Assessment of Bond Integrity, Durability, and Degree of Conversion of a Calcium Fluoride Reinforced Dentin Adhesive
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation of CaF2 Nano-Crystals
2.2. Formulation of the EA and Incorporation of CaF2 Nano-Crystals
2.3. Characterization of CaF2 Nano-Crystals
2.4. Preparation of Teeth and Procedure for Bonding
2.5. μTBS Testing and Failure Mode Analysis
2.6. SEM and EDX Mapping of the Adhesive-Dentin Interface
2.7. Micro-Raman Spectroscopy Investigation
2.8. FTIR and DC Analysis
2.9. Statistical Analysis
3. Results
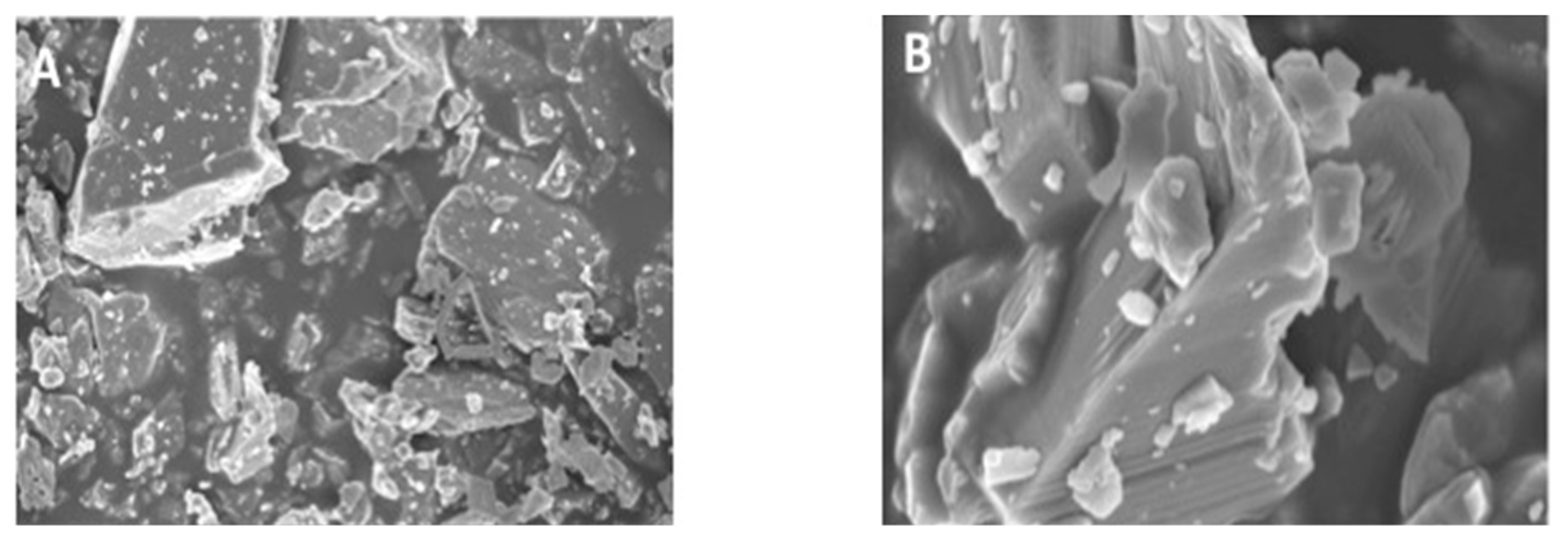
3.1. Morphology of CaF2 Nano-Crystals
3.2. μTBS Testing and Failure Mode Analysis Outcomes
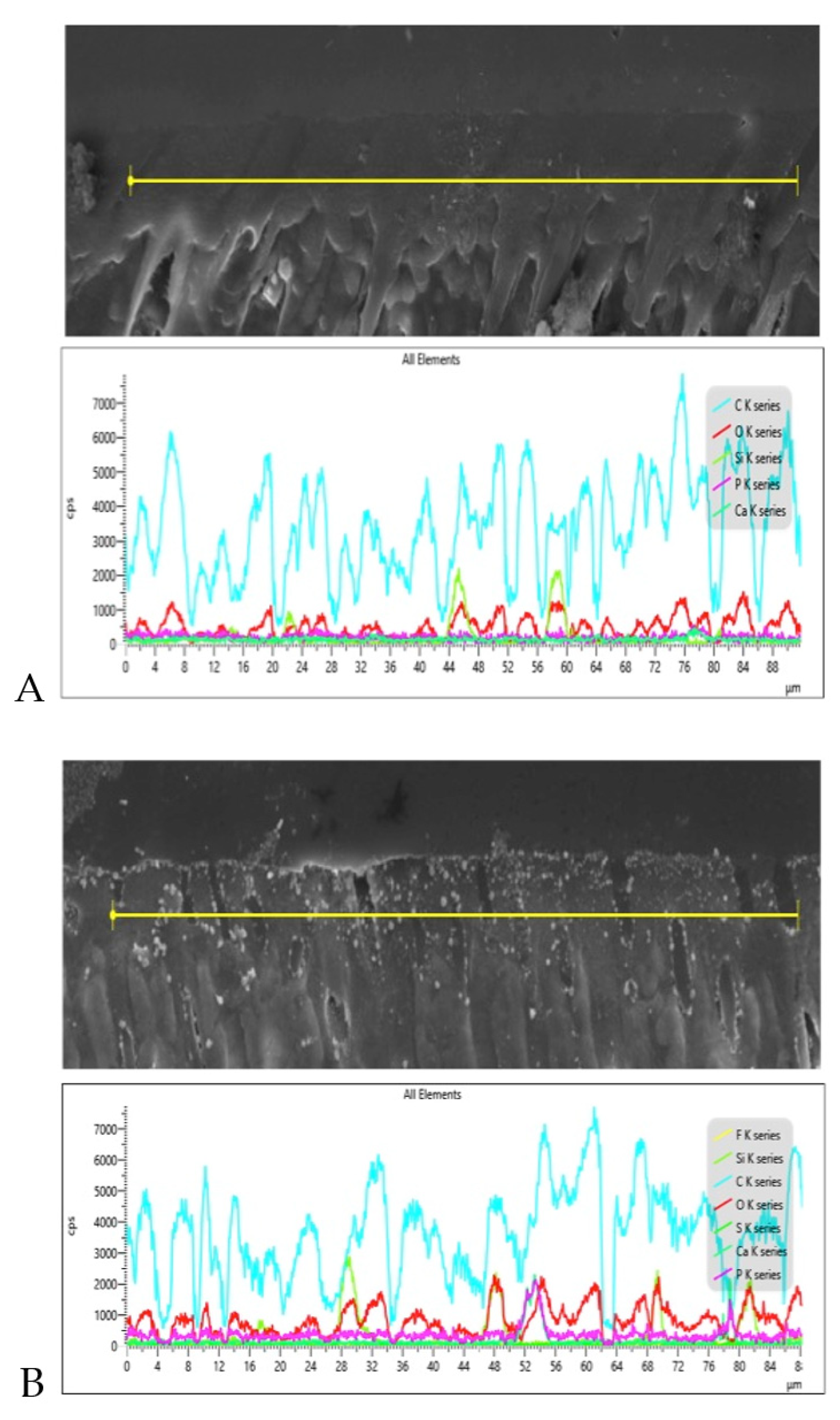
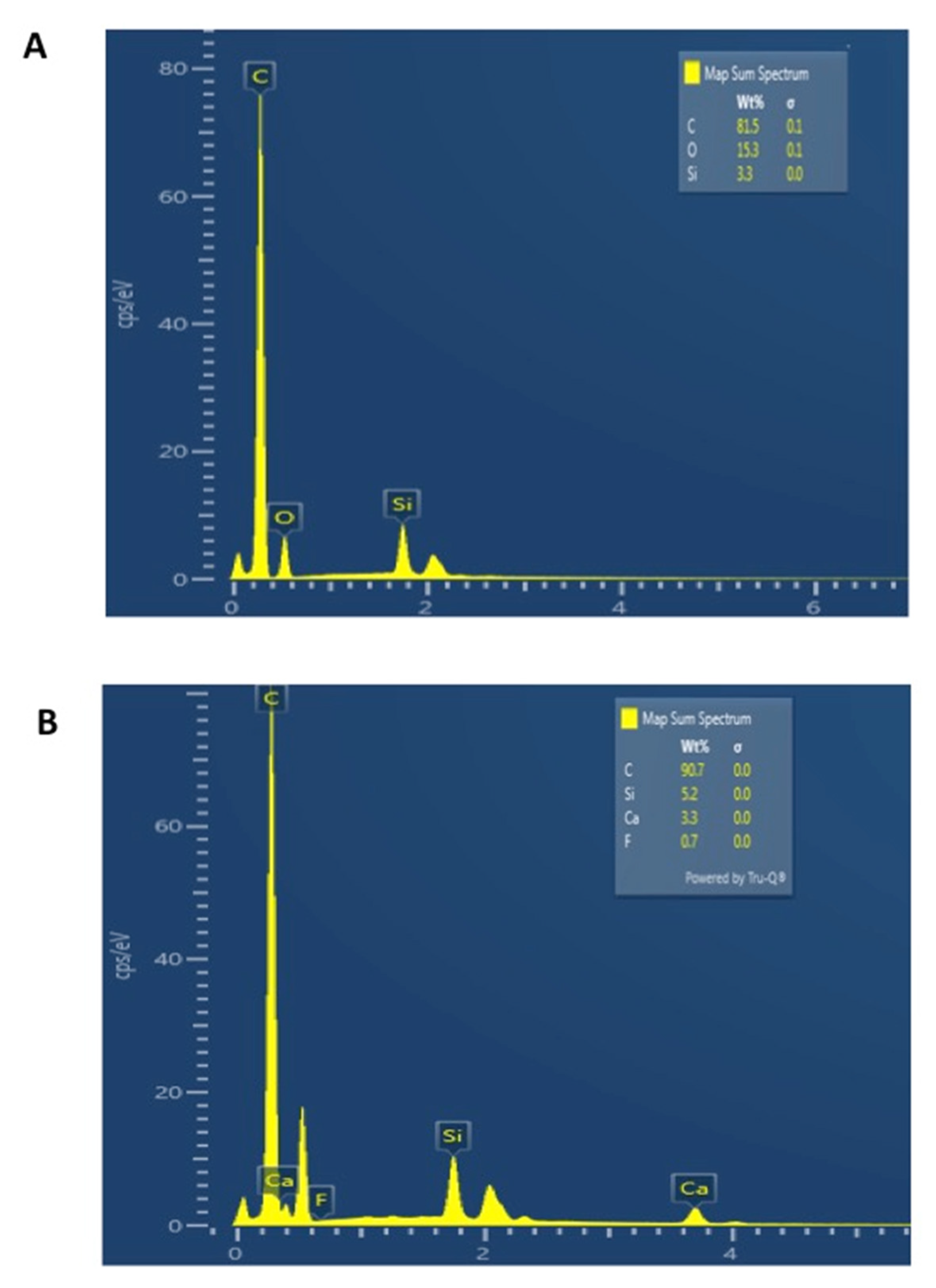
3.3. SEM and EDX Mapping Outcomes
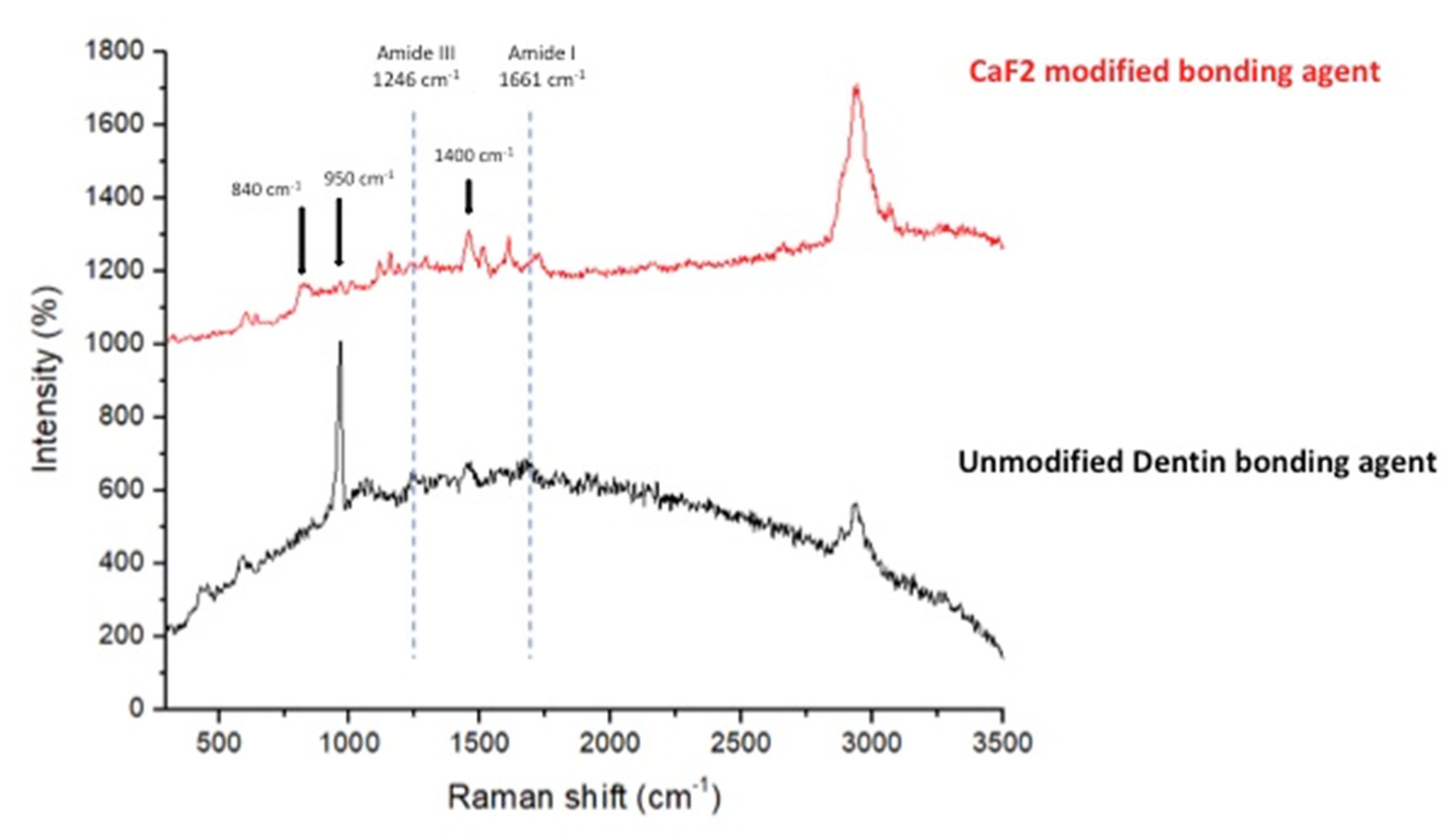
3.4. Micro-Raman Spectroscopy Analysis Results
3.5. FTIR Spectroscopy and DC Analysis Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vergnes, J.N.; Mazevet, M. Oral diseases: A global public health challenge. Lancet 2020, 395, 186. [Google Scholar] [CrossRef]
- Alhussain, A.M.; Alhaddad, A.A.; Ghazwi, M.M.; Farooq, I. Remineralization of artificial carious lesions using a novel fluoride incorporated bioactive glass dentifrice. Dent. Med. Probl. 2018, 55, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Ruiz, A.J.; Perez-Guzman, N.; Rubio-Aparicio, M.; Sanchez-Meca, J. Success rate of proximal tooth-coloured direct restorations in primary teeth at 24 months: A meta-analysis. Sci. Rep. 2020, 10, 6409. [Google Scholar] [CrossRef] [PubMed]
- Aminoroaya, A.; Esmaeely Neisiany, R.; Nouri Khorasani, S.; Panahi, P.; Das, O.; Ramakrishna, S. A Review of Dental Composites: Methods of Characterizations. ACS Biomater. Sci. Eng. 2020, 6, 3713–3744. [Google Scholar] [CrossRef]
- Sakaguchi, R.L. Review of the current status and challenges for dental posterior restorative composites: Clinical, chemistry, and physical behavior considerations. Summary of discussion from the Portland Composites Symposium (POCOS), 17–19 June 2004, Oregon Health and Science University, Portland, Oregon. Dent. Mater. 2005, 21, 3–6. [Google Scholar] [PubMed]
- Bourbia, M.; Finer, Y. Biochemical Stability and Interactions of Dental Resin Composites and Adhesives with Host and Bacteria in the Oral Cavity: A Review. J. Can. Dent. Assoc. 2018, 84, 1–7. [Google Scholar]
- Ferracane, J.L. Models of Caries Formation around Dental Composite Restorations. J. Dent. Res. 2017, 96, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Leitune, V.C.; Collares, F.M.; Trommer, R.M.; Andrioli, D.G.; Bergmann, C.P.; Samuel, S.M. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J. Dent. 2013, 41, 321–327. [Google Scholar] [CrossRef]
- Manuja, N.; Nagpal, R.; Pandit, I.K. Dental adhesion: Mechanism, techniques and durability. J. Clin. Pediatr. Dent. 2012, 36, 223–234. [Google Scholar] [CrossRef]
- Peumans, M.; Kanumilli, P.; De Munck, J.; Van Landuyt, K.; Lambrechts, P.; Van Meerbeek, B. Clinical effectiveness of contemporary adhesives: A systematic review of current clinical trials. Dent. Mater. 2005, 21, 864–881. [Google Scholar] [CrossRef]
- Huang, B.; Siqueira, W.L.; Cvitkovitch, D.G.; Finer, Y. Esterase from a cariogenic bacterium hydrolyzes dental resins. Acta Biomater. 2018, 71, 330–338. [Google Scholar] [CrossRef]
- Santos, G.C., Jr.; Santos, M.J.; Rizkalla, A.S. Adhesive cementation of etchable ceramic esthetic restorations. J. Can. Dent. Assoc. 2009, 75, 379–384. [Google Scholar]
- Kazak, M.; Donmez, N. Development of dentin bonding systems from past to present. Bez. Sci. 2019, 7, 322–330. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, S.; Zhou, X.; Hannig, M.; Rupf, S.; Feng, J.; Peng, X.; Cheng, L. Modifying Adhesive Materials to Improve the Longevity of Resinous Restorations. Int. J. Mol. Sci. 2019, 20, 723. [Google Scholar] [CrossRef] [PubMed]
- Farooq, I.; Bugshan, A. The role of salivary contents and modern technologies in the remineralization of dental enamel: A narrative review. F1000Research 2020, 9, 171. [Google Scholar] [CrossRef]
- ten Cate, J.M. Review on fluoride, with special emphasis on calcium fluoride mechanisms in caries prevention. Eur. J. Oral Sci. 1997, 105, 461–465. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Khan, S.; Hasan, S.; Khan, M.E.; Misba, L.; Khan, A.U. Calcium fluoride nano-crystals induced suppression of Streptococcus mutans biofilm: An in vitro and in vivo approach. Appl. Microbiol. Biotechnol. 2016, 100, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Lukomska-Szymanska, M.; Zarzycka, B.; Grzegorczyk, J.; Sokolowski, K.; Poltorak, K.; Sokolowski, J.; Lapinska, B. Antibacterial Properties of Calcium Fluoride-Based Composite Materials: In Vitro Study. Biomed. Res. Int. 2016, 2016, 1048320. [Google Scholar] [PubMed]
- Mitwalli, H.; Balhaddad, A.A.; AlSahafi, R.; Oates, T.W.; Melo, M.A.S.; Xu, H.H.K.; Weir, M.D. Novel CaF2 Nanocomposites with Antibacterial Function and Fluoride and Calcium Ion Release to Inhibit Oral Biofilm and Protect Teeth. J. Funct. Biomater. 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Li, Y.; Weir, M.D.; Baras, B.H.; Wang, H.; Wang, S.; Sun, J.; Melo, M.A.S.; Ruan, J.; Xu, H.H.K. Novel pit and fissure sealant containing nano-CaF2 and dimethylaminohexadecyl methacrylate with double benefits of fluoride release and antibacterial function. Dent. Mater. 2020, 36, 1241–1253. [Google Scholar] [CrossRef]
- Yi, J.; Dai, Q.; Weir, M.D.; Melo, M.A.S.; Lynch, C.D.; Oates, T.W.; Zhang, K.; Zhao, Z.; Xu, H.H.K. A nano-CaF2-containing orthodontic cement with antibacterial and remineralization capabilities to combat enamel white spot lesions. J. Dent. 2019, 89, 103172. [Google Scholar] [CrossRef]
- Mahrous, A.; Radwan, M.M.; Kamel, S.M. Micro-Shear Bond Strength of Novel MDP Calcium-Fluoride-Releasing Self-Adhesive Resin Cement after Thermocycling. Int. J. Periodontics Restor. Dent. 2020, 40, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Essam, M.; Niazy, M.A.; Farouk, H.; Mostafa, A.A. The Remineralizing Effect of Incorporating Ca-Phosphate and Ca-Fluoride Nano-crystals into the Self-Etch Adhesives Used in Restoring Class I Cavities. Al-Azhar Dent. J. Girls 2019, 6, 231–238. [Google Scholar] [CrossRef]
- Xu, H.H.; Moreau, J.L.; Sun, L.; Chow, L.C. Strength and fluoride release characteristics of a calcium fluoride based dental nanocomposite. Biomaterials 2008, 29, 4261–4267. [Google Scholar] [CrossRef]
- Alqarawi, F.K.; Alkahtany, M.F.; Almadi, K.H.; Ben Gassem, A.A.; Alshahrani, F.A.; AlRefeai, M.H.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Different Conditioning Treatments on the Bond Integrity of Root Dentin to rGO Infiltrated Dentin Adhesive. SEM, EDX, FTIR and MicroRaman Study. Polymers 2021, 13, 1555. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yamaguchi, K.; Tsubota, K.; Takamizawa, T.; Kurokawa, H.; Rikuta, A.; Ando, S.; Miyazaki, M. Effect of metal conditioners on polymerization behavior of bonding agents. J. Oral Sci. 2005, 47, 171–175. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Hamdan, R.S.; Almutairi, B.; Kattan, H.F.; Alsuwailem, N.A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Hydroxyapatite Nanospheres in Dentin Adhesive on the Dentin Bond Integrity and Degree of Conversion: A Scanning Electron Microscopy (SEM), Raman, Fourier Transform-Infrared (FTIR), and Microtensile Study. Polymers 2020, 12, 2948. [Google Scholar] [CrossRef]
- AlFawaz, Y.F.; Almutairi, B.; Kattan, H.F.; Zafar, M.S.; Farooq, I.; Naseem, M.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Hydroxyapatite Containing Resin Adhesive Enhanced with Graphene Oxide Nano-Particles-An SEM, EDX, Micro-Raman, and Microtensile Bond Strength Study. Polymers 2020, 12, 2978. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, B.; Kattan, H.F.; BinMahfooz, A.M.; Qutub, O.A.; Basunbul, G.; ArRejaie, A.S.; Farooq, I.; Vohra, F.; Abduljabbar, T. Synergistic effect of graphene oxide/calcium phosphate nanofiller in a dentin adhesive on its dentin bond integrity and degree of conversion. A scanning electron microscopy, energy dispersive X-ray spectroscopy, Fourier transform infrared, micro-Raman, and bond strength study. Microsc. Res. Tech. 2021, in press. [Google Scholar]
- Koeser, J.; Carvalho, T.S.; Pieles, U.; Lussi, A. Preparation and optimization of calcium fluoride particles for dental applications. J. Mater. Sci. Mater. Med. 2014, 25, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Bozec, L.; Perez, R.A.; Kim, H.W.; Knowles, J.C. Nanotechnology in dentistry: Prevention, diagnosis, and therapy. Int. J. Nanomed. 2015, 10, 6371–6394. [Google Scholar] [CrossRef]
- Firoozmand, L.M.; Noleto, L.E.; Gomes, I.A.; Bauer, J.R.; Ferreira, M.C. Effect of Fluoride and Simplified Adhesive Systems on the Bond Strength of Primary Molars and Incisors. Braz. Dent. J. 2015, 26, 368–373. [Google Scholar] [CrossRef]
- Wagner, A.; Belli, R.; Stotzel, C.; Hilpert, A.; Muller, F.A.; Lohbauer, U. Biomimetically- and hydrothermally-grown HAp nano-crystals as reinforcing fillers for dental adhesives. J. Adhes. Dent. 2013, 15, 413–422. [Google Scholar] [PubMed]
- Noorani, T.Y.; Luddin, N.; Rahman, I.A.; Masudi, S.M. In Vitro Cytotoxicity Evaluation of Novel Nano-Hydroxyapatite-Silica Incorporated Glass Ionomer Cement. J. Clin. Diagn. Res. 2017, 11, ZC105–ZC109. [Google Scholar] [CrossRef] [PubMed]
- Can-Karabulut, D.C.; Oz, F.T.; Karabulut, B.; Batmaz, I.; Ilk, O. Adhesion to primary and permanent dentin and a simple model approach. Eur. J. Dent. 2009, 3, 32–41. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO/TS 11405 Dentistry—Testing of Adhesion to Tooth Structure, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Helvatjoglu-Antoniades, M.; Koliniotou-Kubia, E.; Dionyssopoulos, P. The effect of thermal cycling on the bovine dentine shear bond strength of current adhesive systems. J. Oral Rehabil. 2004, 31, 911–917. [Google Scholar] [CrossRef]
- Alhenaki, A.M.; Attar, E.A.; Alshahrani, A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Filled and Unfilled Resin Adhesive Enhanced with Silica Nano-crystals-An SEM, EDX, Micro-Raman, FTIR and Micro-Tensile Bond Strength Study. Polymers 2021, 13, 1093. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, T.R.; de Oliveira, M.; Arrais, C.A.; Ambrosano, G.M.; Rueggeberg, F.; Giannini, M. The effect of photopolymerization on the degree of conversion, polymerization kinetic, biaxial flexure strength, and modulus of self-adhesive resin cements. J. Prosthet. Dent. 2015, 113, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, M.; Balazsi, R.; Soanca, A.; Roman, A.; Sarosi, C.; Prodan, D.; Vlassa, M.; Cojocaru, I.; Saceleanu, V.; Cristescu, I. Evaluation of the Degree of Conversion, Residual Monomers and Mechanical Properties of Some Light-Cured Dental Resin Composites. Materials 2019, 12, 2109. [Google Scholar] [CrossRef]


| μTBS (MPa) (Mean ± SD) | Failure Mode Analysis (%) | |||||
|---|---|---|---|---|---|---|
| Group (n = 10) | NTC | TC | p-Value * | Adhesive | Cohesive | Mixed |
| CAFA | 32.63 ± 3.15 a A | - | <0.01 | 80 | 0 | 20 |
| - | 29.47 ± 3.33 a A | 100 | 0 | 0 | ||
| EA | 28.80 ± 2.75 a B | 80 | 0 | 20 | ||
| - | 24.04 ± 3.69 b B | 90 | 0 | 10 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlRefeai, M.H.; AlHamdan, E.M.; Al-Saleh, S.; Farooq, I.; Abrar, E.; Vohra, F.; Abduljabbar, T. Assessment of Bond Integrity, Durability, and Degree of Conversion of a Calcium Fluoride Reinforced Dentin Adhesive. Polymers 2021, 13, 2418. https://doi.org/10.3390/polym13152418
AlRefeai MH, AlHamdan EM, Al-Saleh S, Farooq I, Abrar E, Vohra F, Abduljabbar T. Assessment of Bond Integrity, Durability, and Degree of Conversion of a Calcium Fluoride Reinforced Dentin Adhesive. Polymers. 2021; 13(15):2418. https://doi.org/10.3390/polym13152418
Chicago/Turabian StyleAlRefeai, Mohammad H., Eman M. AlHamdan, Samar Al-Saleh, Imran Farooq, Eisha Abrar, Fahim Vohra, and Tariq Abduljabbar. 2021. "Assessment of Bond Integrity, Durability, and Degree of Conversion of a Calcium Fluoride Reinforced Dentin Adhesive" Polymers 13, no. 15: 2418. https://doi.org/10.3390/polym13152418
APA StyleAlRefeai, M. H., AlHamdan, E. M., Al-Saleh, S., Farooq, I., Abrar, E., Vohra, F., & Abduljabbar, T. (2021). Assessment of Bond Integrity, Durability, and Degree of Conversion of a Calcium Fluoride Reinforced Dentin Adhesive. Polymers, 13(15), 2418. https://doi.org/10.3390/polym13152418







