Polyimide-Derived Carbon-Coated Li4Ti5O12 as High-Rate Anode Materials for Lithium Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Material
2.2. Preparation of Pyromellitic Dianhydride/4,4′-Oxydianiline Polyimide (PMDA/ODA PI) and PMDA/ODA/p-Phenylenediamine (p-PDA) PI
2.3. Preparation of Carbon-Coated Li4Ti5O12 (LTO)
2.4. Characterization
2.5. Electrochemical Analysis
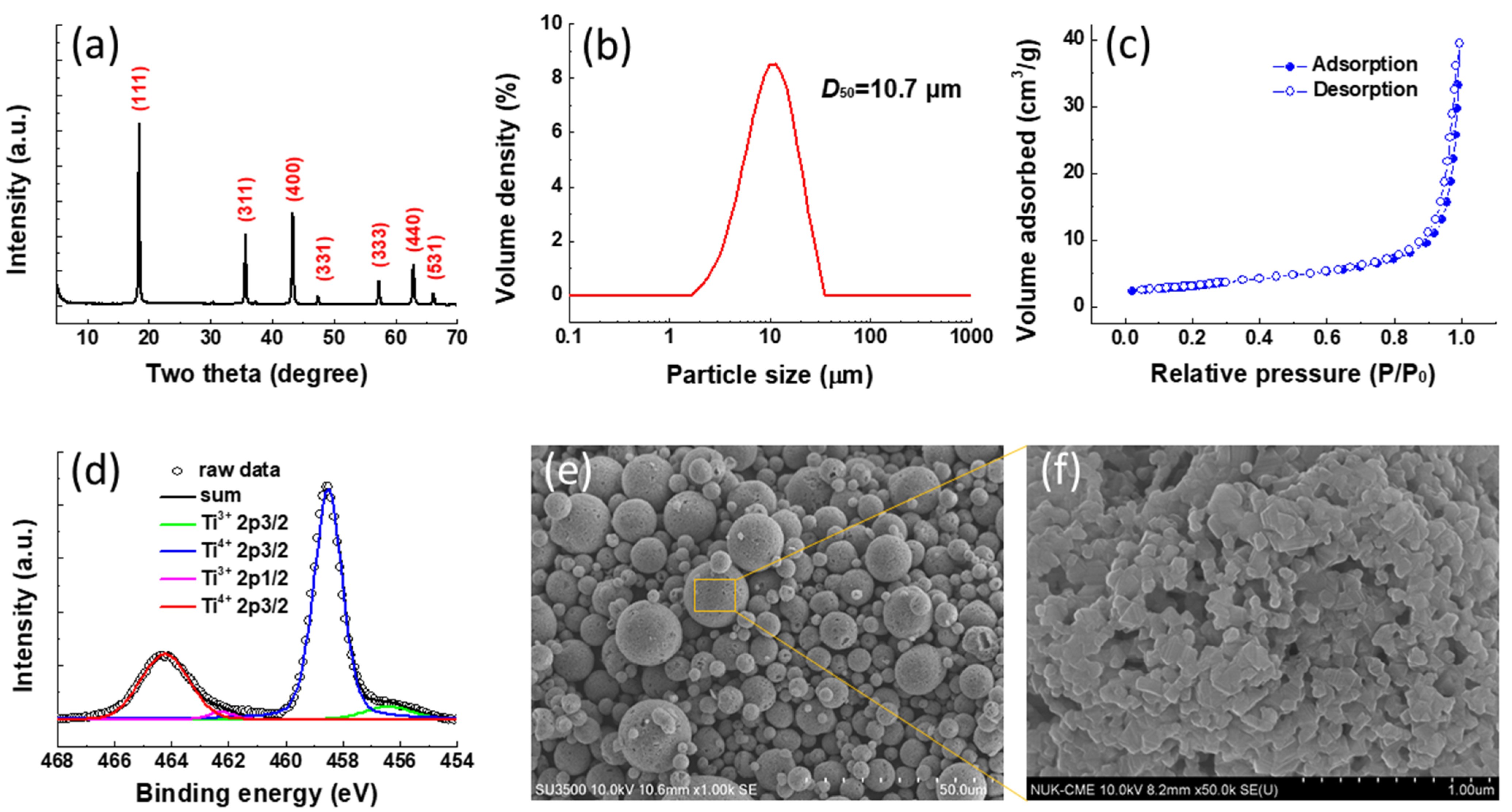
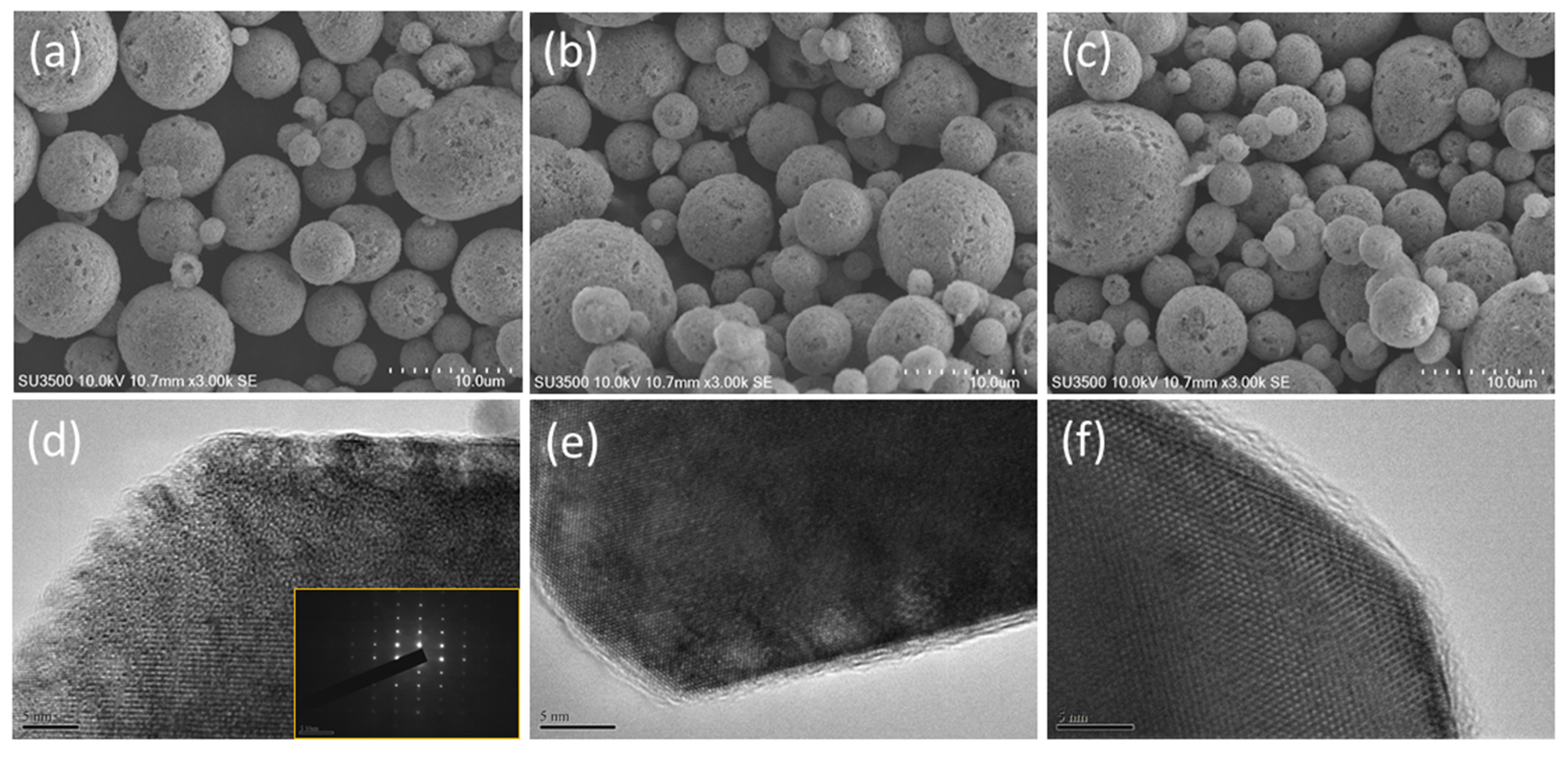
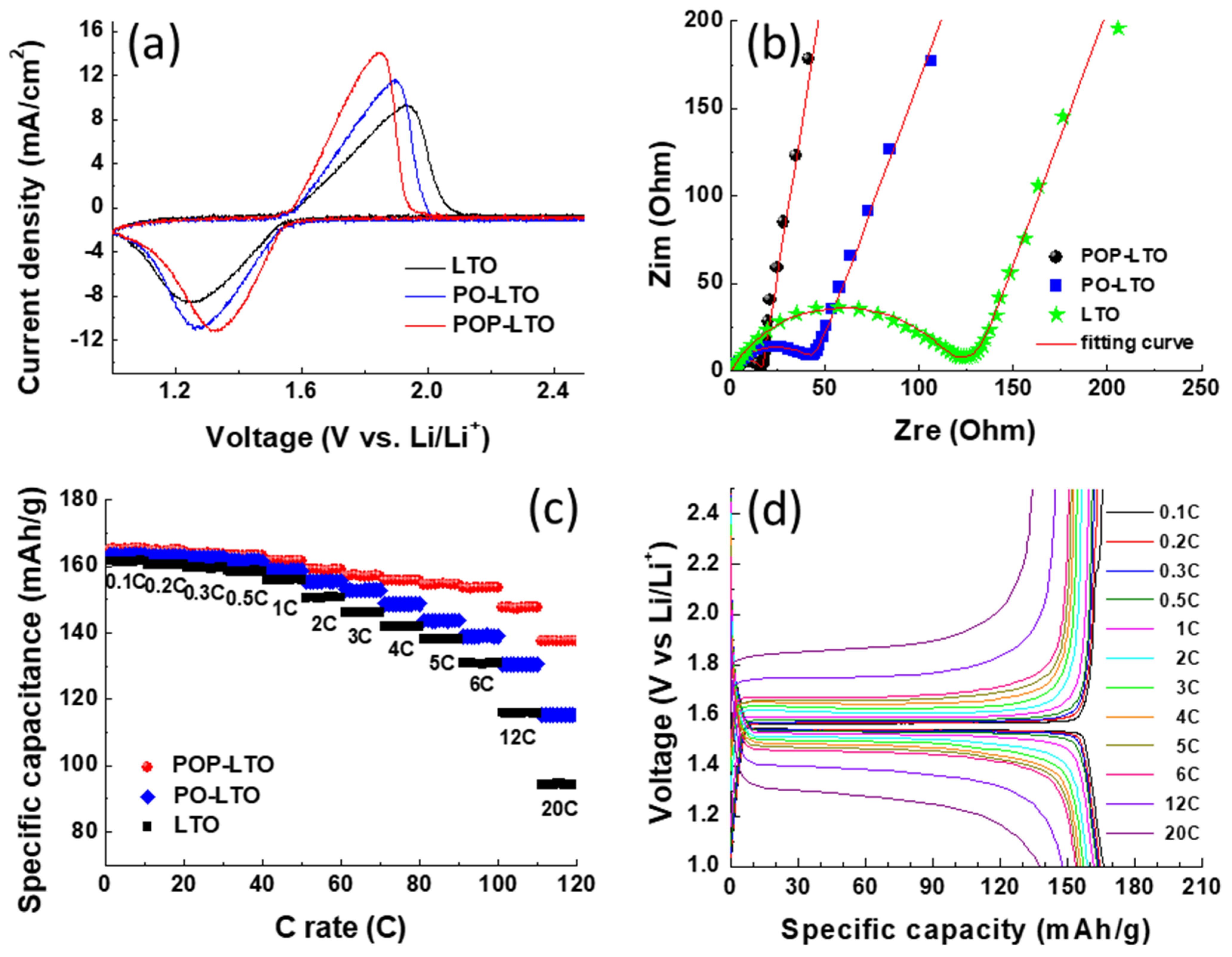
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-ion batteries: Current status and future perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X. Recent developments in the doped-Li4Ti5O12 anode materials of lithium-ion batteries for improving the rate capability. Int. J. Electrochem. Sci. 2013, 8, 6449–6456. [Google Scholar]
- Wu, L.; Leng, X.; Liu, Y.; Wei, S.; Li, C.; Wang, G.; Lian, J.; Jiang, Q.; Nie, A.; Zhang, T.Y. A strategy for synthesis of nanosheets consisting of alternating spinel Li4Ti5O12 and rutile TiO2 lamellas for high-rate anodes of lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 4649–4657. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.M.; Wang, K.X.; Wu, X.Y.; Zhang, H.J.; Bartlett, B.M.; Chen, J.S. Li4Ti5O12/TiO2 hollow spheres composed nanoflakes with preferentially exposed Li4Ti5O12 (011) facets for high-rate lithium ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 19791–19796. [Google Scholar] [CrossRef]
- Park, K.S.; Benayad, A.; Kang, D.J.; Doo, S.G. Nitridation-driven conductive Li4Ti5O12 for lithium ion batteries. J. Am. Chem. Soc. 2008, 130, 14930–14931. [Google Scholar] [CrossRef] [PubMed]
- Long, D.H.; Jeong, M.G.; Lee, Y.S.; Choi, W.; Lee, J.K.; Oh, I.H.; Jung, H.G. Coating lithium titanate with nitrogen-doped carbon by simple refluxing for high-power lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 10250–10257. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.R.; Nam, K.M.; Lee, Y.; Song, K.; Park, J.T.; Kang, Y.M. Phosphidation of Li4Ti5O12 nanoparticles and their electrochemical and biocompatible superiority for lithium rechargeable batteries. Chem. Commun. 2011, 47, 11474–11476. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Kou, L.; Li, J.; Huang, J.; Yang, J.; Wang, Y. Nitrogen-doped carbon-coated V2O5 nanocomposite as cathode materials for lithium-ion battery. J. Mater. Sci. 2018, 53, 10270–10279. [Google Scholar] [CrossRef]
- Kim, T.; Kim, H.; You, T.S.; Kim, J. Carbon-coated V2O5 nanoparticles derived from metal-organic frameworks as a cathode material for rechargeable lithium batteries. J. Alloys Compd. 2017, 727, 522–530. [Google Scholar] [CrossRef]
- Lu, Q.; Jianhua Fang, J.; Yang, J.; Feng, X.; Wang, J.; Nuli, Y. A polyimide ion-conductive protection layer to suppress side reactions on Li4Ti5O12 electrodes at elevated temperature. RSC Adv. 2014, 4, 10280–10283. [Google Scholar] [CrossRef]
- Belharouak, I.; Amine, C.J.K. Synthesis and electrochemical analysis of vapor-deposited carbon-coated LiFePO4. Electrochem. Commun. 2005, 7, 983–988. [Google Scholar] [CrossRef]
- Chen, Z.; Dahn, J.R. Reducing carbon in LiFePO4/C composite electrodes to maximize specific energy, volumetric energy, and tap density. J. Electrochem. Soc. 2002, 149, A1184–A1189. [Google Scholar] [CrossRef]
- Cho, J.; Kim, Y.J.; Park, B. Novel LiCoO2 cathode material with Al2O3 coating for a Li ion cell. Chem. Mater. 2000, 12, 3788–3791. [Google Scholar] [CrossRef]
- Jang, S.B.; Kang, S.H.; Amine, K.; Bae, Y.C.; Sun, Y.K. Synthesis and improved electrochemical performance of Al(OH)3-coated Li[Ni1/3Mn1/3Co1/3]O2 cathode materials at elevated temperature. Electrochim. Acta 2005, 50, 4168–4173. [Google Scholar] [CrossRef]
- Appapillai, A.T.; Mansour, A.N.; Cho, J.; Shao-Horn, Y. Microstructure of LiCoO2 with and without “AlPO4” nanoparticle coating: Combined STEM and XPS studies. Chem. Mater. 2007, 19, 5748–5757. [Google Scholar] [CrossRef]
- Ying, J.; Wan, C.; Jiang, C. Surface treatment of LiNi0.8Co0.2O2 cathode material for lithium secondary batteries. J. Power Sources 2001, 102, 162–166. [Google Scholar] [CrossRef]
- Li, H.; Zhou, H. Enhancing the performances of Li-ion batteries by carbon-coating: Present and future. Chem. Commun. 2012, 48, 1201–1217. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-induced graphene. Acc. Chem. Res. 2018, 51, 1609–1620. [Google Scholar] [CrossRef]
- Inagaki, M.; Harada, S.; Sato, T.; Nakajima, T.; Horino, Y.; Morita, K. Carbonization of polyimide film “Kapton”. Carbon 1989, 27, 253–257. [Google Scholar] [CrossRef]
- Peng, Z.; Ye, R.; Mann, J.A.; Zakhidov, D.; Li, Y.; Smalley, P.R.; Lin, J.; Tour, J.M. Flexible boron-doped laser induced graphene microsupercapacitors. ACS Nano 2015, 9, 5868–5875. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.F.; Fernandes, A.J.S.; Leitão, C.; Deuermeier, J.; Marques, A.C.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-induced graphene strain sensors produced by ultraviolet irradiation of polyimide. Adv. Funct. Mater. 2018, 28, 1805271. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, M.; Wang, L.; Li, Y.; Yakobson, B.I.; Tour, J.M. Oxidized laser-induced graphene for efficient oxygen electrocatalysis. Adv. Mater. 2018, 30, 1707319. [Google Scholar] [CrossRef] [PubMed]
- Bobinger, M.R.; Romero, F.J.; Salinas-Castillo, A.; Becherer, M.; Lugli, P.; Morales, D.P.; Rodríguez, N.; Rivadeneyra, A. Flexible and robust laser-induced graphene heaters photothermally scribed on bare polyimide substrates. Carbon 2019, 144, 116–126. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, M.; Niu, H.; Chang, J.; Liu, W.; Yang, H.; Cao, W.; Wu, D. Structural relationship between random copolyimides and their carbon fibers. J. Mater. Sci. 2017, 52, 1883–1897. [Google Scholar] [CrossRef]
- Ando, S.; Matsuura, T.; Sasaki, S. Coloration of aromatic polyimides and electronic properties of their source materials. Polym. J. 1997, 29, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, T.; Hasuda, Y.; Nishi, S.; Yamadat, N. Polyimide derived from 2,2’-Bis(trifluoromethyl)-4,4’-diaminobiphenyl.1. Synthesis and characterization of polyimides prepared with 2,2-Bis(3,4-dicarboxyphenyl)hexafluoropropane dianhydride or pyromellitic dianhydride. Macromolecules 1991, 24, 5001–5005. [Google Scholar] [CrossRef]
- Kim, S.; Jang, K.S.; Choi, H.D.; Choi, S.H.; Kwon, S.J.; Kim, I.D.; Lim, J.A.; Hong, J.M. Porous polyimide membranes prepared by wet phase inversion for use in low dielectric applications. Int. J. Mol. Sci. 2013, 24, 8698–8707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, K.M.; Li, Y.; Yoo, D.G.; Lee, N.K.; Lee, H.G.; Ando, S.; Ha, C.S. Effects of crosslinking agents on the physical properties of polyimide/amino-functionalized graphene oxide hybrid films. Polym. Int. 2018, 67, 588–597. [Google Scholar] [CrossRef]
- Ji, D.; Li, T.; Zou, Y.; Chu, M.; Zhou, K.; Liu, J.; Tian, G.; Zhang, Z.; Zhang, X.; Li, L.; et al. Copolymer dielectrics with balanced chain-packing density and surface polarity for high-performance flexible organic electronics. Nat. Commun. 2018, 9, 2339. [Google Scholar] [CrossRef] [PubMed]
- Ektessabi, A.N.; Hakamata, S. XPS study of ion beam modified polyimide films. Thin Solid Film. 2000, 377–378, 621–625. [Google Scholar] [CrossRef]
- Liu, J.N.; Sil, M.C.; Cheng, R.; Feng, S.P.; Chen, C.M. Surface silanization of polyimide for autocatalytic metallization. JOM 2020, 72, 3529–3537. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Berda, E.B.; Chen, C.; Wang, K.; Chen, S.; Zou, B.; Liu, B.; Zhou, Q.; Li, F.; et al. The elastic properties and piezochromism of polyimide films under high pressure. Polymer 2016, 90, 1–8. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Niu, H.Q.; Qi, S.L.; Tian, G.F.; Wang, X.D.; Wu, D.Z. Structure evolutions involved in the carbonization of polyimide fibers with different chemical constitution. Mater. Today Commun. 2014, 1, 1–8. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef] [Green Version]
- Sarno, M.; Baldino, L.; Scudieri, C.; Cardea, S.; Ciambelli, P.; Reverchon, E. SC-CO2 assisted process for high energy density aerogel supercapacitor: The effect of GO loading. Nanotechnology 2017, 28, 204001. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, V.E.; Gofman, I.V.; Maritcheva, T.A.; Yudin, V.E.; Eto, K.; Takeichi, T.; Kaburagi, Y.; Hishiyama, Y. The effect of different orientations in rigid rod polyimide films on the graphitized products. Carbon 2007, 45, 839–846. [Google Scholar] [CrossRef]
- Song, H.; Jeong, T.G.; Moon, Y.H.; Chun, H.H.; Chung, K.Y.; Kim, H.S.; Cho, B.W.; Kim, Y.T. Stabilization of oxygen-deficient structure for conducting Li4Ti5O12–δ by molybdenum doping in a reducing atmosphere. Sci. Rep. 2014, 4, 4350. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, S.-C.; Huang, T.-T.; Wu, Y.-J.; Lu, C.-Z.; Weng, H.C.; Huang, J.-H.; Chang-Jian, C.-W.; Liu, T.-Y. Polyimide-Derived Carbon-Coated Li4Ti5O12 as High-Rate Anode Materials for Lithium Ion Batteries. Polymers 2021, 13, 1672. https://doi.org/10.3390/polym13111672
Hsu S-C, Huang T-T, Wu Y-J, Lu C-Z, Weng HC, Huang J-H, Chang-Jian C-W, Liu T-Y. Polyimide-Derived Carbon-Coated Li4Ti5O12 as High-Rate Anode Materials for Lithium Ion Batteries. Polymers. 2021; 13(11):1672. https://doi.org/10.3390/polym13111672
Chicago/Turabian StyleHsu, Shih-Chieh, Tzu-Ten Huang, Yen-Ju Wu, Cheng-Zhang Lu, Huei Chu Weng, Jen-Hsien Huang, Cai-Wan Chang-Jian, and Ting-Yu Liu. 2021. "Polyimide-Derived Carbon-Coated Li4Ti5O12 as High-Rate Anode Materials for Lithium Ion Batteries" Polymers 13, no. 11: 1672. https://doi.org/10.3390/polym13111672







