Sunaryono; Arif; Hidayat; Mufti, N.; Hidayat, N.; Susanto, H.; et al. Preparation and Characterization of Magnetite Nanoparticles Combined with Polyaniline and Activated Carbon. IOP Conf. Ser. Earth Environ. Sci. 2019, 276, 012041. [Google Scholar] [CrossRef]
- Xu, F.; Ma, L.; Huo, Q.; Gan, M.; Tang, J. Microwave absorbing properties and structural design of microwave absorbers based on polyaniline and polyaniline/magnetite nanocomposite. J. Magn. Magn. Mater. 2015, 374, 311–316. [Google Scholar] [CrossRef]
- Batool, R.; Akhtar, M.A.; Hayat, A.; Han, D.; Niu, L.; Ahmad, M.A.; Nawaz, M.H. A nanocomposite prepared from magnetite nanoparticles, polyaniline and carboxy-modified graphene oxide for non-enzymatic sensing of glucose. Microchim. Acta 2019, 186, 267. [Google Scholar] [CrossRef]
- Zanotto, C.; Ratuchne, F.; de Castro, E.G.; Marques, P.T. Structural Characterization of Magnetite and its Influence on the Formation of Composites with Polyaniline. Orbital: Electron. J. Chem. 2019, 11, 427–432. [Google Scholar] [CrossRef]
- Farias-Mancilla, R.; Elizalde-Galindo, J.T.; Vigueras-Santiago, E.; Hernández-Escobar, C.A.; Vega-Rios, A.; Zaragoza-Contreras, E.A. Synthesis and Characterization of Polyaniline/Magnetite Nanocomposite. Int. J. Theor. Appl. Nanotechnol. 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Kim, J.H.; Fang, F.F.; Choi, H.J.; Seo, Y. Magnetic composites of conducting polyaniline/nano-sized magnetite and their magnetorheology. Mater. Lett. 2008, 62, 2897–2899. [Google Scholar] [CrossRef]
- Benda, D.; Šňupárek, J.; Čermák, V. Inverse suspension polymerization of the hydrophilic acrylic monomers in the static continuous phase. J. Dispers. Sci. Technol. 1997, 18, 115–121. [Google Scholar] [CrossRef]
- Wang, G.; Li, M.; Chen, X. Inverse suspension polymerization of sodium acrylate. J. Appl. Polym. Sci. 1997, 65, 789–794. [Google Scholar] [CrossRef]
- Choudhary, M.S. Inverse Suspension Polymerization of Partially Neutralized and Lightly Cross-Linked Acrylic Acid: Effect of Reaction Parameters. Macromol. Symp. 2009, 277, 171–176. [Google Scholar] [CrossRef]
- Meouche, W.; Branger, C.; Beurroies, I.; Denoyel, R.; Margaillan, A. Inverse Suspension Polymerization as a New Tool for the Synthesis of Ion-Imprinted Polymers. Macromol. Rapid Commun. 2012, 33, 928–932. [Google Scholar] [CrossRef]
- Takai1, Z.I.; Mustafa, M.K.; Asman, S.; Sekak, K.A. Preparation and Characterization of Magnetite (Fe3O4) nanoparticles By Sol-Gel Method. Int. J. Nanoelectron. Mater. 2019, 12, 37–46. [Google Scholar]
- Ansari, F.; Sobhani, A.; Salavati-Niasari, M. Green synthesis of magnetic chitosan nanocomposites by a new sol–gel auto-combustion method. J. Magn. Magn. Mater. 2016, 410, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Corona-Rivera, M.A.; Ovando-Medina, V.M.; Martínez-Gutiérrez, H.; Silva-Aguilar, F.E.; Pérez, E.; Antonio-Carmona, I.D. Morphology and conductivity tuning of polyaniline using short-chain alcohols by heterophase polymerization. Colloid Polym. Sci. 2015, 293, 605–615. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Chang, M.-Y.; Wang, Y.-Z.; Huang, Y.-C.; Ho, K.-S.; Hsieh, T.-H.; Kuo, Y.-C. Polyaniline Based Pt-Electrocatalyst for a Proton Exchanged Membrane Fuel Cell. Polymers 2020, 12, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.Z.; Huang, W.Y.; Hsieh, T.H.; Jheng, L.C.; Ho, K.S.; Huang, S.W.; Chao, L. FeNxC Based Catalysts Prepared by the Calcination of Iron-Ethylenediamine@Polyaniline as the Cathode-Catalyst of Proton Exchange Membrane Fuel Cell. Polymers 2019, 11, 1368. [Google Scholar] [CrossRef] [Green Version]
- Tseng, P.-H.; Wang, Y.-Z.; Hsieh, T.-H.; Ho, K.-S.; Huang, P.-C.; Lo, W.-T. Facile way to prepare one dimensional Ag@oligoaniline wires. J. Taiwan Inst. Chem. Eng. 2017, 81, 445–454. [Google Scholar] [CrossRef]
- Hsieh, B.-Z.; Chuang, H.-Y.; Chao, L.; Li, Y.-J.; Huang, Y.-J.; Tseng, P.-H.; Hsieh, T.-H.; Ho, K.-S. Formation mechanism of a nanotubular polyanilines prepared by an emulsion polymerization without organic solvent. Polymer 2008, 49, 4218–4225. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Zhang, L.; He, R.; Gu, H.-C. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Shen, S.-Y.; Wu, Y.-J.; Ho, K.-S.; Hsieh, T.-H.; Ho, T.-H.; Wang, Y.-Z.; Tseng, P.-H.; Hsu, Y.-C. Branched and curved nanotubular polyaniline synthesized by emulsion polymerization in presence of zinc salts of n-dodecylbenzenesulfonic acid. Polymer 2011, 52, 2609–2617. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
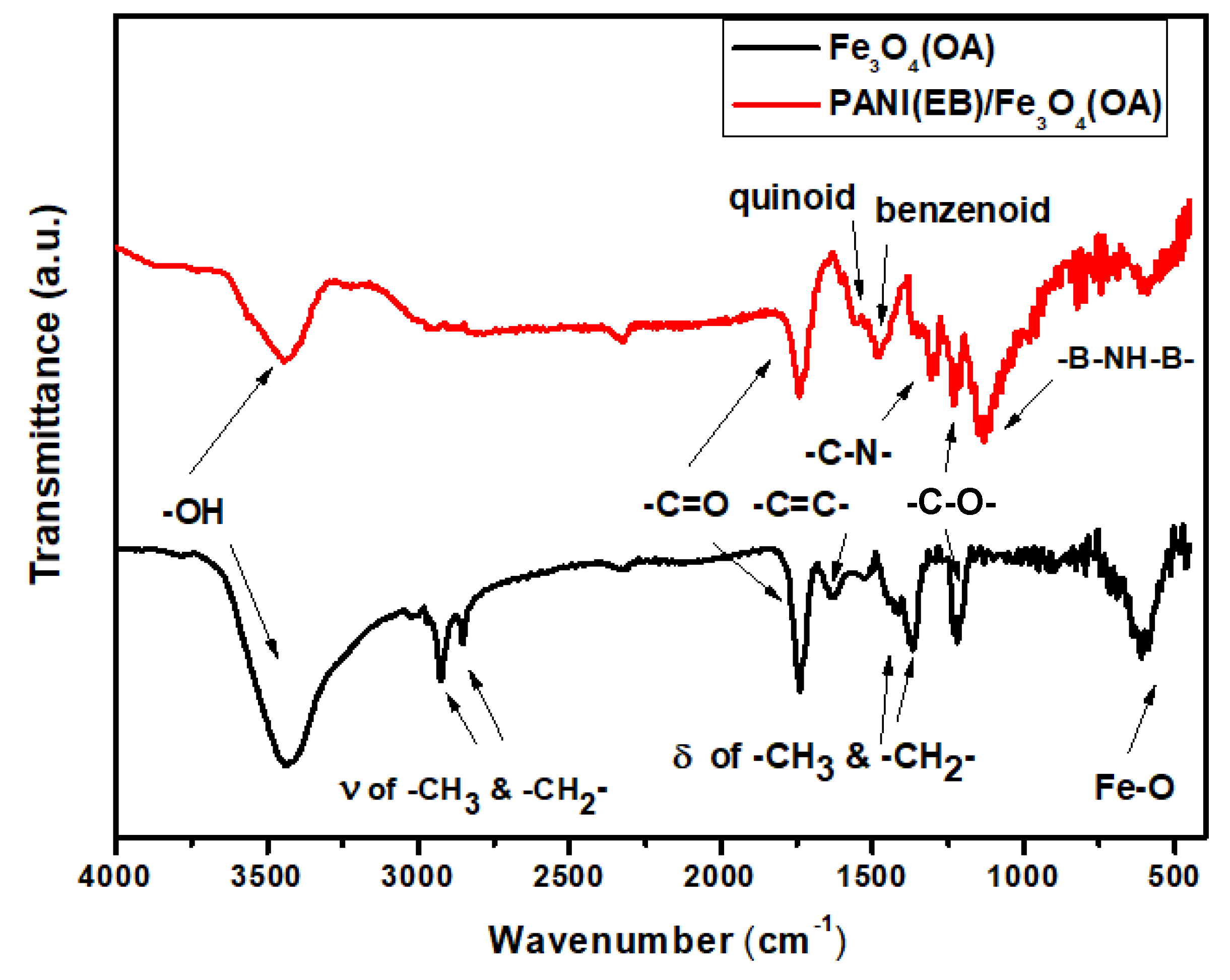







| –OH | 3400 cm−1 |
| –CH3, –CH2 (stretching) | 2900, 3000 cm−1 |
| –C=O | 1750 cm−1 |
| –C=C– | 1650 cm−1 |
| Quinoid ring | 1570 cm−1 |
| Benzoid ring | 1476, cm−1 |
| –CH3, –CH2 (bending) | 1400, 1350 cm−1 |
| –C–N– | 1307 cm−1 |
| –C–OH | 1250 cm−1 |
| –B–NH–B– | 1135 cm−1 |
| Fe–O | 590 cm−1 |
| Properties | Ms (a) (emu g−1) | Weight (b) (%) | ID/IG (c) | |||
|---|---|---|---|---|---|---|
| T (°C) | PANI(EB) | FeNC | PANI(EB) | FeNC | ||
| Room Temp. | 17.7 | 100 | 100 (PANI(EB)/Fe3O4(OA)) | 1.32 | 1.30 (PANI(EB)/Fe3O4(OA)) | |
| 600 | 49.3 | 15.3 | 37.2 | 0.96 | 0.89 | |
| 700 | 43.2 | 13.0 | 36.5 | 0.86 | 0.95 | |
| 800 | 31.0 | 13.1 | 36.0 | 0.82 | 0.97 | |
| 900 | 229.1 | 13.3 | 35.9 | 0.79 | 0.98 | |
| 950 | 244.9 | 13.5 | 35.9 | 0.74 | 0.98 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-Z.; Cheng, Y.-W.; Ho, L.-C.; Huang, W.-Y.; Ho, K.-S.; Syu, Y.-T. Superparamagnetic, High Magnetic ?-Fe & ??-Fe16N2 Mixture Prepared from Inverse Suspension-Polymerized Fe3O4@polyaniline Composite. Polymers 2021, 13, 2380. https://doi.org/10.3390/polym13142380
Wang Y-Z, Cheng Y-W, Ho L-C, Huang W-Y, Ho K-S, Syu Y-T. Superparamagnetic, High Magnetic ?-Fe & ??-Fe16N2 Mixture Prepared from Inverse Suspension-Polymerized Fe3O4@polyaniline Composite. Polymers. 2021; 13(14):2380. https://doi.org/10.3390/polym13142380
Chicago/Turabian StyleWang, Yen-Zen, Yu-Wei Cheng, Lin-Chia Ho, Wen-Yao Huang, Ko-Shan Ho, and Yu-Ting Syu. 2021. "Superparamagnetic, High Magnetic ?-Fe & ??-Fe16N2 Mixture Prepared from Inverse Suspension-Polymerized Fe3O4@polyaniline Composite" Polymers 13, no. 14: 2380. https://doi.org/10.3390/polym13142380
APA StyleWang, Y.-Z., Cheng, Y.-W., Ho, L.-C., Huang, W.-Y., Ho, K.-S., & Syu, Y.-T. (2021). Superparamagnetic, High Magnetic ?-Fe & ??-Fe16N2 Mixture Prepared from Inverse Suspension-Polymerized Fe3O4@polyaniline Composite. Polymers, 13(14), 2380. https://doi.org/10.3390/polym13142380









