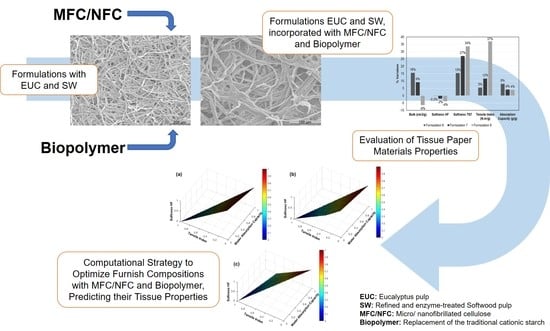
An Innovative Computational Strategy to Optimize Different Furnish Compositions of Tissue Materials Using Micro/Nanofibrillated Cellulose and Biopolymer as Additives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enzymatic and Mechanical Treatments
2.3. Tissue Formulations
2.4. Preparation of Tissue Structures
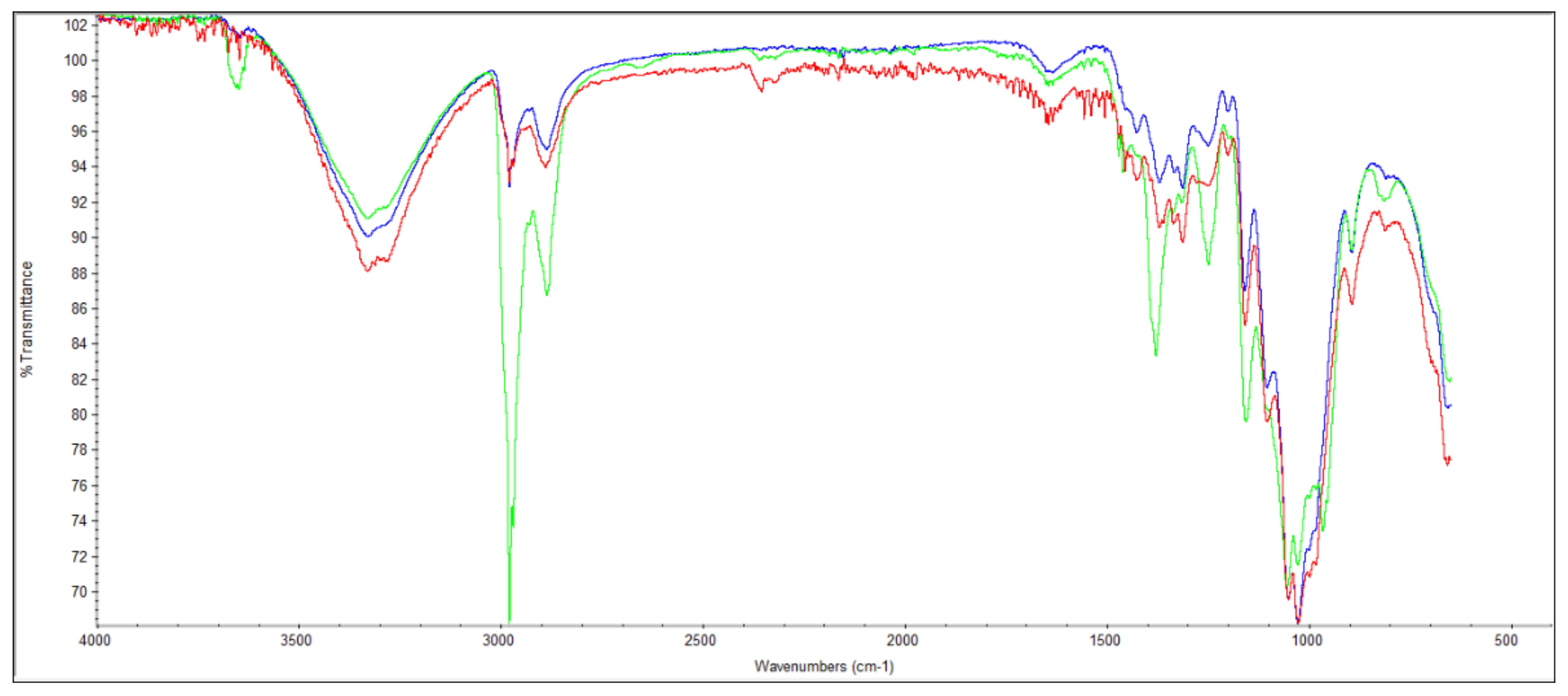
2.5. Characterization of the Morphological, Drainability and Chemical Properties
2.6. Characterization of the Tissue Properties
2.6.1. Structural Properties
2.6.2. Softness Properties
2.6.3. Strength Properties
2.6.4. Absorbency Properties
2.7. Computational Studies
3. Results and Discussion
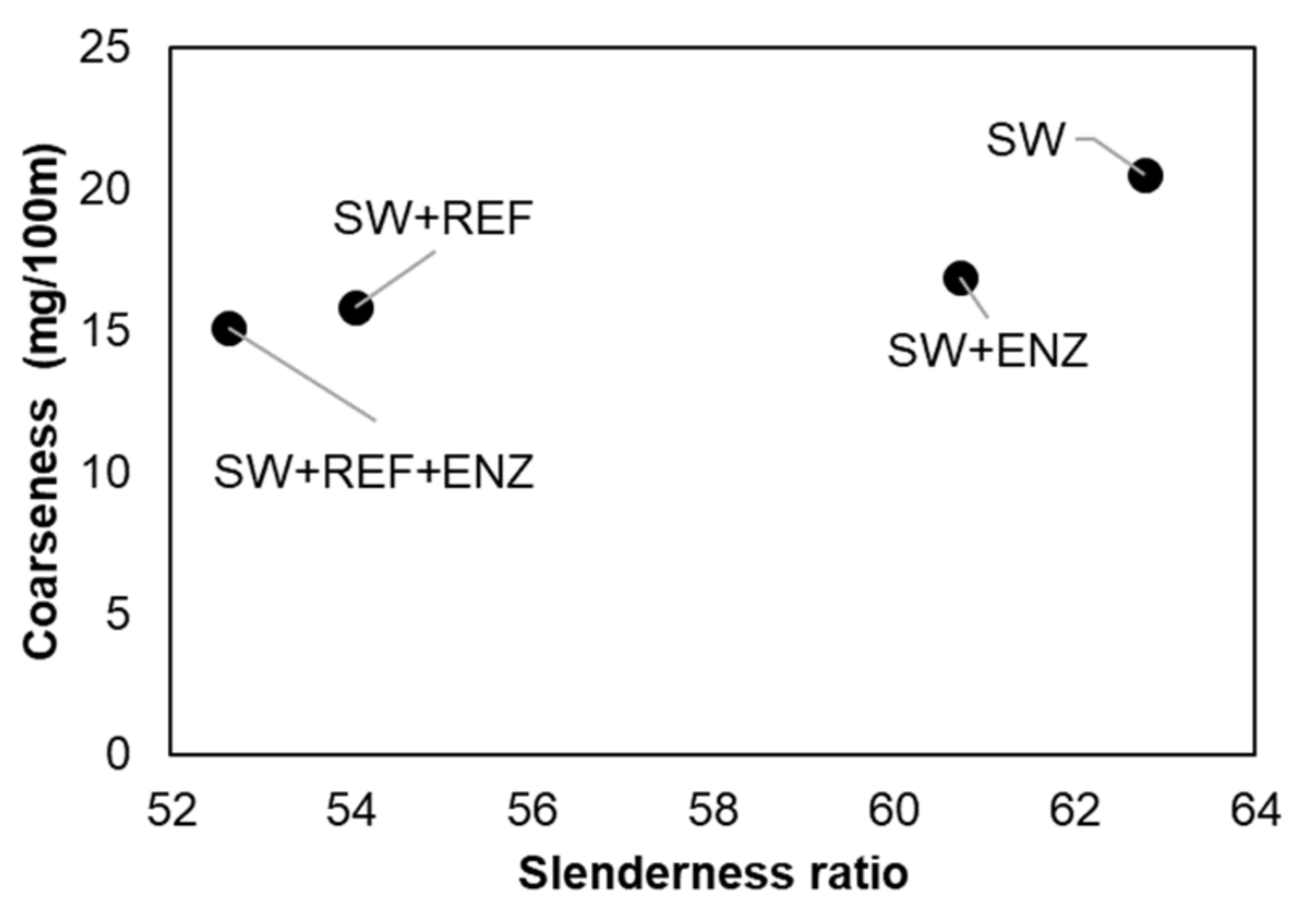
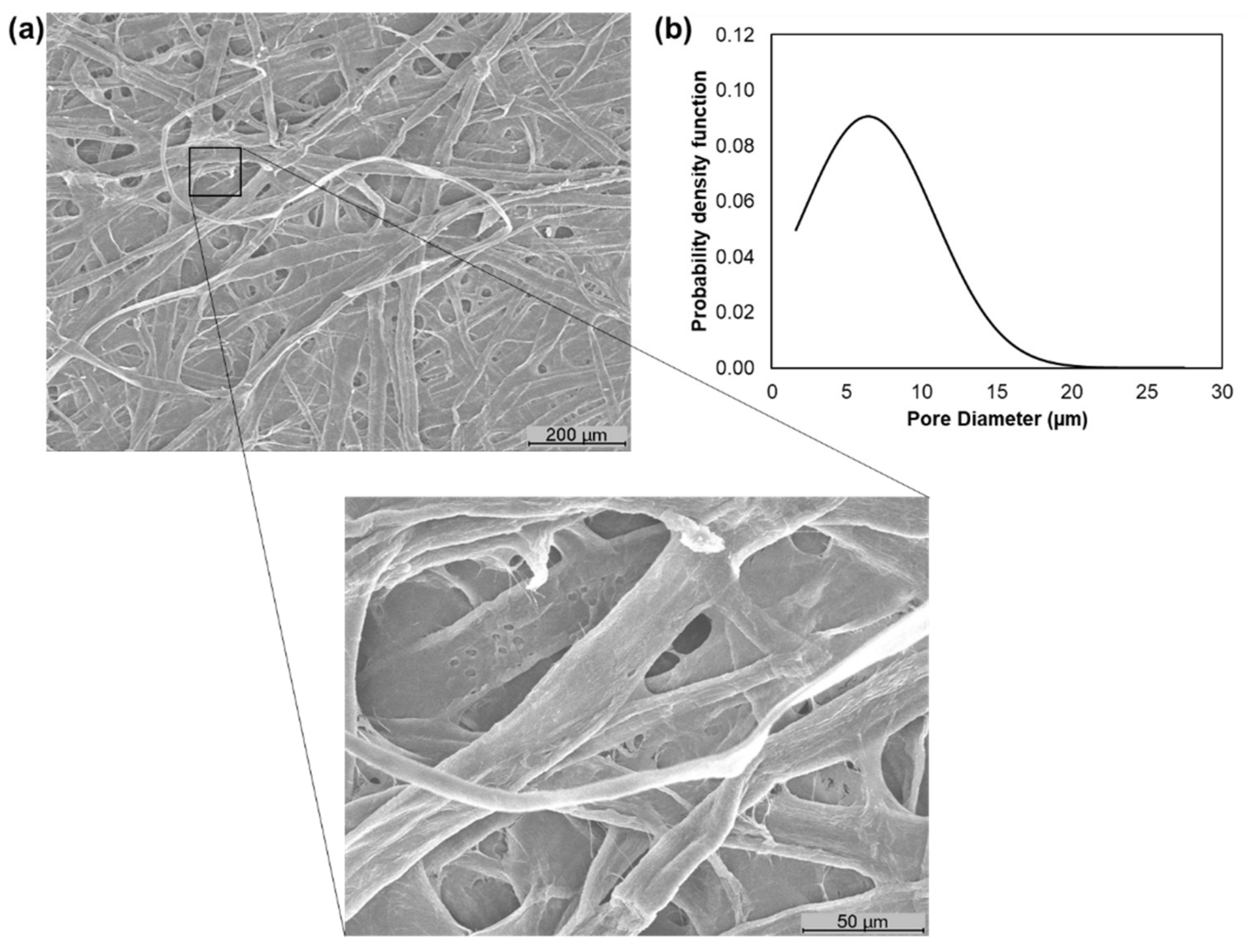
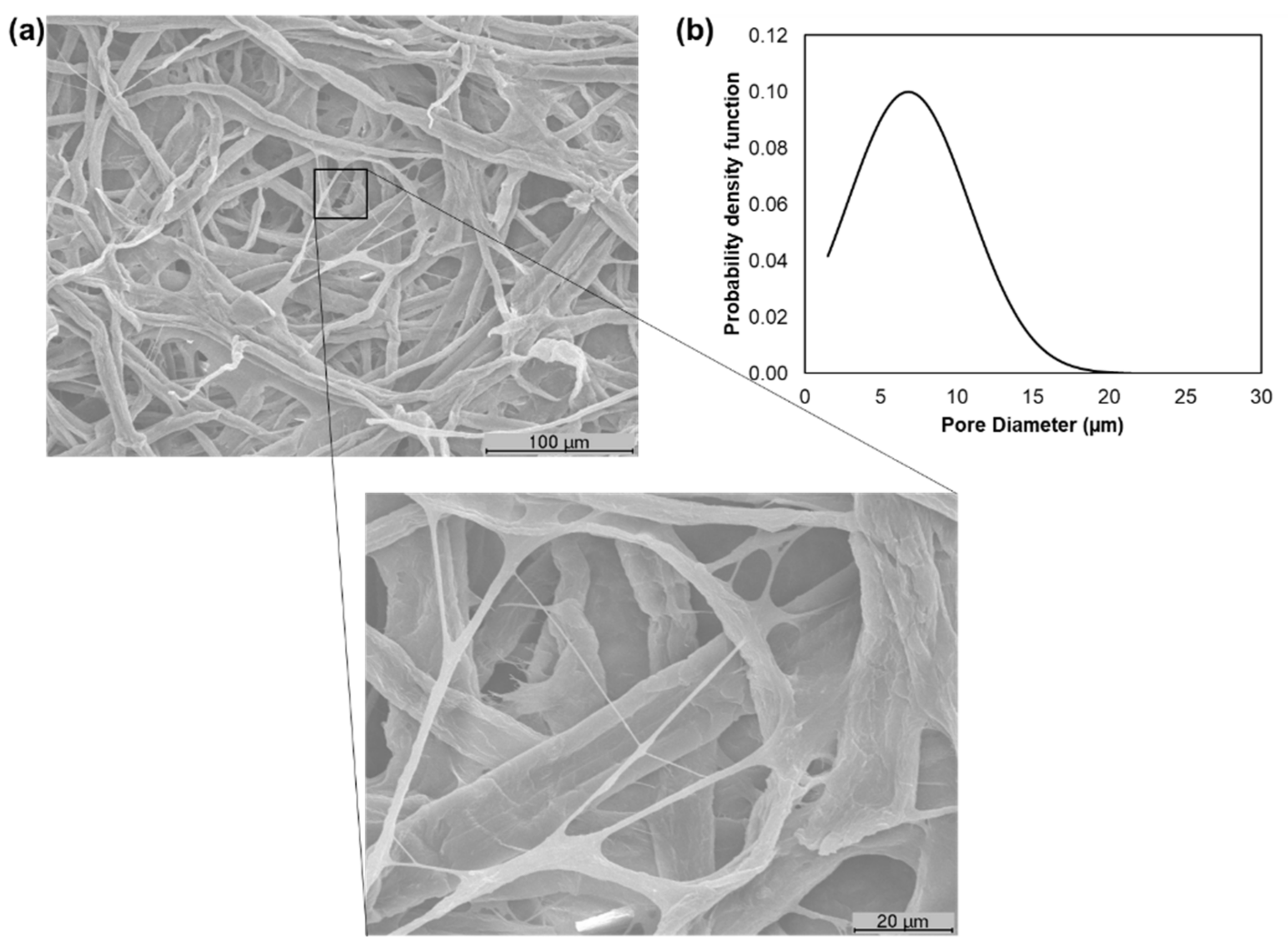
3.1. Characterization of Pulp Fibers and Tissue Formulations
3.2. Characterization of the Properties of the Final End-Use Tissue
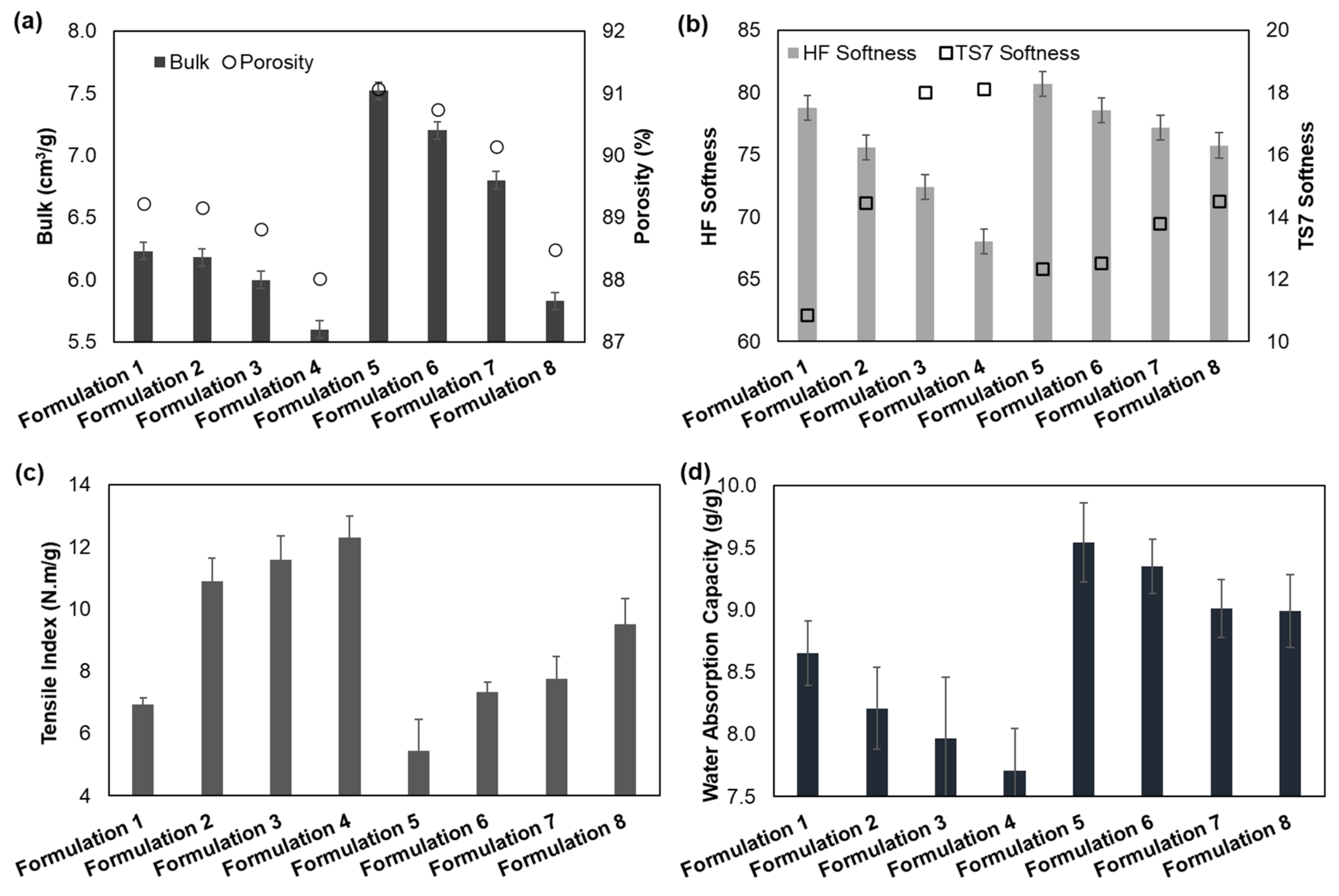
3.2.1. Structural Properties
3.2.2. Softness Properties
3.2.3. Strength Properties
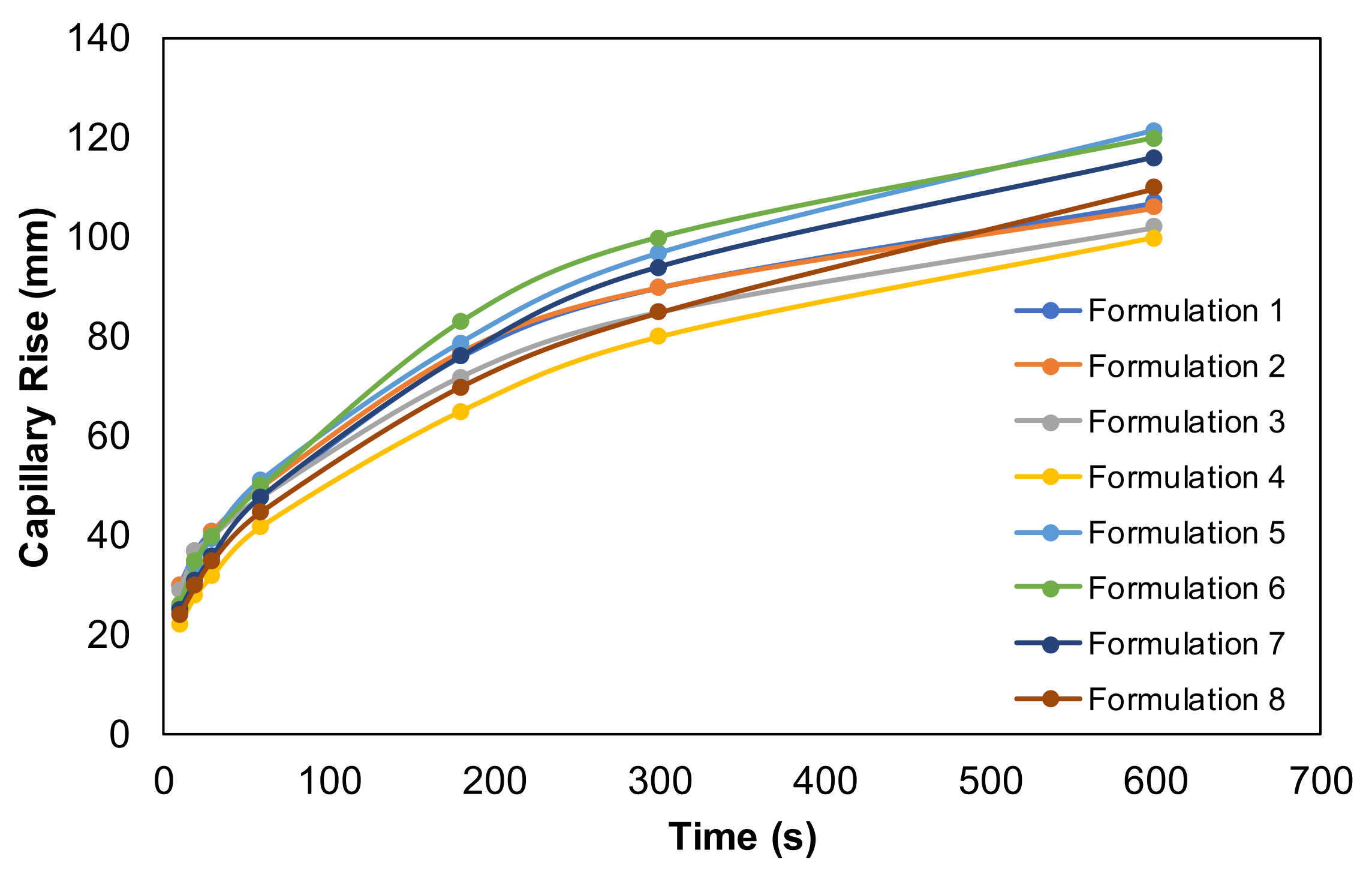
3.2.4. Absorbency Properties
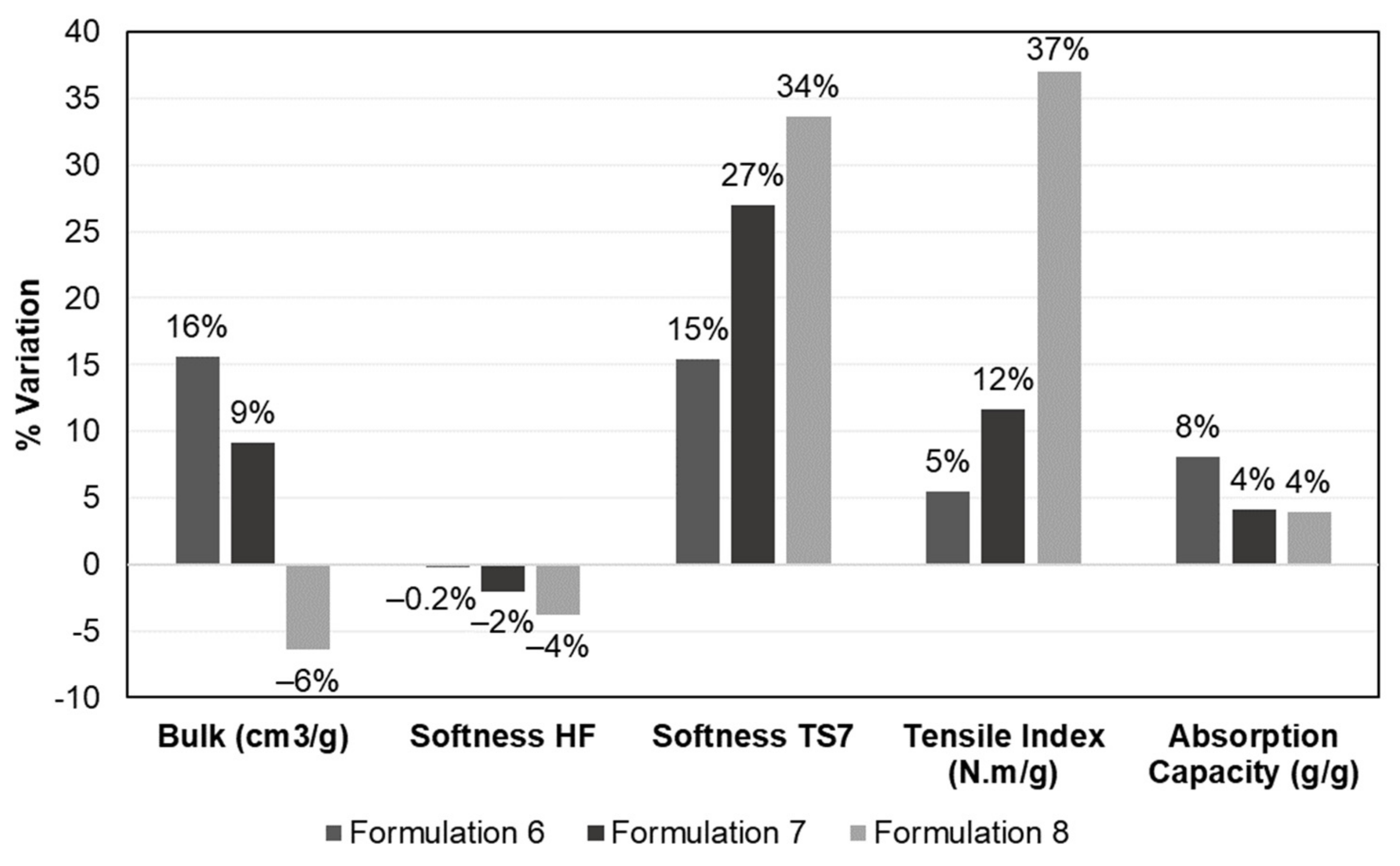
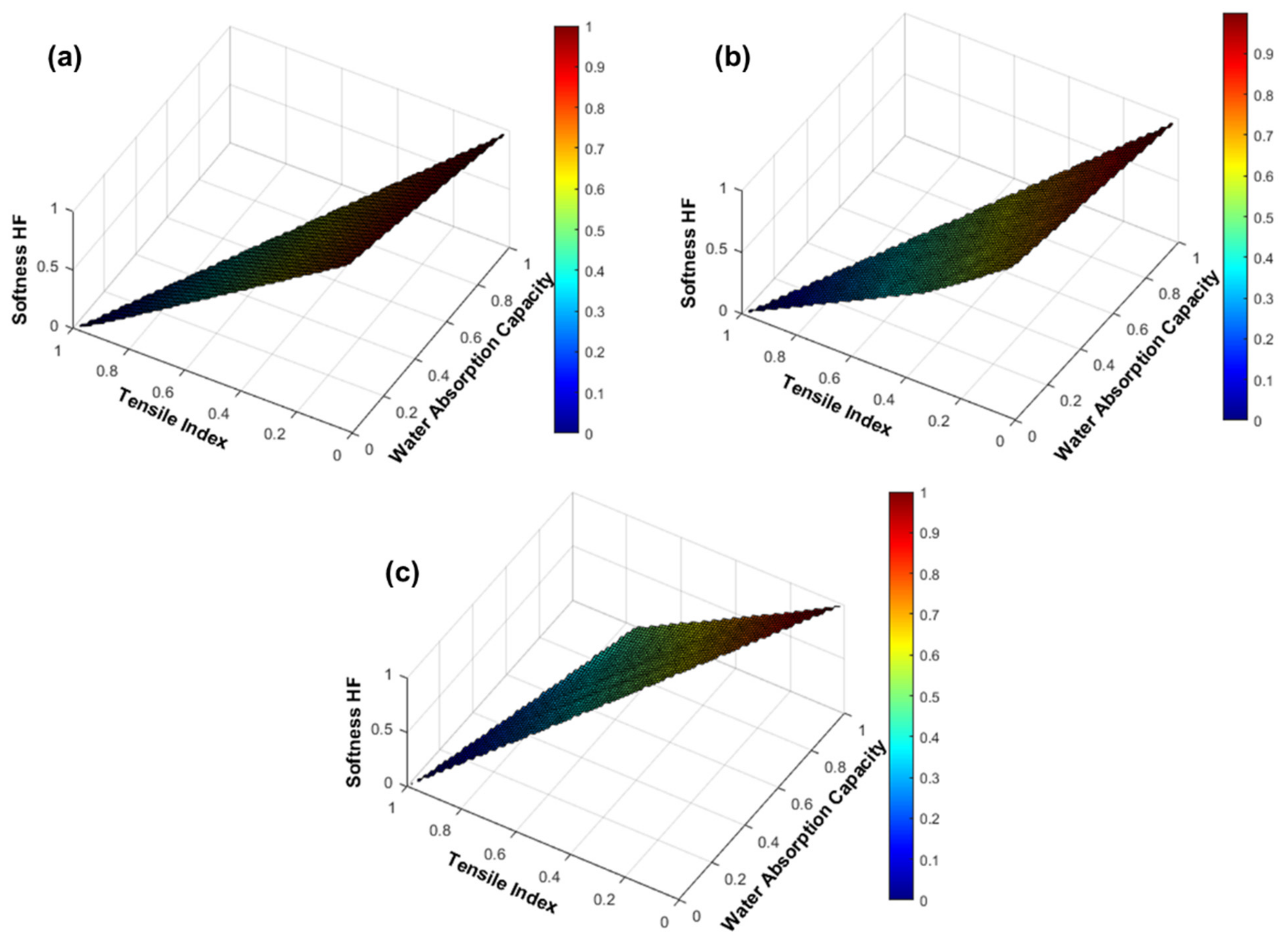
3.3. Computational Prediction of the CBA and CMF Performance in Formulations with 100% Eucalyptus Fibers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raunio, J.-P.; Löyttynieml, T.; Ritala, R. Online quality evaluation of tissue paper structure on new generation tissue machines. Nord. Pulp Pap. Res. J. 2018, 33, 133–141. [Google Scholar] [CrossRef]
- de Assis, T.; Reisinger, L.W.; Pal, L.; Pawlak, J.; Jameel, H.; Gonzalez, R.W. Understanding the Effect of Machine Technology and Cellulosic Fibers on Tissue Properties—A Review. BioResources 2018, 13, 4593–4629. [Google Scholar] [CrossRef]
- Boudreau, J.; Germgård, U. Influence of various pulp properties on the adhesion between tissue paper and Yankee cylinder surface. BioResources 2014, 9, 2107–2114. [Google Scholar] [CrossRef][Green Version]
- de Assis, T.; Pawlak, J.; Pal, L.; Jameel, H.; Reisinger, L.W.; Kavalew, D.; Campbell, C.; Pawlowska, L.; Gonzalez, R.W. Comparison between uncreped and creped handsheets on tissue paper properties using a creping simulator unit. Cellulose 2020, 27, 5981–5999. [Google Scholar] [CrossRef]
- Fišerová, M.; Gigac, J.; Stankovská, M.; Opálená, E. Influence of Bleached Softwood and Hardwood Kraft Pulps on Tissue Paper Properties. Cellul. Chem. Technol. 2019, 53, 469–477. [Google Scholar] [CrossRef]
- Morais, F.P.; Bértolo, R.A.C.; Curto, J.M.R.; Amaral, M.E.C.C.; Carta, A.M.M.S.; Evtyugin, D.V. Comparative characterization of eucalyptus fibers and softwood fibers for tissue papers applications. Mater. Lett. X 2019, 4, 100028. [Google Scholar] [CrossRef]
- Morais, F.P.; Bértolo, R.A.C.; Curto, J.M.R.; Amaral, M.E.C.C.; Carta, A.M.M.S.; Evtyugin, D.V. Characterization data of pulp fibres performance in tissue papers applications. Data Brief 2020, 29, 105253. [Google Scholar] [CrossRef]
- Stankovská, M.; Gigac, J.; Fišerová, M.; Opálená, E. Blending impact of hardwood pulps with softwood pulp on tissue paper properties. Wood Res. 2020, 65, 447–458. [Google Scholar] [CrossRef]
- Gigac, J.; Fišerová, M. Influence of pulp refining on tissue paper properties. Tappi J. 2008, 7, 27–32. [Google Scholar]
- Chang, C.-H.; Yu, S.-T.; Perng, Y.-S. Effects of furnish and refining on properties of household paper. Cellul. Chem. Technol. 2018, 52, 433–440. [Google Scholar]
- Demuner, B.J.; Junior, N.P.; Antunes, A.M.S. Technology prospecting on enzymes for the pulp and paper industry. J. Technol. Manag. Innov. 2011, 6, 148–158. [Google Scholar] [CrossRef]
- Morais, F.P.; Carta, A.M.M.S.; Amaral, M.E.; Curto, J.M.R. Cellulose Fiber Enzymatic Modification to Improve the Softness, Strength, and Absorption Properties of Tissue Papers. BioResources 2021, 16, 846–861. [Google Scholar] [CrossRef]
- Park, J.Y.; Melani, L.; Lee, H.; Kim, H.J. Effect of chemical additives on softness components of hygiene paper. Nord. Pulp Pap. Res. J. 2019, 34, 173–181. [Google Scholar] [CrossRef]
- Valencia, C.; Valencia, Y.; Tovar, C.D.G. Synthesis and Application of a Cationic Polyamine as Yankee Dryer Coating Agent for the Tissue Paper-Making Process. Polymers 2020, 12, 173. [Google Scholar] [CrossRef]
- Chan, E.; Woods, B.M.; Salaam, L.T.E. Soft Tissue Paper Having a Polyhydroxy Compound and Lotion Applied onto a Surface Thereof. U.S. Patent 7,972,475 B2, 5 July 2011. [Google Scholar]
- Siqueira, E.J.; Salon, M.-C.B.; Belgacem, M.N.; Mauret, E. Carboxymethylcellulose (CMC) as a model compound of cellulose fibers and polyamideamine epichlorohydrin (PAE)-CMC interactions as a model of PAE-fibers interactions of PAE-based wet strength papers. J. Appl. Polym. Sci. 2015, 132, 42144. [Google Scholar] [CrossRef]
- Adhikari, B.B.; Appadu, P.; Kislitsin, V.; Chae, M.; Choi, P.; Bressler, D.C. Enhancing the Adhesive Strength of a Plywood Adhesive Developed from Hydrolyzed Specified Risk Materials. Polymers 2016, 8, 285. [Google Scholar] [CrossRef]
- Miller, J.H.; Sumnicht, D.W.; Oriaran, T.P.; Schuh, B.J.; Ramirez, A.J.; Lee, J.A. High Softness, High Durability Bath Tissues with Temporary Wet Strength. U.S. Patent 9,309,627 B2, 12 April 2016. [Google Scholar]
- Miller, J.H.; Sumnicht, D.W.; Oriaran, T.P.; Schuh, B.J.; Ramirez, A.J.; Lee, J.A. Multi-Ply Bath Tissue with Temporary Wet Strength Resin and/or a Particular Lignin Content. U.S. Patent 9,879,382, 30 January 2018. [Google Scholar]
- Chen, Z.; Zhang, L.; He, Z. Rethinking the determination of wet strength of paper. BioResources 2018, 13, 2184–2186. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Li, N.; Chen, Z.; Ding, C.; Zheng, Q.; Xu, J.; Meng, Q. Synthesis of High-Water-Resistance Lignin-Phenol Resin Adhesive with Furfural as a Crosslinking Agent. Polymers 2020, 12, 2805. [Google Scholar] [CrossRef]
- Ginebreda, A.; Guilén, D.; Barceló, D.; Darbra, R.M. Additives in the Paper Industry. In Global Risk-Based Management of Chemical Additives I: Production, Usage and Environmental Occurrence, 1st ed.; Bilitewski, B., Darbra, R.M., Barceló, D., Eds.; Hdb Env Chem; Springer: Berlin/Heidelberg, Germany, 2012; pp. 11–34. [Google Scholar]
- Shen, J.; Fatehi, P.; Ni, Y. Biopolymers for surface engineering of paper-based products. Cellulose 2014, 21, 3145–3160. [Google Scholar] [CrossRef]
- Morais, F.P.; Carta, A.M.M.S.; Amaral, M.E.; Curto, J.M.R. Micro/nano-fibrillated cellulose (MFC/NFC) fibers as an additive to maximize eucalyptus fibers on tissue paper production. Cellulose 2021, 28, 6587–6605. [Google Scholar] [CrossRef]
- Guan, M.; An, X.; Liu, H. Cellulose nanofiber (CNF) as a versatile filler for the preparation of bamboo pulp based tissue paper handsheets. Cellulose 2019, 26, 2613–2624. [Google Scholar] [CrossRef]
- Patiño-Masó, J.; Serra-Parareda, F.; Tarrés, Q.; Mutjé, P.; Espinach, F.X.; Delgado-Aguilar, M. TEMPO-Oxidized Cellulose Nanofibers: A Potential Bio-Based Superabsorbent for Diaper Production. Nanomaterials 2019, 9, 1271. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, F.; Wang, Y.; Zwilling, J.D.; Venditti, R.; Jameel, H.; Rojas, O.; Gonzalez, R. Micro- and nanofibrillated cellulose from virgin and recycled fibers: A comparative study of its effects on the properties of hygiene tissue paper. Carbohyd. Polym. 2021, 254, 117430. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, F.; Starkey, H.; Wang, Y.; de Assis, C.A.; Venditti, R.; Pal, L.; Jameel, H.; Hubbe, M.A.; Rojas, O.J.; Gonzalez, R. Using Micro- and Nanofibrillated Cellulose as a Means to Reduce Weight of Paper Products: A Review. BioResources 2020, 15, 4553–4590. [Google Scholar] [CrossRef]
- González, I.; Boufi, S.; Pèlach, M.A.; Alcalà, M.; Vilaseca, F.; Mutjé, P. Nanofibrillated cellulose as paper additive in Eucalyptus pulps. BioResources 2012, 7, 5167–5180. [Google Scholar] [CrossRef]
- de Assis, T.C.A.; Iglesias, M.C.; Bilodeau, M.; Johnson, D.; Phillips, R.; Peresin, M.S.; Bilek, E.M.; Rojas, O.J.; Venditti, R.; Gonzalez, R. Cellulose micro- and nanofibrils (CMNF) manufacturing—financial and risk assessment. Biofuels Bioprod. Biorefining 2018, 12, 251–264. [Google Scholar] [CrossRef]
- Hotaling, N.A.; Bharti, K.; Kriel, H.; Simon, C.G., Jr. DiameterJ: A Validated Open Source Nanofiber Diameter Measurement Tool. Biomaterials 2015, 61, 327–338. [Google Scholar] [CrossRef]
- Conceição, E.L.T.; Curto, J.M.R.; Simões, R.M.S.; Portugal, A.T.G. Coding a simulation model of the 3D structure of paper. In Computational Modeling of Objects Represented in Images. CompIMAGE 2010. Lecture Notes in Computer Science, 1st ed.; Barneva, R.P., Brimkov, V.E., Hauptman, H.A., Natal Jorge, R.M., Tavares, J.M.R.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 6026, pp. 299–310. [Google Scholar]
- Curto, J.M.R.; Conceição, E.L.T.; Portugal, A.T.G.; Simões, R.M.S. Three dimensional modeling of fibrous materials and experimental validation. Mater. Werkst. 2011, 42, 370–374. [Google Scholar] [CrossRef]
- Morais, F.P.; Carta, A.M.M.S.; Amaral, M.E.; Curto, J.M.R. 3D Fiber Models to Simulate and Optimize Tissue Materials. BioResources 2020, 15, 8833–8848. [Google Scholar] [CrossRef]
- de Assis, T.; Huang, S.; Driemeier, C.E.; Donohoe, B.S.; Kim, C.; Kim, S.H.; Gonzalez, R.; Jameel, H.; Park, S. Toward an understanding of the increase in enzymatic hydrolysis by mechanical refining. Biotechnol. Biofuels 2018, 11, 289. [Google Scholar] [CrossRef]
- Martins, V.D.F.; Cerqueira, M.A.; Fuciños, P.; Garrido-Maestu, A.; Curto, J.M.R.; Pastrana, L.M. Active bi-layer cellulose-based films: Development and characterization. Cellulose 2018, 25, 6361–6375. [Google Scholar] [CrossRef]
- Trepanier, R. Pulp fiber quality and the relationship with paper tissue properties. In Proceedings of the issue Conference & Expo 2017: The Power of TAPPI & RISI, Miami Beach, FL, USA, 3–6 October 2017; pp. 1–4. [Google Scholar]
- Maurer, H.W. Starch in the Paper Industry. In Starch—Chemistry and Technology. Food Science and Technology, 3rd ed.; BeMiller, J., Whistler, R., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 657–713. [Google Scholar]
- Beuther, J.C.; Veith, M.W.; Zwick, K.J. Characterization of Absorbent Flow Rate in Towel and Tissue. J. Eng. Fibers Fabr. 2010, 5, 1–7. [Google Scholar] [CrossRef]




| Eucalyptus Pulp | Softwood Pulp with Mechanical and Enzymatic Treatment | CBA (Commercial Biopolymer Additive) | CMF (Micro/Nanofibrillated Cellulose) | |
|---|---|---|---|---|
| Formulation 1 | 75% | 25% | - | - |
| Formulation 2 | 75% | 25% | 2% | - |
| Formulation 3 | 75% | 25% | - | 2% |
| Formulation 4 | 75% | 25% | 2% | 2% |
| Formulation 5 | 90% | 10% | - | - |
| Formulation 6 | 90% | 10% | 2% | - |
| Formulation 7 | 90% | 10% | - | 2% |
| Formulation 8 | 90% | 10% | 2% | 2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morais, F.P.; Carta, A.M.M.S.; Amaral, M.E.; Curto, J.M.R. An Innovative Computational Strategy to Optimize Different Furnish Compositions of Tissue Materials Using Micro/Nanofibrillated Cellulose and Biopolymer as Additives. Polymers 2021, 13, 2397. https://doi.org/10.3390/polym13152397
Morais FP, Carta AMMS, Amaral ME, Curto JMR. An Innovative Computational Strategy to Optimize Different Furnish Compositions of Tissue Materials Using Micro/Nanofibrillated Cellulose and Biopolymer as Additives. Polymers. 2021; 13(15):2397. https://doi.org/10.3390/polym13152397
Chicago/Turabian StyleMorais, Flávia P., Ana M. M. S. Carta, Maria E. Amaral, and Joana M. R. Curto. 2021. "An Innovative Computational Strategy to Optimize Different Furnish Compositions of Tissue Materials Using Micro/Nanofibrillated Cellulose and Biopolymer as Additives" Polymers 13, no. 15: 2397. https://doi.org/10.3390/polym13152397
APA StyleMorais, F. P., Carta, A. M. M. S., Amaral, M. E., & Curto, J. M. R. (2021). An Innovative Computational Strategy to Optimize Different Furnish Compositions of Tissue Materials Using Micro/Nanofibrillated Cellulose and Biopolymer as Additives. Polymers, 13(15), 2397. https://doi.org/10.3390/polym13152397